Molecular Iodine Exhibited Differential Antiproliferative Actions in Progenitor and Stem Populations from Chemoresistant Cancer Cells
Abstract
1. Introduction
2. Results
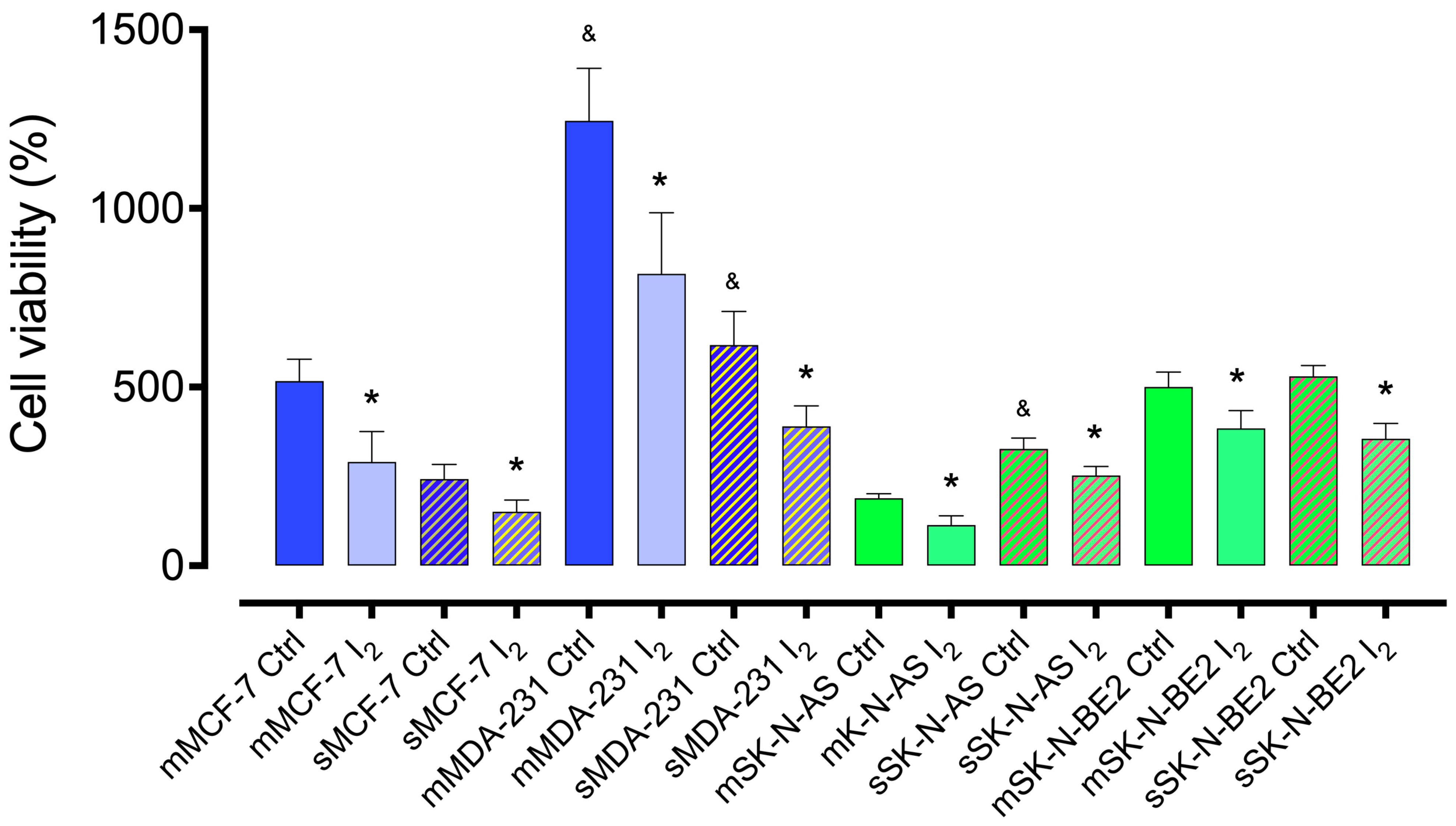
2.1. I2 Supplementation Diminishes the Cell Line’s Viability
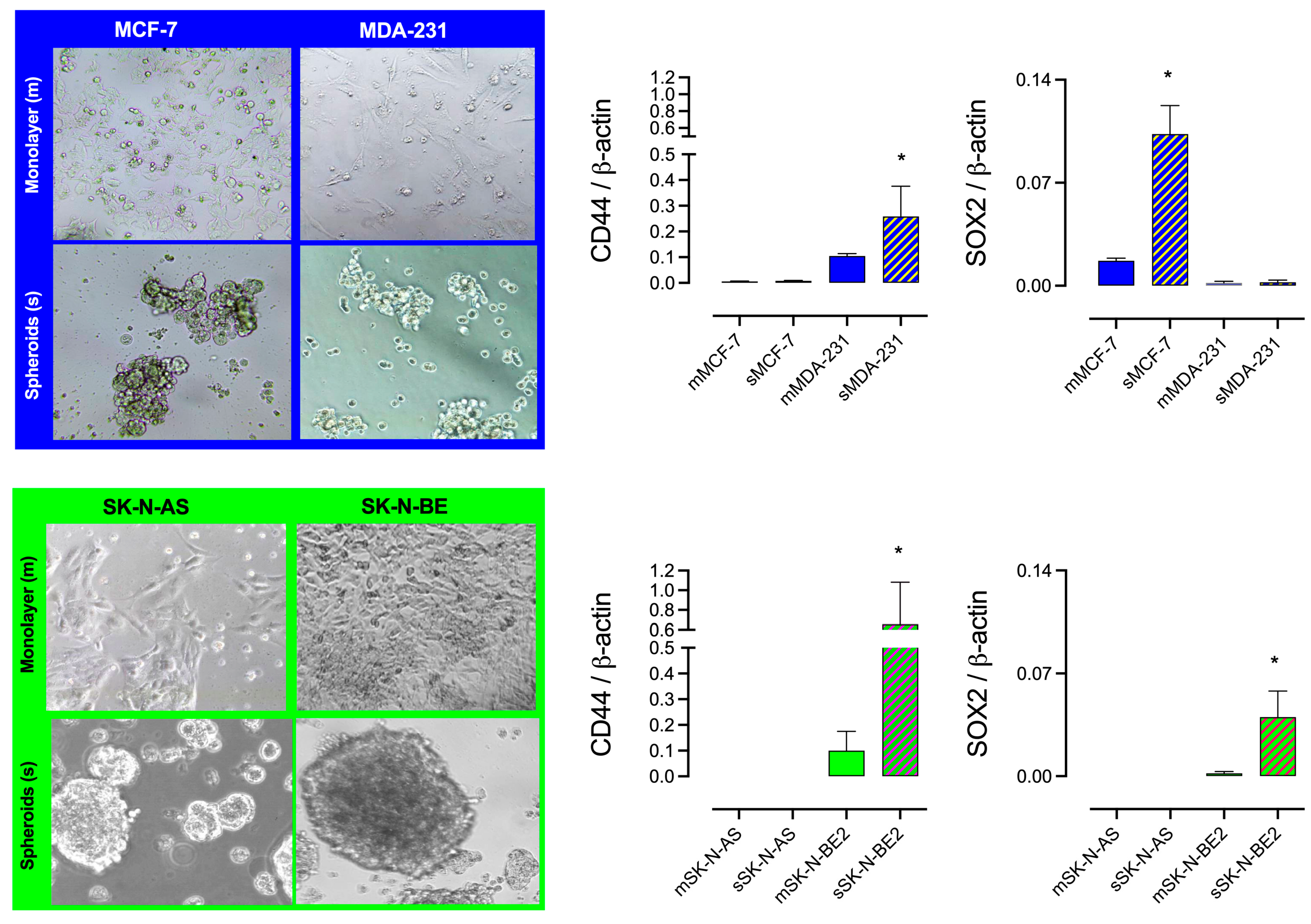
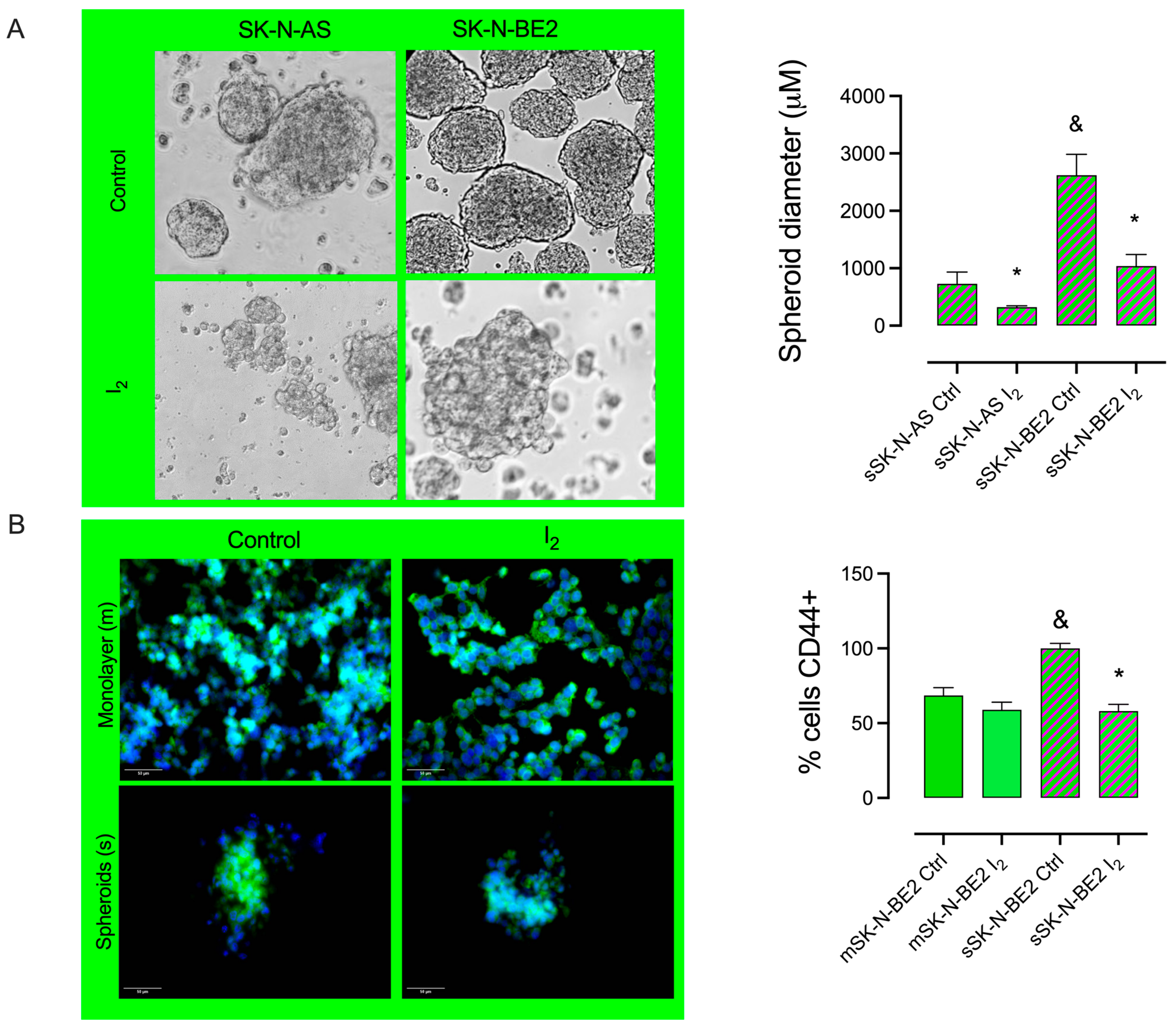
2.2. I2 Supplementation Diminishes the Formation of Spheroid and Stem Marker Expression
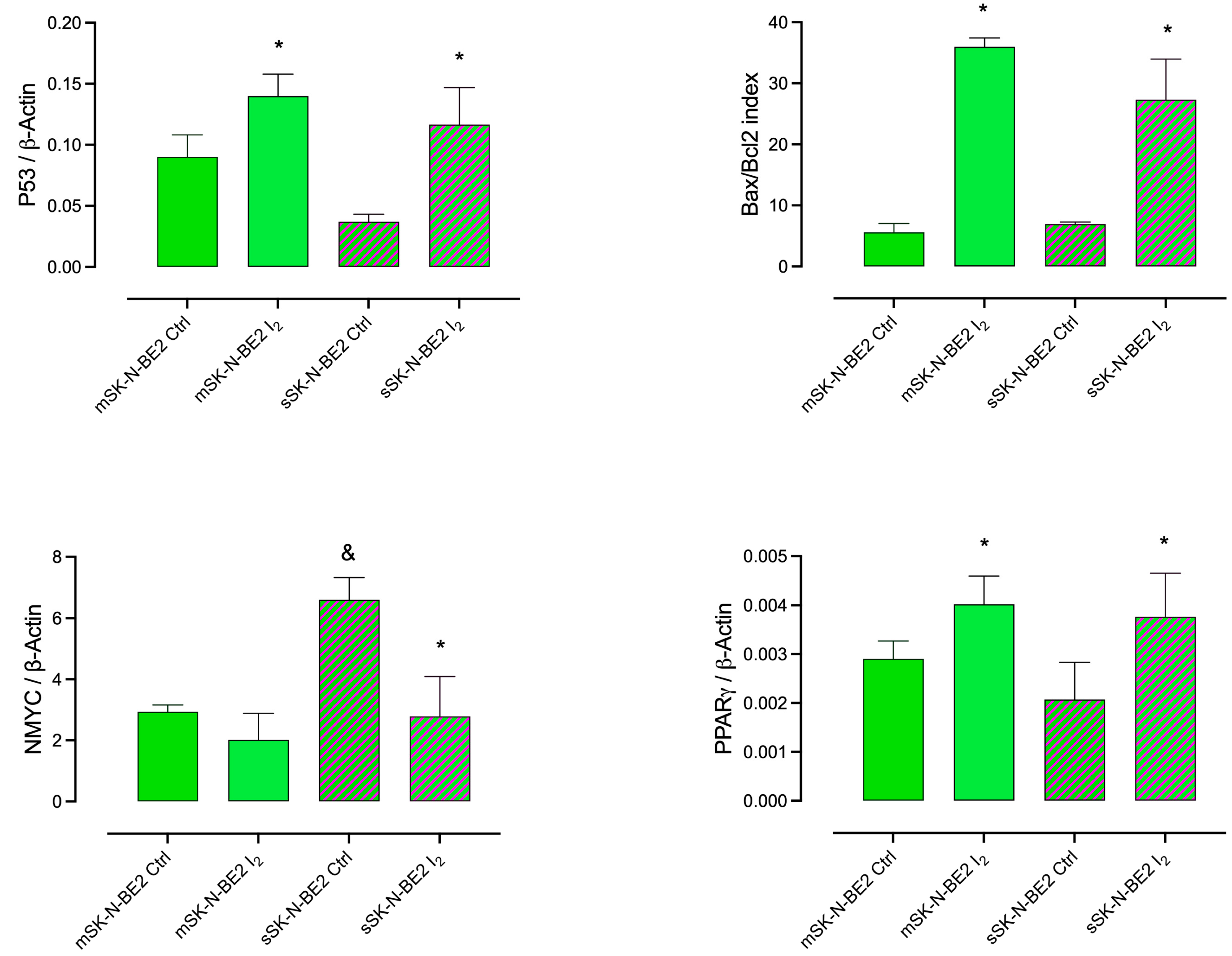
2.3. Induction of Apoptotic and Differentiation Markers in Response to the I2 Supplement in SK-N-BE2 Populations
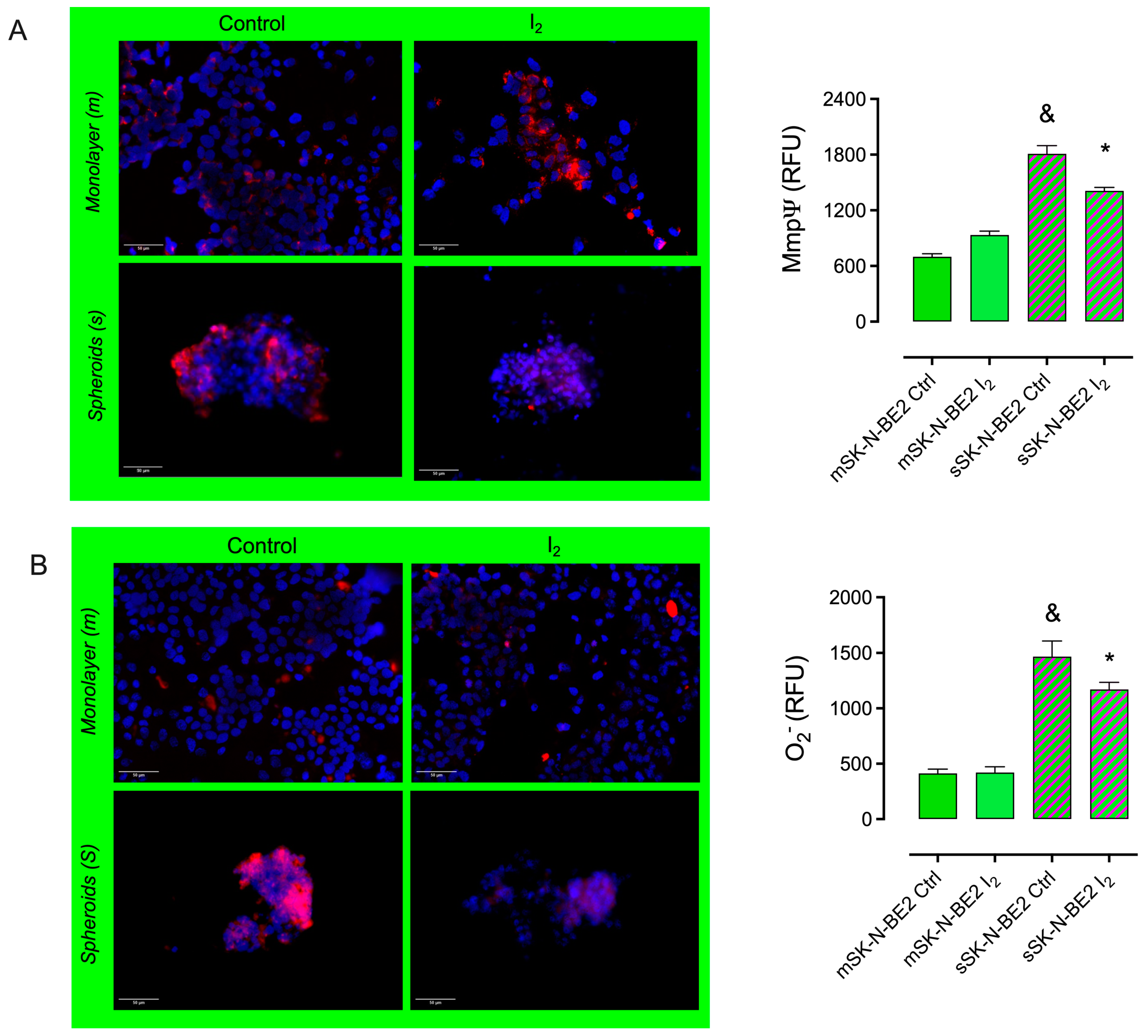
2.4. The I2 Supplement Decreases the Oxidative Mitochondrial Status
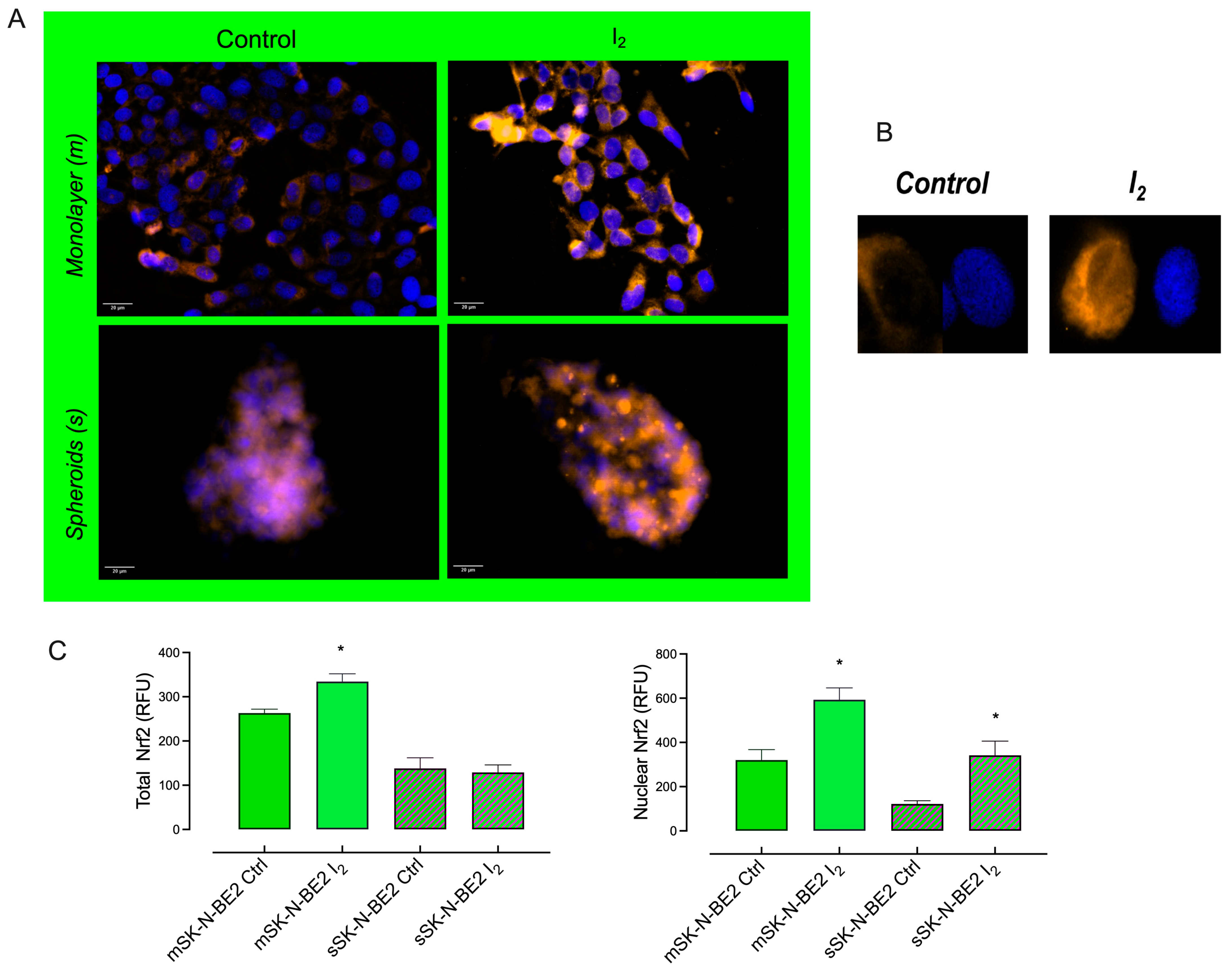
2.5. I2 Supplement Induces the Expression of Antioxidant and Autophagic Pathways
2.6. I2 Supplementation Decreases Invasive Potential Markers in the Stem Population
