The p130Cas-Crk/CrkL Axis: A Therapeutic Target for Invasive Cancers Unveiled by Collaboration Among p130Cas, Crk, and CrkL
Abstract
1. Introduction
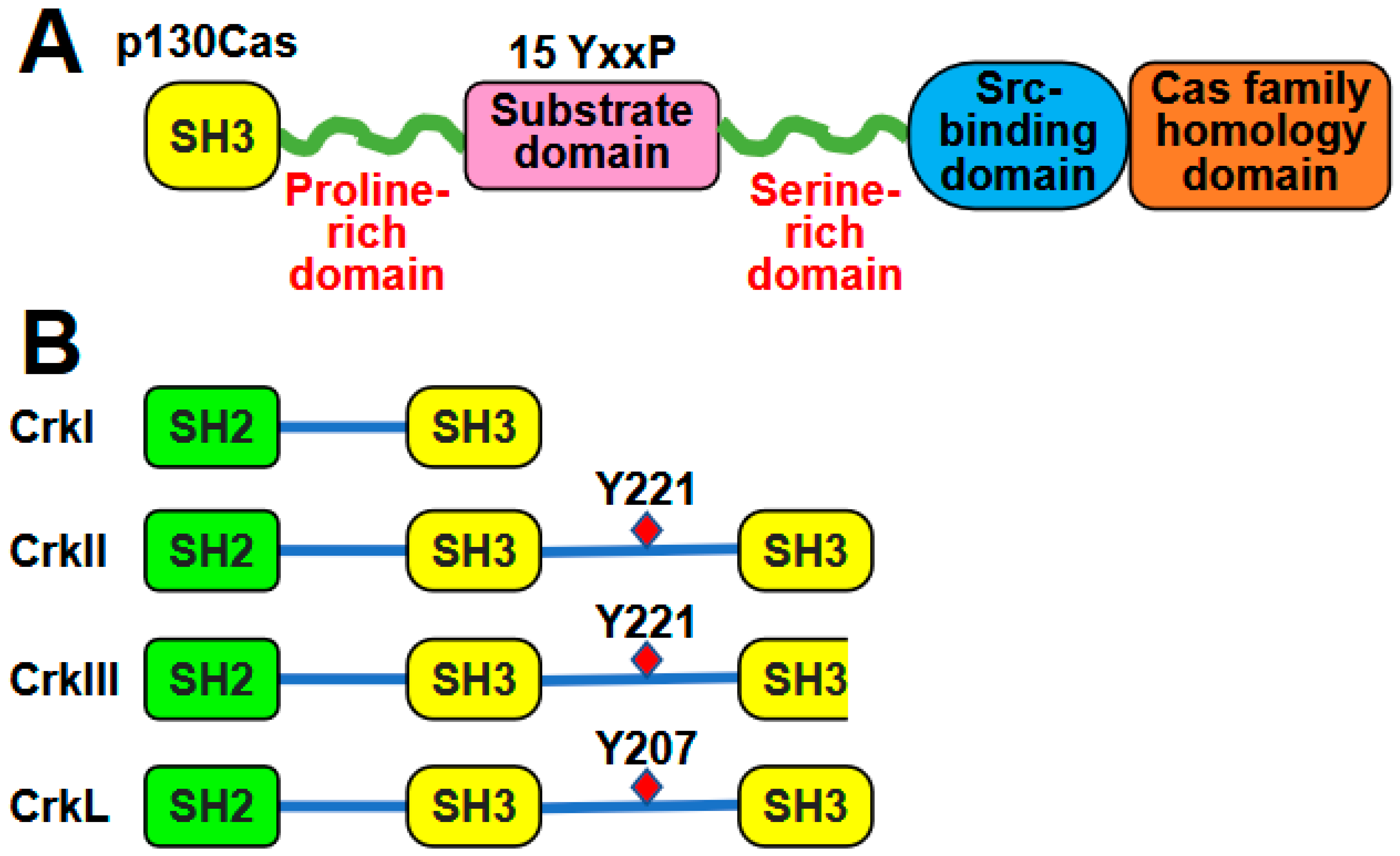
2. Structures of p130Cas and Crk Family Proteins
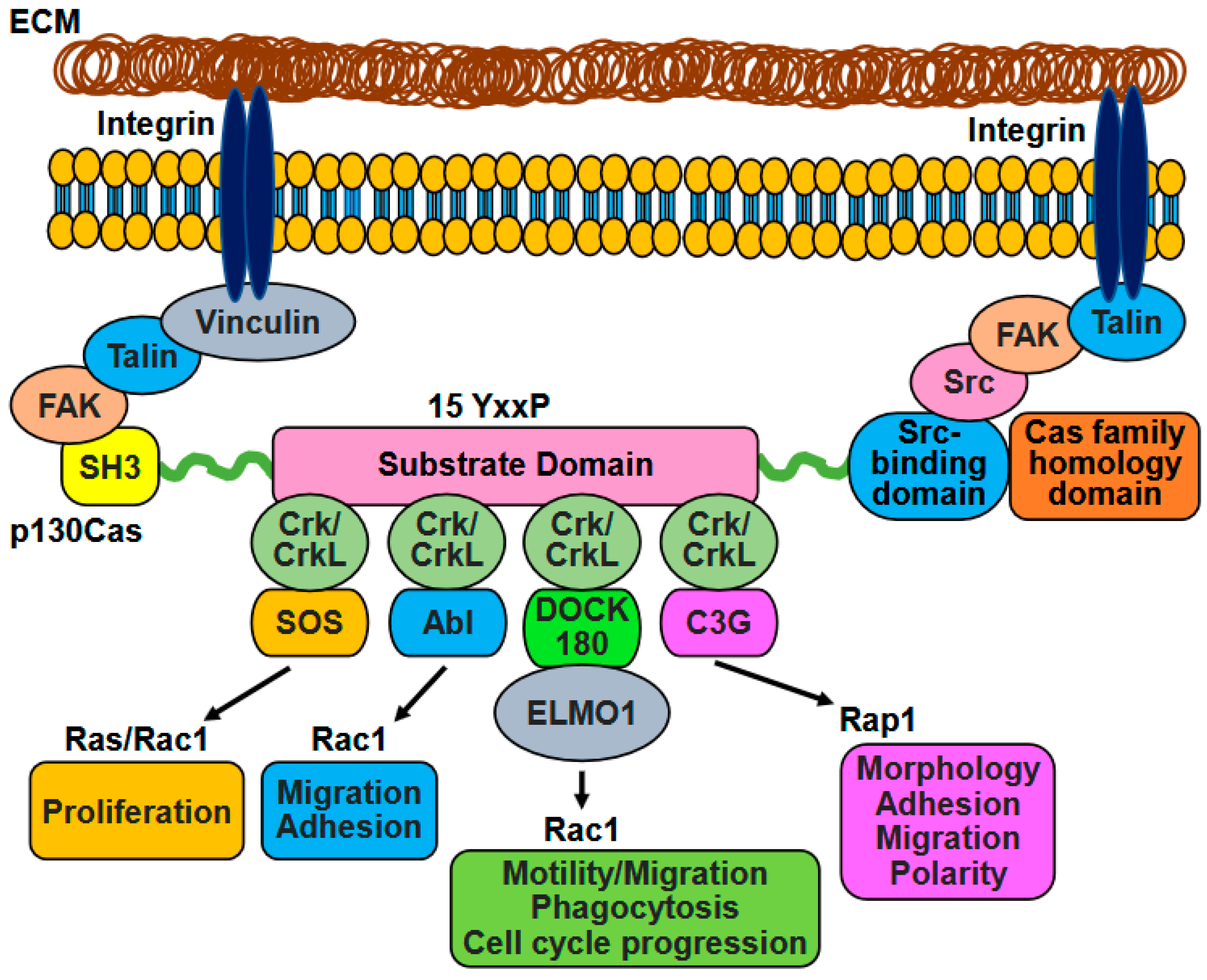
3. p130Cas-Crk/CrkL Interaction
4. Functions of p130Cas and Crk Family Proteins in Normal Cells
5. Functions of p130Cas and Crk Family Proteins in Tumor Cells
5.1. Expression of p130Cas, Crk, and CrkL in Tumor Cells
5.1.1. p130Cas Expression in Tumor Cells
5.1.2. Crk and CrkL Expression in Tumor Cells
5.1.3. Cooperative Roles of p130Cas and Crk/CrkL Overexpression in Tumor Cells
5.2. Cellular Transformation
5.2.1. Contribution of p130Cas to Cellular Transformation
5.2.2. Contribution of Crk and CrkL to Cellular Transformation
5.2.3. Cooperation Between p130Cas and Crk/CrkL for Cellular Transformation
5.3. Cell Motility and Migration
5.3.1. Contribution of p130Cas to Tumor Cell Motility and Migration
5.3.2. Contribution of Crk and CrkL to Tumor Cell Motility and Migration
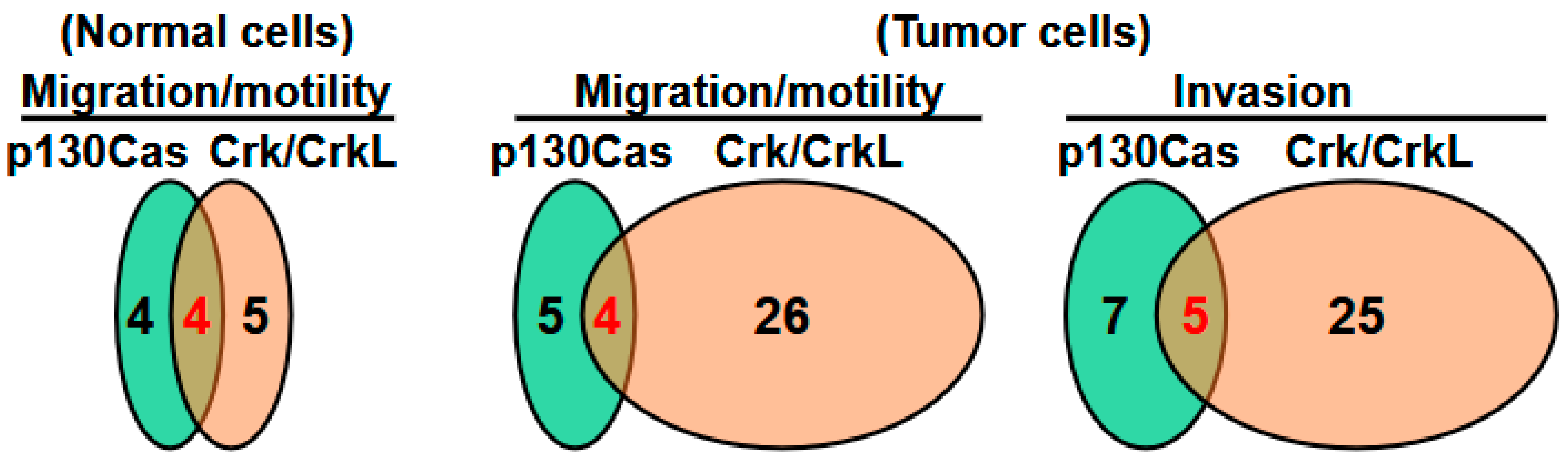
5.3.3. Cooperation Between p130Cas and Crk/CrkL for Tumor Cell Motility and Migration
5.4. Invasion and Metastasis
5.4.1. Contribution of p130Cas to Invasion and Metastasis
5.4.2. Contribution of Crk and CrkL to Invasion and Metastasis
5.4.3. Cooperation Between p130Cas and Crk/CrkL for Invasion and Metastasis
6. Conclusion I: Critical Roles of the p130Cas-Crk/CrkL Axis
7. Conclusion II: The p130Cas-Crk/CrkL Axis as a Potential Therapeutic Target for Invasive Cancers
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, A.; Pellet-Many, C.; Zachary, I.C.; Evans, I.M.; Frankel, P. p130Cas: A key signalling node in health and disease. Cell Signal. 2013, 25, 766–777. [Google Scholar] [CrossRef] [PubMed]
- Centonze, G.; Natalini, D.; Salemme, V.; Costamagna, A.; Cabodi, S.; Defilippi, P. p130Cas/BCAR1 and p140Cap/SRCIN1 adaptors: The Yin Yang in breast cancer? Front. Cell Dev. Biol. 2021, 9, 729093. [Google Scholar] [CrossRef] [PubMed]
- Honda, H.; Oda, H.; Nakamoto, T.; Honda, Z.-I.; Sakai, R.; Suzuki, T.; Saito, T.; Nakamura, K.; Nakao, K.; Ishikawa, T. Cardiovascular anomaly, impaired actin bundling and resistance to Src-induced transformation in mice lacking p130Cas. Nat. Genet. 1998, 19, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, T.; Sakai, R.; Honda, H.; Ogawa, S.; Ueno, H.; Suzuki, T.; Aizawa, S.-I.; Yazaki, Y.; Hirai, H. Requirements for localization of p130cas to focal adhesions. Mol. Cell. Biol. 1997, 17, 3884–3897. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Hunter, T. Integrin signalling and tyrosine phosphorylation: Just the FAKs? Trends Cell Biol. 1998, 8, 151–157. [Google Scholar] [CrossRef]
- Lu, Y.; Brush, J.; Stewart, T.A. NSP1 defines a novel family of adaptor proteins linking integrin and tyrosine kinase receptors to the c-Jun N-terminal kinase/stress-activated protein kinase signaling pathway. J. Biol. Chem. 1999, 274, 10047–10052. [Google Scholar] [CrossRef]
- Manié, S.N.; Beck, A.R.; Astier, A.; Law, S.F.; Canty, T.; Hirai, H.; Druker, B.J.; Avraham, H.; Haghayeghi, N.; Sattler, M. Involvement of p130Cas and p105HEF1, a novel Cas-like docking protein, in a cytoskeleton-dependent signaling pathway initiated by ligation of integrin or antigen receptor on human B cells. J. Biol. Chem. 1997, 272, 4230–4236. [Google Scholar] [CrossRef]
- Klemke, R.L.; Leng, J.; Molander, R.; Brooks, P.C.; Vuori, K.; Cheresh, D.A. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J. Cell Biol. 1998, 140, 961–972. [Google Scholar] [CrossRef]
- Persson, C.; Carballeira, N.; Wolf-Watz, H.; Fällman, M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 1997, 16, 2307–2318. [Google Scholar] [CrossRef]
- Brinkman, A.; van der Flier, S.; Kok, E.M.; Dorssers, L.C. BCAR1, a human homologue of the adapter protein p130Cas, and antiestrogen resistance in breast cancer cells. J. Natl. Cancer Inst. 2000, 92, 112–120. [Google Scholar] [CrossRef]
- Leal, M.d.P.C.; Sciortino, M.; Tornillo, G.; Colombo, S.; Defilippi, P.; Cabodi, S. p130Cas/BCAR1 scaffold protein in tissue homeostasis and pathogenesis. Gene 2015, 562, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sakai, R.; Iwamatsu, A.; Hirano, N.; Ogawa, S.; Tanaka, T.; Mano, H.; Yazaki, Y.; Hirai, H. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 1994, 13, 3748–3756. [Google Scholar] [CrossRef] [PubMed]
- Birge, R.B.; Kalodimos, C.; Inagaki, F.; Tanaka, S. Crk and CrkL adaptor proteins: Networks for physiological and pathological signaling. Cell Commun. Signal. 2009, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Polte, T.R.; Hanks, S.K. Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc. Natl. Acad. Sci. USA 1995, 92, 10678–10682. [Google Scholar] [CrossRef]