2.7. I2-Preselected SK-N-BE2 Populations Exhibited Minor Capacities of Implantation and Invasion in the In Vivo Zebrafish Xenograft Model
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. In Vitro
4.2.1. Cell Culture
4.2.2. Spheroids (Stem)
4.2.3. Cell Proliferation
4.2.4. Immunocytochemistry
4.2.5. Mitochondrial Function
4.2.6. Transcriptional Response
4.2.7. Flow Cytometry
4.3. In Vivo
4.3.1. Zebrafish Xenograft Model
4.3.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Sig. Transduct. Target Ther. 2020, 5, 8–10. [Google Scholar]
- Ricardo, S.; Vieira, A.F.; Gerhard, R.; Leitão, D.; Pinto, R.; Cameselle-Teijeiro, J.F.; Milanezi, F.; Schmitt, F.; Paredes, J. Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J. Clin. Pathol. 2011, 64, 937–946. [Google Scholar] [CrossRef]
- Huang, M.; Weiss, W.A. Neuroblastoma and MYCN. Cold Spring Harb. Perspect. Med. 2013, 3, a014415. [Google Scholar] [CrossRef]
- Rickman, D.S.; Schulte, J.H.; Eilers, M. The expanding world of N-MYC–driven tumors. Cancer Discov. 2018, 8, 150–163. [Google Scholar] [CrossRef]