- Chodniewicz, D.; Klemke, R.L. Regulation of integrin-mediated cellular responses through assembly of a CAS/Crk scaffold. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2004, 1692, 63–76. [Google Scholar] [CrossRef]
- Franke, F.C.; Slusarenko, B.O.; Engleitner, T.; Johannes, W.; Laschinger, M.; Rad, R.; Nitsche, U.; Janssen, K.P. Novel role for CRK adaptor proteins as essential components of SRC/FAK signaling for epithelial–mesenchymal transition and colorectal cancer aggressiveness. Int. J. Cancer 2020, 147, 1715–1731. [Google Scholar] [CrossRef]
- Tsuda, M.; Tanaka, S. Roles for crk in cancer metastasis and invasion. Genes Cancer 2012, 3, 334–340. [Google Scholar] [CrossRef]
- Takino, T.; Tamura, M.; Miyamori, H.; Araki, M.; Matsumoto, K.; Sato, H.; Yamada, K.M. Tyrosine phosphorylation of the CrkII adaptor protein modulates cell migration. J. Cell Sci. 2003, 116, 3145–3155. [Google Scholar] [CrossRef][Green Version]
- Cho, S.Y.; Klemke, R.L. Extracellular-regulated kinase activation and CAS/Crk coupling regulate cell migration and suppress apoptosis during invasion of the extracellular matrix. J. Cell Biol. 2000, 149, 223–236. [Google Scholar] [CrossRef]
- Reddien, P.W.; Horvitz, H.R. CED-2/CrkII and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nat. Cell Biol. 2000, 2, 131–136. [Google Scholar] [CrossRef]
- Kain, K.H.; Klemke, R.L. Inhibition of cell migration by Abl family tyrosine kinases through uncoupling of Crk-CAS complexes. J. Biol. Chem. 2001, 276, 16185–16192. [Google Scholar] [CrossRef] [PubMed]
- Pellicena, P.; Miller, W.T. Processive phosphorylation of p130Cas by Src depends on SH3-polyproline interactions. J. Biol. Chem. 2001, 276, 28190–28196. [Google Scholar] [CrossRef] [PubMed]
- Briknarová, K.; Nasertorabi, F.; Havert, M.L.; Eggleston, E.; Hoyt, D.W.; Li, C.; Olson, A.J.; Vuori, K.; Ely, K.R. The serine-rich domain from Crk-associated substrate (p130cas) is a four-helix bundle. J. Biol. Chem. 2005, 280, 21908–21914. [Google Scholar] [CrossRef] [PubMed]
- Prosser, S.; Sorokina, E.; Pratt, P.; Sorokin, A. CrkIII: A novel and biologically distinct member of the Crk family of adaptor proteins. Oncogene 2003, 22, 4799–4806. [Google Scholar] [CrossRef]
- Takino, T.; Nakada, M.; Miyamori, H.; Yamashita, J.; Yamada, K.M.; Sato, H. CrkI adapter protein modulates cell migration and invasion in glioblastoma. Cancer Res. 2003, 63, 2335–2337. [Google Scholar]
- Feller, S.M.; Knudsen, B.; Hanafusa, H. c-Abl kinase regulates the protein binding activity of c-Crk. EMBO J. 1994, 13, 2341–2351. [Google Scholar] [CrossRef]
- Kobashigawa, Y.; Sakai, M.; Naito, M.; Yokochi, M.; Kumeta, H.; Makino, Y.; Ogura, K.; Tanaka, S.; Inagaki, F. Structural basis for the transforming activity of human cancer-related signaling adaptor protein CRK. Nat. Struct. Mol. Biol. 2007, 14, 503–510. [Google Scholar] [CrossRef]
- Li, L.; Guris, D.L.; Okura, M.; Imamoto, A. Translocation of CrkL to focal adhesions mediates integrin-induced migration downstream of Src family kinases. Mol. Cell. Biol. 2003, 23, 2883–2892. [Google Scholar] [CrossRef]
- Park, T. Crk and CrkL as therapeutic targets for cancer treatment. Cells 2021, 10, 739. [Google Scholar] [CrossRef]
- Douglas, J.T.; Johnson, D.K.; Roy, A.; Park, T. Use of phosphotyrosine-containing peptides to target SH2 domains: Antagonist peptides of the Crk/CrkL-p130Cas axis. Methods Enzym. 2024, 698, 301–342. [Google Scholar] [CrossRef]
- Park, T.; Curran, T. Essential roles of Crk and CrkL in fibroblast structure and motility. Oncogene 2014, 33, 5121–5132. [Google Scholar] [CrossRef] [PubMed]
- Janoštiak, R.; Pataki, A.C.; Brábek, J.; Rösel, D. Mechanosensors in integrin signaling: The emerging role of p130Cas. Eur. J. Cell Biol. 2014, 93, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Miller, D.J.; Guibao, C.D.; Donato, D.M.; Hanks, S.K.; Zheng, J.J. Structural and functional insights into the interaction between the Cas family scaffolding protein p130Cas and the focal adhesion-associated protein paxillin. J. Biol. Chem. 2017, 292, 18281–18289. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.M.; Cheresh, D.A.; Schwartz, M.A. Protein kinase C regulates alpha v beta 5-dependent cytoskeletal associations and focal adhesion kinase phosphorylation. J. Cell Biol. 1996, 134, 1323–1332. [Google Scholar] [CrossRef]
- Fagerstrom, S.; Pahlman, S.; Nanberg, E. Protein kinase C-dependent tyrosine phosphorylation of p130cas in differentiating neuroblastoma cells. J. Biol. Chem. 1998, 273, 2336–2343. [Google Scholar] [CrossRef]
- Feller, S.M.; Posern, G.; Voss, J.; Kardinal, C.; Sakkab, D.; Zheng, J.; Knudsen, B.S. Physiological signals and oncogenesis mediated through Crk family adapter proteins. J. Cell. Physiol. 1998, 177, 535–552. [Google Scholar] [CrossRef]
- Radha, V.; Mitra, A.; Dayma, K.; Sasikumar, K. Signalling to actin: Role of C3G, a multitasking guanine-nucleotide-exchange factor. Biosci. Rep. 2011, 31, 231–244. [Google Scholar] [CrossRef]
- Cho, Y.J.; Hemmeryckx, B.; Groffen, J.; Heisterkamp, N. Interaction of Bcr/Abl with C3G, an exchange factor for the small GTPase Rap1, through the adapter protein Crkl. Biochem. Biophys. Res. Commun. 2005, 333, 1276–1283. [Google Scholar] [CrossRef]
- Sastry, S.K.; Rajfur, Z.; Liu, B.P.; Cote, J.-F.; Tremblay, M.L.; Burridge, K. PTP-PEST couples membrane protrusion and tail retraction via VAV2 and p190RhoGAP. J. Biol. Chem. 2006, 281, 11627–11636. [Google Scholar] [CrossRef]
- Shin, N.-Y.; Dise, R.S.; Schneider-Mergener, J.; Ritchie, M.D.; Kilkenny, D.M.; Hanks, S.K. Subsets of the major tyrosine phosphorylation sites in Crk-associated substrate (CAS) are sufficient to promote cell migration. J. Biol. Chem. 2004, 279, 38331–38337. [Google Scholar] [CrossRef]
- Payne, S.L.; Hendrix, M.J.; Kirschmann, D.A. Lysyl oxidase regulates actin filament formation through the p130Cas/Crk/DOCK180 signaling complex. J. Cell. Biochem. 2006, 98, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Cabodi, S.; del Pilar Camacho-Leal, M.; Di Stefano, P.; Defilippi, P. Integrin signalling adaptors: Not only figurants in the cancer story. Nat. Rev. Cancer 2010, 10, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Tamada, M.; Dubin-Thaler, B.J.; Cherniavskaya, O.; Sakai, R.; Tanaka, S.; Sheetz, M.P. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 2006, 127, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Kook, S.; Shim, S.R.; Choi, S.J.; Ahnn, J.; Kim, J.I.; Eom, S.H.; Jung, Y.K.; Paik, S.G.; Song, W.K. Caspase-mediated cleavage of p130cas in etoposide-induced apoptotic Rat-1 cells. Mol. Biol. Cell 2000, 11, 929–939. [Google Scholar] [CrossRef]
- Nagai, Y.; Osawa, K.; Fukushima, H.; Tamura, Y.; Aoki, K.; Ohya, K.; Yasuda, H.; Hikiji, H.; Takahashi, M.; Seta, Y. p130Cas, Crk-associated substrate, plays important roles in osteoclastic bone resorption. J. Bone Miner. Res. 2013, 28, 2449–2462. [Google Scholar] [CrossRef]
- Abassi, Y.A.; Rehn, M.; Ekman, N.; Alitalo, K.; Vuori, K. p130Cas Couples the tyrosine kinase Bmx/Etk with regulation of the actin cytoskeleton and cell migration. J. Biol. Chem. 2003, 278, 35636–35643. [Google Scholar] [CrossRef]
- Smith, H.W.; Marra, P.; Marshall, C.J. uPAR promotes formation of the p130Cas–Crk complex to activate Rac through DOCK180. J. Cell Biol. 2008, 182, 777–790. [Google Scholar] [CrossRef]
- Lu, C.; Wu, F.; Qiu, W.; Liu, R. P130Cas substrate domain is intrinsically disordered as characterized by single-molecule force measurements. Biophys. Chem. 2013, 180, 37–43. [Google Scholar] [CrossRef]
- Jimi, E.; Honda, H.; Nakamura, I. The unique function of p130Cas in regulating the bone metabolism. Pharmacol. Ther. 2022, 230, 107965. [Google Scholar] [CrossRef]
- Defilippi, P.; Di Stefano, P.; Cabodi, S. p130Cas: A versatile scaffold in signaling networks. Trends Cell Biol. 2006, 16, 257–263. [Google Scholar] [CrossRef]
- Honda, H.; Nakamoto, T.; Sakai, R.; Hirai, H. p130(Cas), an assembling molecule of actin filaments, promotes cell movement, cell migration, and cell spreading in fibroblasts. Biochem. Biophys. Res. Commun. 1999, 262, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Brabek, J.; Constancio, S.S.; Shin, N.-Y.; Pozzi, A.; Weaver, A.M.; Hanks, S.K. CAS promotes invasiveness of Src-transformed cells. Oncogene 2004, 23, 7406–7415. [Google Scholar] [CrossRef] [PubMed]
- Cary, L.A.; Han, D.C.; Polte, T.R.; Hanks, S.K.; Guan, J.L. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 1998, 140, 211–221. [Google Scholar] [CrossRef]
- Matsuda, M.; Tanaka, S.; Nagata, S.; Kojima, A.; Kurata, T.; Shibuya, M. Two species of human CRK cDNA encode proteins with distinct biological activities. Mol. Cell Biol. 1992, 12, 3482–3489. [Google Scholar] [CrossRef]
- George, B.; Fan, Q.; Dlugos, C.P.; Soofi, A.A.; Zhang, J.; Verma, R.; Park, T.-J.; Wong, H.; Curran, T.; Nihalani, D. Crk1/2 and CrkL form a hetero-oligomer and functionally complement each other during podocyte morphogenesis. Kidney Int. 2014, 85, 1382–1394. [Google Scholar] [CrossRef]
- Huang, Y.; Clarke, F.; Karimi, M.; Roy, N.H.; Williamson, E.K.; Okumura, M.; Mochizuki, K.; Chen, E.J.; Park, T.-J.; Debes, G.F. CRK proteins selectively regulate T cell migration into inflamed tissues. J. Clin. Investig. 2015, 125, 1019–1032. [Google Scholar] [CrossRef]
- Kumar, S.; Lu, B.; Davra, V.; Hornbeck, P.; Machida, K.; Birge, R.B. Crk Tyrosine Phosphorylation Regulates PDGF-BB-inducible Src Activation and Breast Tumorigenicity and Metastasis. Mol. Cancer Res. 2018, 16, 173–183. [Google Scholar] [CrossRef]
- Shi, L.; Racedo, S.E.; Diacou, A.; Park, T.; Zhou, B.; Morrow, B.E. Crk and Crkl have shared functions in neural crest cells for cardiac outflow tract septation and vascular smooth muscle differentiation. Hum. Mol. Genet. 2022, 31, 1197–1215. [Google Scholar] [CrossRef]