- Zhu, S.; Lee, J.S.; Guo, F.; Shin, J.; Perez-Atayde, A.R.; Kutok, J.L.; Rodig, S.J.; Neuberg, D.S.; Helman, D.; Feng, H.; et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012, 21, 362–373. [Google Scholar] [CrossRef]
- Dey, S.; Dinakar, Y.H.; Soundarya, R.; Jain, V.; Jain, R. Navigating the therapeutic landscape for breast cancer: Targeting breast cancer stem cells. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 2387–2406. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ohata, H.; Sato, A.; Yamawaki, K.; Enomoto, T.; Okamoto, K. Tumor-derived spheroids: Relevance to cancer stem cells and clinical applications. Cancer Sci. 2017, 108, 283–289. [Google Scholar] [CrossRef]
- Vitale, M.; Di Matola, T.; D’Ascoli, F.; Salzano, S.; Bogazzi, F.; Fenzi, G.; Rossi, G. Iodide excess induces apoptosis in thyroid cells through a p53-independent mechanism involving oxidative stress. Endocrinology 2000, 141, 598–605. [Google Scholar] [CrossRef]
- Zhang, L.; Sharma, S.; Zhu, L.X.; Kogai, T.; Hershman, J.M.; Brent, G.A.; Dubinett, S.M.; Huang, M. Nonradioactive iodide effectively induces apoptosis in genetically modified lung cancer cells. Cancer Res. 2003, 63, 5065–5072. [Google Scholar]
- Torremante, E.P.; Rosner, H. Antiproliferative effects of molecular iodine in cancers. Curr. Chem. Biol. 2011, 5, 168–176. [Google Scholar]
- Aceves, C.; Mendieta, I.; Anguiano, B.; Delgado-Gonzalez, E. Molecular iodine has extrathyroidal effects as an antioxidant, differentiator, and immunomodulator. Int. J. Mol. Sci. 2021, 22, 1228. [Google Scholar] [CrossRef]
- Shrivastava, A.; Tiwari, M.; Sinha, R.A.; Kumar, A.; Balapure, A.K.; Bajpai, V.K.; Sharma, R.; Mitra, K.; Tandon, A.; Godbole, M.M. Molecular Iodine Induces Caspase-Independent Apoptosis in Human Breast Carcinoma Cells Involving the Mitochondria mediated Pathway. J. Biol. Chem. 2006, 281, 19762–19771. [Google Scholar] [CrossRef]