- Moon, A.M.; Guris, D.L.; Seo, J.H.; Li, L.; Hammond, J.; Talbot, A.; Imamoto, A. Crkl deficiency disrupts Fgf8 signaling in a mouse model of 22q11 deletion syndromes. Dev. Cell 2006, 10, 71–80. [Google Scholar] [CrossRef]
- Shi, L.; Song, H.; Zhou, B.; Morrow, B.E. Crk/Crkl regulates early angiogenesis in mouse embryos by accelerating endothelial cell maturation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Nabekura, T.; Chen, Z.; Schroeder, C.; Park, T.; Vivier, E.; Lanier, L.L.; Liu, D. Crk Adaptor Proteins Regulate NK Cell Expansion and Differentiation during Mouse Cytomegalovirus Infection. J. Immunol. 2018, 200, 3420–3428. [Google Scholar] [CrossRef] [PubMed]
- Kain, K.H.; Gooch, S.; Klemke, R.L. Cytoplasmic c-Abl provides a molecular ‘Rheostat’controlling carcinoma cell survival and invasion. Oncogene 2003, 22, 6071–6080. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, T.-J.; Curran, T. Crk and Crk-like play essential overlapping roles downstream of disabled-1 in the Reelin pathway. J. Neurosci. 2008, 28, 13551–13562. [Google Scholar] [CrossRef]
- Sanders, M.A.; Basson, M.D. p130cas but not paxillin is essential for Caco-2 intestinal epithelial cell spreading and migration on collagen IV. J. Biol. Chem. 2005, 280, 23516–23522. [Google Scholar] [CrossRef]
- Gustavsson, A.; Yuan, M.; Fällman, M. Temporal dissection of β1-integrin signaling indicates a role for p130Cas-Crk in filopodia formation. J. Biol. Chem. 2004, 279, 22893–22901. [Google Scholar] [CrossRef]
- Tamada, M.; Sheetz, M.P.; Sawada, Y. Activation of a signaling cascade by cytoskeleton stretch. Dev. Cell 2004, 7, 709–718. [Google Scholar] [CrossRef]
- Oktay, M.; Wary, K.K.; Dans, M.; Birge, R.B.; Giancotti, F.G. Integrin-mediated activation of focal adhesion kinase is required for signaling to Jun NH2-terminal kinase and progression through the G1 phase of the cell cycle. J. Cell Biol. 1999, 145, 1461–1469. [Google Scholar] [CrossRef]
- Huang, Z.; Yazdani, U.; Thompson-Peer, K.L.; Kolodkin, A.L.; Terman, J.R. Crk-associated substrate (Cas) signaling protein functions with integrins to specify axon guidance during development. Development 2007, 134, 2337–2347. [Google Scholar] [CrossRef]
- Tornillo, G.; Elia, A.R.; Castellano, I.; Spadaro, M.; Bernabei, P.; Bisaro, B.; Camacho-Leal, M.d.P.; Pincini, A.; Provero, P.; Sapino, A. p130Cas alters the differentiation potential of mammary luminal progenitors by deregulating c-Kit activity. Stem Cells 2013, 31, 1422–1433. [Google Scholar] [CrossRef]
- Cabodi, S.; Tinnirello, A.; Di Stefano, P.; Bisaro, B.; Ambrosino, E.; Castellano, I.; Sapino, A.; Arisio, R.; Cavallo, F.; Forni, G.; et al. p130Cas as a new regulator of mammary epithelial cell proliferation, survival, and HER2-neu oncogene-dependent breast tumorigenesis. Cancer Res. 2006, 66, 4672–4680. [Google Scholar] [CrossRef]
- Hallock, P.T.; Xu, C.-F.; Park, T.-J.; Neubert, T.A.; Curran, T.; Burden, S.J. Dok-7 regulates neuromuscular synapse formation by recruiting Crk and Crk-L. Genes Dev. 2010, 24, 2451–2461. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Curran, T. Requirement for Crk and CrkL during postnatal lens development. Biochem. Biophys. Res. Commun. 2020, 529, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, B.; Benkaci, S.; Shi, L.; Lu, P.; Park, T.; Morrow, B.E.; Wang, Y.; Zhou, B. Crk and Crkl Are Required in the Endocardial Lineage for Heart Valve Development. J. Am. Heart Assoc. 2023, 12, e029683. [Google Scholar] [CrossRef] [PubMed]
- Yeo, M.G.; Sung, B.H.; Oh, H.J.; Park, Z.Y.; Marcantonio, E.E.; Song, W.K. Focal adhesion targeting of v-Crk is essential for FAK phosphorylation and cell migration in mouse embryo fibroblasts deficient src family kinases or p130CAS. J. Cell. Physiol. 2008, 214, 604–613. [Google Scholar] [CrossRef]
- Yokoyama, N.; Miller, W.T. Protein phosphatase 2A interacts with the Src kinase substrate p130(CAS). Oncogene 2001, 20, 6057–6065. [Google Scholar] [CrossRef][Green Version]
- Li, H.; Li, L.; Qiu, X.; Zhang, J.; Hua, Z. The interaction of CFLAR with p130Cas promotes cell migration. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2023, 1870, 119390. [Google Scholar] [CrossRef]
- Cunningham-Edmondson, A.C.; Hanks, S.K. p130Cas substrate domain signaling promotes migration, invasion, and survival of estrogen receptor-negative breast cancer cells. Breast Cancer Targets Ther. 2009, 39–52. [Google Scholar]
- Wendt, M.K.; Smith, J.A.; Schiemann, W.P. p130Cas is required for mammary tumor growth and transforming growth factor-β-mediated metastasis through regulation of Smad2/3 activity. J. Biol. Chem. 2009, 284, 34145–34156. [Google Scholar] [CrossRef]
- Mao, C.g.; Jiang, S.S.; Shen, C.; Long, T.; Jin, H.; Tan, Q.Y.; Deng, B. BCAR1 promotes proliferation and cell growth in lung adenocarcinoma via upregulation of POLR2A. Thorac. Cancer 2020, 11, 3326–3336. [Google Scholar] [CrossRef]
- Mao, C.-G.; Jiang, S.-S.; Wang, X.-Y.; Tao, S.-L.; Jiang, B.; Mao, C.-Y.; Yang, Y.-L.; Hu, Z.-Y.; Long, T.; Jin, H. BCAR1 plays critical roles in the formation and immunoevasion of invasive circulating tumor cells in lung adenocarcinoma. Int. J. Biol. Sci. 2021, 17, 2461. [Google Scholar] [CrossRef]
- Ta, H.Q.; Thomas, K.S.; Schrecengost, R.S.; Bouton, A.H. A novel association between p130Cas and resistance to the chemotherapeutic drug adriamycin in human breast cancer cells. Cancer Res. 2008, 68, 8796–8804. [Google Scholar] [CrossRef] [PubMed]
- Nick, A.M.; Stone, R.L.; Armaiz-Pena, G.; Ozpolat, B.; Tekedereli, I.; Graybill, W.S.; Landen, C.N.; Villares, G.; Vivas-Mejia, P.; Bottsford-Miller, J. Silencing of p130cas in ovarian carcinoma: A novel mechanism for tumor cell death. J. Natl. Cancer Inst. 2011, 103, 1596–1612. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Anand, S.; Murphy, E.A.; Desgrosellier, J.S.; Stupack, D.G.; Shattil, S.J.; Schlaepfer, D.D.; Cheresh, D.A. EGFR-dependent pancreatic carcinoma cell metastasis through Rap1 activation. Oncogene 2012, 31, 2783–2793. [Google Scholar] [CrossRef] [PubMed]
- Tornillo, G.; Bisaro, B.; del Pilar Camacho-Leal, M.; Galiè, M.; Provero, P.; Di Stefano, P.; Turco, E.; Defilippi, P.; Cabodi, S. p130Cas promotes invasiveness of three-dimensional ErbB2-transformed mammary acinar structures by enhanced activation of mTOR/p70S6K and Rac1. Eur. J. Cell Biol. 2011, 90, 237–248. [Google Scholar] [CrossRef]
- Wang, Y.; McNiven, M.A. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK–p130Cas complex. J. Cell Biol. 2012, 196, 375–385. [Google Scholar] [CrossRef]
- Kumar, S.; Lu, B.; Dixit, U.; Hossain, S.; Liu, Y.; Li, J.; Hornbeck, P.; Zheng, W.; Sowalsky, A.G.; Kotula, L.; et al. Reciprocal regulation of Abl kinase by Crk Y251 and Abi1 controls invasive phenotypes in glioblastoma. Oncotarget 2015, 6, 37792–37807. [Google Scholar] [CrossRef]
- Park, T.; Large, N.; Curran, T. Quantitative assessment of glioblastoma phenotypes in vitro establishes cell migration as a robust readout of Crk and CrkL activity. J. Biol. Chem. 2021, 296, 100390. [Google Scholar] [CrossRef]
- Pezeshkpour, G.H.; Moatamed, F.; Lewis, M.; Hoang, B.; Rettig, M.; Mortazavi, F. CRK SH3N Domain Diminishes Cell Invasiveness of Non-Small Cell Lung Cancer. Genes Cancer 2013, 4, 315–324. [Google Scholar] [CrossRef][Green Version]
- Linghu, H.; Tsuda, M.; Makino, Y.; Sakai, M.; Watanabe, T.; Ichihara, S.; Sawa, H.; Nagashima, K.; Mochizuki, N.; Tanaka, S. Involvement of adaptor protein Crk in malignant feature of human ovarian cancer cell line MCAS. Oncogene 2006, 25, 3547–3556. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, S.P.; Fathers, K.E.; Chan, G.; Zuo, D.; Halwani, F.; Meterissian, S.; Park, M. CrkI and CrkII function as key signaling integrators for migration and invasion of cancer cells. Mol. Cancer Res. 2005, 3, 183–194. [Google Scholar] [CrossRef]
- Wang, J.; Che, Y.L.; Li, G.; Liu, B.; Shen, T.M.; Wang, H.; Linghu, H. Crk and CrkL present with different expression and significance in epithelial ovarian carcinoma. Mol. Carcinog. 2011, 50, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Tsuda, M.; Makino, Y.; Ichihara, S.; Sawa, H.; Minami, A.; Mochizuki, N.; Nagashima, K.; Tanaka, S. Adaptor molecule Crk is required for sustained phosphorylation of Grb2-associated binder 1 and hepatocyte growth factor-induced cell motility of human synovial sarcoma cell lines. Mol. Cancer Res. 2006, 4, 499–510. [Google Scholar] [CrossRef]
- Wang, L.; Tabu, K.; Kimura, T.; Tsuda, M.; Linghu, H.; Tanino, M.; Kaneko, S.; Nishihara, H.; Tanaka, S. Signaling adaptor protein Crk is indispensable for malignant feature of glioblastoma cell line KMG4. Biochem. Biophys. Res. Commun. 2007, 362, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Fathers, K.E.; Bell, E.S.; Rajadurai, C.V.; Cory, S.; Zhao, H.; Mourskaia, A.; Zuo, D.; Madore, J.; Monast, A.; Mes-Masson, A.M.; et al. Crk adaptor proteins act as key signaling integrators for breast tumorigenesis. Breast Cancer Res. 2012, 14, R74. [Google Scholar] [CrossRef]
- Yanagi, H.; Wang, L.; Nishihara, H.; Kimura, T.; Tanino, M.; Yanagi, T.; Fukuda, S.; Tanaka, S. CRKL plays a pivotal role in tumorigenesis of head and neck squamous cell carcinoma through the regulation of cell adhesion. Biochem. Biophys. Res. Commun. 2012, 418, 104–109. [Google Scholar] [CrossRef]
- Uemura, S.; Wang, L.; Tsuda, M.; Suzuka, J.; Tanikawa, S.; Sugino, H.; Nakamura, T.; Mitsuhashi, T.; Hirano, S.; Tanaka, S. Signaling adaptor protein Crk is involved in malignant feature of pancreatic cancer associated with phosphorylation of c-Met. Biochem. Biophys. Res. Commun. 2020, 524, 378–384. [Google Scholar] [CrossRef]