- Nava-Villalba, M.; Núñez-Anita, R.E.; Bontempo, A.; Aceves, C. Activation of peroxisome proliferator-activated receptor gamma is crucial for antitumoral effects of 6-iodolactone. Mol. Cancer 2015, 14, 168–172. [Google Scholar] [CrossRef]
- Bontempo, A.; Ugalde-Villanueva, B.; Delgado-González, E.; Rodríguez, A.L.; Aceves, C. Molecular iodine impairs chemoresistance mechanisms, enhances Doxorubicin retention and induces down regulation of CD44+/CD24+ and E-Cad+/Vim+ subpopulations in MCF-7 cells resistant to low doses of Doxorubicin. Oncol. Rep. 2017, 38, 2867–2876. [Google Scholar] [CrossRef]
- Bigoni-Ordóñez, G.D.; Ortiz-Sánchez, E.; Rosendo-Chalma, P.; Valencia-González, H.A.; Aceves, C.; García-Carrancá, A. Molecular iodine inhibits the expression of stemness markers on cancer stem-like cells of established cell lines derived from cervical cancer. BMC Cancer 2018, 18, 928. [Google Scholar] [CrossRef]
- Alfaro, Y.; Delgado, G.; Cárabes, A.; Anguiano, B.; Aceves, C. Iodine and doxorubicin, a good combination for mammary cancer treatment: Antineoplastic adjuvancy, chemoresistance inhibition, and cardioprotection. Mol. Cancer 2013, 12, 45–56. [Google Scholar] [CrossRef]
- Mendieta, I.; Rodríguez-Gómez, G.M.; Rueda-Zarazúa, B.; Rodríguez-Castelán, J.; Delgado-González, E.; Anguiano, B.; Aceves, C. Molecular iodine synergized and sensitized neuroblastoma cells to the antineoplastic effect of all-trans retinoic acid. Endoc-Relat. Cancer 2020, 27, 699–710. [Google Scholar] [CrossRef]
- Álvarez-León, W.; Mendieta, I.; Delgado-González, E.; Anguiano, B.; Aceves, C. Molecular iodine/cyclophosphamide synergism on chemoresistant neuroblastoma models. Int. J. Mol. Sci 2021, 22, 8936. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hemat. Oncol. 2018, 11, 64–72. [Google Scholar] [CrossRef]