- Ji, H.; Li, B.; Zhang, S.; He, Z.; Zhou, Y.; Ouyang, L. Crk-like adapter protein is overexpressed in cervical carcinoma, facilitates proliferation, invasion and chemoresistance, and regulates Src and Akt signaling. Oncol. Lett. 2016, 12, 3811–3817. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, X.; Hu, B.; Wang, X.J.; Wang, Q.; Wang, W.L. The effects of Micro-429 on inhibition of cervical cancer cells through targeting ZEB1 and CRKL. Biomed. Pharmacother. 2016, 80, 311–321. [Google Scholar] [CrossRef]
- Song, Q.; Yi, F.; Zhang, Y.; Jun Li, D.K.; Wei, Y.; Yu, H.; Zhang, Y. CRKL regulates alternative splicing of cancer-related genes in cervical cancer samples and HeLa cell. BMC Cancer 2019, 19, 499. [Google Scholar] [CrossRef]
- Zhao, T.; Miao, Z.; Wang, Z.; Xu, Y.; Wu, J.; Liu, X.; You, Y.; Li, J. Overexpression of CRKL correlates with malignant cell proliferation in breast cancer. Tumour Biol. 2013, 34, 2891–2897. [Google Scholar] [CrossRef]
- Kumar, S.; Davra, V.; Obr, A.E.; Geng, K.; Wood, T.L.; De Lorenzo, M.S.; Birge, R.B. Crk adaptor protein promotes PD-L1 expression, EMT and immune evasion in a murine model of triple-negative breast cancer. Oncoimmunology 2017, 7, e1376155. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Sun, M.Z.; Guo, C.; Shi, J.; Chen, X.; Liu, S. CRKL overexpression suppresses in vitro proliferation, invasion and migration of murine hepatocarcinoma Hca-P cells. Biomed. Pharmacother. 2015, 69, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shang, J.; Li, J.; Liu, W.; Zhang, Z.; Yuan, J.; Yang, M. The long noncoding RNA PCAT-1 links the microRNA miR-215 to oncogene CRKL-mediated signaling in hepatocellular carcinoma. J. Biol. Chem. 2017, 292, 17939–17949. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, Q.Z.; Fu, L.; Stoecker, M.; Wang, E.; Wang, E.H. Overexpression of CRKL correlates with poor prognosis and cell proliferation in non-small cell lung cancer. Mol. Carcinog. 2013, 52, 890–899. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsuda, M.; Tanaka, S.; Ohba, Y.; Kawaguchi, H.; Majima, T.; Sawa, H.; Minami, A. Adaptor protein Crk induces Src-dependent activation of p38 MAPK in regulation of synovial sarcoma cell proliferation. Mol. Cancer Res. 2009, 7, 1582–1592. [Google Scholar] [CrossRef]
- Yeung, C.L.; Ngo, V.N.; Grohar, P.J.; Arnaldez, F.I.; Asante, A.; Wan, X.; Khan, J.; Hewitt, S.M.; Khanna, C.; Staudt, L.M.; et al. Loss-of-function screen in rhabdomyosarcoma identifies CRKL-YES as a critical signal for tumor growth. Oncogene 2013, 32, 5429–5438. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Li, P.; Su, L.; Yu, B.; Cai, Q.; Li, J.; Yu, Y.; Liu, B.; Zhu, Z. CRKL promotes cell proliferation in gastric cancer and is negatively regulated by miR-126. Chem. Biol. Interact. 2013, 206, 230–238. [Google Scholar] [CrossRef]
- Cai, L.; Wang, H.; Yang, Q. CRKL overexpression promotes cell proliferation and inhibits apoptosis in endometrial carcinoma. Oncol. Lett. 2017, 13, 51–56. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, R.; Tsuda, M.; Wang, L.; Maishi, N.; Abe, T.; Kimura, T.; Tanino, M.; Nishihara, H.; Hida, K.; Ohba, Y.; et al. Adaptor protein CRK induces epithelial-mesenchymal transition and metastasis of bladder cancer cells through HGF/c-Met feedback loop. Cancer Sci. 2015, 106, 709–717. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, X.; Guo, C.; Sun, M.Z.; Liu, S. CRKII overexpression promotes the in vitro proliferation, migration and invasion potential of murine hepatocarcinoma Hca-P cells. Oncol. Lett. 2019, 17, 5169–5174. [Google Scholar] [CrossRef]
- Franke, F.C.; Muller, J.; Abal, M.; Medina, E.D.; Nitsche, U.; Weidmann, H.; Chardonnet, S.; Ninio, E.; Janssen, K.P. The Tumor Suppressor SASH1 Interacts With the Signal Adaptor CRKL to Inhibit Epithelial-Mesenchymal Transition and Metastasis in Colorectal Cancer. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 33–53. [Google Scholar] [CrossRef] [PubMed]
- Dhupkar, P.; Zhao, H.; Mujoo, K.; An, Z.; Zhang, N. Crk II silencing down-regulates IGF-IR and inhibits migration and invasion of prostate cancer cells. Biochem. Biophys. Rep. 2016, 8, 382–388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Senechal, K.; Halpern, J.; Sawyers, C.L. The CRKL adaptor protein transforms fibroblasts and functions in transformation by the BCR-ABL oncogene. J. Biol. Chem. 1996, 271, 23255–23261. [Google Scholar] [CrossRef] [PubMed]
- Koptyra, M.; Park, T.J.; Curran, T. Crk and CrkL are required for cell transformation by v-fos and v-ras. Mol. Carcinog. 2016, 55, 97–104. [Google Scholar] [CrossRef]
- Lv, S.; Qin, J.; Yi, R.; Coreman, M.; Shi, R.; Kang, H.; Yao, C. CrkL efficiently mediates cell proliferation, migration, and invasion induced by TGF-beta pathway in glioblastoma. J. Mol. Neurosci. 2013, 51, 1046–1051. [Google Scholar] [CrossRef]
- Petit, V.; Boyer, B.; Lentz, D.; Turner, C.E.; Thiery, J.P.; Vallés, A.M. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J. Cell Biol. 2000, 148, 957–970. [Google Scholar] [CrossRef]
- Cheng, S.; Guo, J.; Yang, Q.; Yang, X. Crk-like adapter protein regulates CCL19/CCR7-mediated epithelial-to-mesenchymal transition via ERK signaling pathway in epithelial ovarian carcinomas. Med. Oncol. 2015, 32, 47. [Google Scholar] [CrossRef]
- Yamada, S.; Yanamoto, S.; Kawasaki, G.; Rokutanda, S.; Yonezawa, H.; Kawakita, A.; Nemoto, T.K. Overexpression of CRKII increases migration and invasive potential in oral squamous cell carcinoma. Cancer Lett. 2011, 303, 84–91. [Google Scholar] [CrossRef]
- Han, G.; Wu, D.; Yang, Y.; Li, Z.; Zhang, J.; Li, C. CrkL meditates CCL20/CCR6-induced EMT in gastric cancer. Cytokine 2015, 76, 163–169. [Google Scholar] [CrossRef]
- Rettig, M.; Trinidad, K.; Pezeshkpour, G.; Frost, P.; Sharma, S.; Moatamed, F.; Tamanoi, F.; Mortazavi, F. PAK1 kinase promotes cell motility and invasiveness through CRK-II serine phosphorylation in non-small cell lung cancer cells. PLoS ONE 2012, 7, e42012. [Google Scholar] [CrossRef]
- Li, X.; Wang, F.; Qi, Y. MiR-126 inhibits the invasion of gastric cancer cell in part by targeting Crk. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2031–2037. [Google Scholar] [PubMed]
- Elmansuri, A.Z.; Tanino, M.A.; Mahabir, R.; Wang, L.; Kimura, T.; Nishihara, H.; Kinoshita, I.; Dosaka-Akita, H.; Tsuda, M.; Tanaka, S. Novel signaling collaboration between TGF-beta and adaptor protein Crk facilitates EMT in human lung cancer. Oncotarget 2016, 7, 27094–27107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kirsch, K.H.; Kensinger, M.; Hanafusa, H.; August, A. A p130 Cas tyrosine phosphorylated substrate domain decoy disrupts v-Crk signaling. BMC Cell Biol. 2002, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Qi, L.; Zhang, X.; Li, Y.; Chen, M.; Zu, X. CrkI and p130(Cas) complex regulates the migration and invasion of prostate cancer cells. Cell Biochem. Funct. 2011, 29, 625–629. [Google Scholar] [CrossRef]
- Spencer, K.S.; Graus-Porta, D.; Leng, J.; Hynes, N.E.; Klemke, R.L. ErbB2 is necessary for induction of carcinoma cell invasion by ErbB family receptor tyrosine kinases. J. Cell Biol. 2000, 148, 385–397. [Google Scholar] [CrossRef]
- Fromont, G.; Vallancien, G.; Validire, P.; Levillain, P.; Cussenot, O. BCAR1 expression in prostate cancer: Association with 16q23 LOH status, tumor progression and EGFR/KAI1 staining. Prostate 2007, 67, 268–273. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Q.G.; Yang, W.; Zhang, Z.L.; Yao, Y.M. Relation among p130Cas, E-cadherin and beta-catenin expression, clinicopathologic significance and prognosis in human hepatocellular carcinoma. Hepatobiliary Pancreat. Dis. Int. 2008, 7, 490–496. [Google Scholar]
- Salgia, R.; Pisick, E.; Sattler, M.; Li, J.L.; Uemura, N.; Wong, W.K.; Burky, S.A.; Hirai, H.; Chen, L.B.; Griffin, J.D. p130CAS forms a signaling complex with the adapter protein CRKL in hematopoietic cells transformed by the BCR/ABL oncogene. J. Biol. Chem. 1996, 271, 25198–25203. [Google Scholar] [CrossRef]
- Sriram, G.; Birge, R.B. Emerging roles for crk in human cancer. Genes Cancer 2010, 1, 1132–1139. [Google Scholar] [CrossRef]
- Tikhmyanova, N.; Little, J.L.; Golemis, E.A. CAS proteins in normal and pathological cell growth control. Cell Mol. Life Sci. 2010, 67, 1025–1048. [Google Scholar] [CrossRef]
- Brabek, J.; Constancio, S.S.; Siesser, P.F.; Shin, N.Y.; Pozzi, A.; Hanks, S.K. Crk-associated substrate tyrosine phosphorylation sites are critical for invasion and metastasis of SRC-transformed cells. Mol. Cancer Res. 2005, 3, 307–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gumienny, T.L.; Brugnera, E.; Tosello-Trampont, A.C.; Kinchen, J.M.; Haney, L.B.; Nishiwaki, K.; Walk, S.F.; Nemergut, M.E.; Macara, I.G.; Francis, R.; et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell 2001, 107, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Brugnera, E.; Haney, L.; Grimsley, C.; Lu, M.; Walk, S.F.; Tosello-Trampont, A.-C.; Macara, I.G.; Madhani, H.; Fink, G.R.; Ravichandran, K.S. Unconventional Rac-GEF activity is mediated through the Dock180–ELMO complex. Nat. Cell Biol. 2002, 4, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J.; Paterson, H.F.; Johnston, C.L.; Diekmann, D.; Hall, A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992, 70, 401–410. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho GTPases and cell migration. J. Cell Sci. 2001, 114, 2713–2722. [Google Scholar] [CrossRef]
- Helfer, B.; Boswell, B.C.; Finlay, D.; Cipres, A.; Vuori, K.; Bong Kang, T.; Wallach, D.; Dorfleutner, A.; Lahti, J.M.; Flynn, D.C.; et al. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 2006, 66, 4273–4278. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef]
- Nakamoto, T.; Yamagata, T.; Sakai, R.; Ogawa, S.; Honda, H.; Ueno, H.; Hirano, N.; Yazaki, Y.; Hirai, H. CIZ, a zinc finger protein that interacts with p130(cas) and activates the expression of matrix metalloproteinases. Mol. Cell Biol. 2000, 20, 1649–1658. [Google Scholar] [CrossRef]
| Normal Cells | ||
|---|---|---|
| p130Cas Only | Crk/CrkL Only | p130Cas-Crk/CrkL |
| Cell Morphology [51] Cytoskeleton [3,45,52] Cell Adhesion [51] Cell Migration [8,40,51,53] Cell Invasion [52] | Cell Morphology [54] Cytoskeleton [31,55] Cell Adhesion [56,57] Cell Proliferation [58] Apoptosis [58,59] Cell Differentiation [58,60,61] Cell Motility and Migration [31,56,59,62,63] | Cell Spreading [64] Cytoskeleton [8,65,66] Apoptosis [62] Cell Cycle [67] Cell Migration [8,21,62,64] |
| Tumor Cells | |||
|---|---|---|---|
| p130Cas Only | Crk/CrkL Only | p130Cas-Crk/CrkL | |
| Cell Spreading: Breast cancer [76] Cytoskeleton: Breast cancer [76,77] Cell Proliferation: Breast cancer [70,77,78] Lung cancer [79,80] Cell Survival and Apoptosis: Breast cancer [70,77,81] Lung cancer [80] Ovarian cancer [82] Cell Cycle: Breast cancer [81] Cell Transformation: Lung cancer [79,80] Fibroblasts [52] Cell Migration: Breast cancer [76,77] Lung cancer [80] Pancreatic cancer [83] Ovarian cancer [82] Cell Invasion: Breast cancer [77,78,84] Lung cancer [80] Pancreatic cancer [85] Ovarian cancer [82] | Cell Morphology: Glioma/glioblastoma [86,87] Lung cancer [88] Ovarian cancer [89] Colorectal cancer [16] Cytoskeleton: Breast cancer [90] Ovarian cancer [91] Synovial sarcoma [92] Cell Adhesion: Glioma/glioblastoma [57,87,93] Breast cancer [57,90,94] Head and neck cancer [95] Pancreatic cancer [96] Cell Proliferation: Cervical cancer [97,98,99] Breast cancer [100,101] Glioma/glioblastoma [87,93] Liver cancer [102,103] Lung cancer [104] Synovial sarcoma [105] Rhabdomyosarcoma [106] Gastric cancer [107] Endometrial carcinoma [108] Bladder cancer [109] Head and neck cancer [95] Colorectal cancer [16] Cell Survival and Apoptosis: Endometrial carcinoma [108] Cervical cancer [98] Cell Cycle: Breast cancer [100] Lung cancer [104] Synovial sarcoma [105] Gastric cancer [107] Endometrial carcinoma [108] Cell Transformation and Colony Formation: Liver cancer [102,103,110] Ovarian cancer [89,91] Synovial sarcoma [105] Lung cancer [104] Glioma/glioblastoma [93] Cervical cancer [98] Colorectal cancer [111] Prostate cancer [112] Pancreatic cancer [96] Endometrial carcinoma [108] Breast cancer [57] Fibroblasts [113,114] | Cell Motility and Migration: Glioma/glioblastoma [57,86,87,93,115] Bladder cancer [109,116] Ovarian cancer [89,117] Liver cancer [102,110] Colorectal cancer [16,111] Breast cancer [57,90,94] Lung cancer [88,90] Cervical cancer [90,98] Oral squamous cell carcinoma [118] Head and neck cancer [95] Synovial sarcoma [92] Prostate cancer [112] Gastric cancer [119] Pancreatic cancer [96] Cell Invasion: Glioma/glioblastoma [57,86,87,115] Ovarian cancer [89,91,117] Lung cancer [88,90,120] Cervical cancer [90,97,98] Breast cancer [57,90,94] Gastric cancer [119,121] Liver cancer [102,110] Colorectal cancer [16,111] Bladder cancer [109] Prostate cancer [112] Oral squamous cell carcinoma [118] Epithelial-Mesenchymal Transition: Breast cancer [101] Bladder cancer [109] Colorectal cancer [111] Gastric cancer [119] Lung cancer [122] Ovarian cancer [117] | Cell Transformation: Glioma/glioblastoma [25] Fibroblasts [123] Cell Migration: Prostate cancer [124] Glioma/glioblastoma [25] Breast cancer [125] Fibrosarcoma [18] Cell Invasion: Breast cancer [125] Glioma/glioblastoma [25] Pancreatic cancer [62] Prostate cancer [124] Colorectal cancer [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhadi, P.; Park, T. The p130Cas-Crk/CrkL Axis: A Therapeutic Target for Invasive Cancers Unveiled by Collaboration Among p130Cas, Crk, and CrkL. Int. J. Mol. Sci. 2025, 26, 4017. https://doi.org/10.3390/ijms26094017
Farhadi P, Park T. The p130Cas-Crk/CrkL Axis: A Therapeutic Target for Invasive Cancers Unveiled by Collaboration Among p130Cas, Crk, and CrkL. International Journal of Molecular Sciences. 2025; 26(9):4017. https://doi.org/10.3390/ijms26094017
Chicago/Turabian StyleFarhadi, Pegah, and Taeju Park. 2025. "The p130Cas-Crk/CrkL Axis: A Therapeutic Target for Invasive Cancers Unveiled by Collaboration Among p130Cas, Crk, and CrkL" International Journal of Molecular Sciences 26, no. 9: 4017. https://doi.org/10.3390/ijms26094017
APA StyleFarhadi, P., & Park, T. (2025). The p130Cas-Crk/CrkL Axis: A Therapeutic Target for Invasive Cancers Unveiled by Collaboration Among p130Cas, Crk, and CrkL. International Journal of Molecular Sciences, 26(9), 4017. https://doi.org/10.3390/ijms26094017