- Han, S.W.; Greene, M.E.; Pitts, J.; Wada, R.K.; Sidell, N. Novel expression and function of peroxisome proliferator-activated receptor gamma (PPARgamma) in human neuroblastoma cells. Clin. Cancer Res. 2001, 7, 98–104. [Google Scholar] [PubMed]
- Vella, S.; Conaldi, P.G.; Florio, T.; Pagano, A. PPAR Gamma in Neuroblastoma: The Translational Perspectives of Hypoglycemic Drugs. PPAR Res. 2016, 30, 38164–38173. [Google Scholar] [CrossRef]
- Ricordati, C.; Gelmini, S.; Ceni, E.; Galli, A.; Balzi, M.; Faraoni, M.; Serio, M.; Peri, E. In vivo effects of rosiglitazone in a human neuroblastoma xenograft. Br. J. Cancer 2010, 102, 685–692. [Google Scholar]
- Asgharzadeh, F.; Memarzia, A.; Alikhani, V.; Beigoli, S.; Boskabady, M.H. Peroxisome proliferator-activated receptors: Key regulators of tumor progression and growth. Transl. Oncol. 2024, 47, 102039–102043. [Google Scholar] [CrossRef]
- Glorieux, C.; Liu, S.; Trachootham, D. Targeting ROS in cancer: Rationale and strategies. Nat. Rev. Drug. Discov. 2024, 23, 583–606. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, M.B.; Frusic-Zlotkin, M.; Soroka, Y.; Ben-Sasson, S.; Bianco-Peled, H.; Kohen, R. A novel role of topical iodine in skin: Activation of the Nrf2 pathway. Free Radic. Biol. Med. 2017, 104, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Enríquez, C.; González, C.; Aguirre-Martínez, G.; Buc Calderon, P. The Multifaceted Roles of NRF2 in Cancer: Friend or Foe? Antioxidants 2024, 13, 70. [Google Scholar] [CrossRef]
- Niharika, K.; Garg, M. Understanding the autophagic functions in cancer stem cell maintenance and therapy resistance. Expert Rev. Mol. Med. 2024, 26, e23. [Google Scholar] [CrossRef]
- Zou, J.; Li, W.; Misra, A.; Yue, F.; Song, K.; Chen, Q.; Guo, G.; Yi, J.; Kimata, J.T.; Liu, L. The viral restriction factor tetherin prevents leucine-rich pentatricopeptide repeat-containing protein (LRPPRC) from association with beclin1 and B-cell CLL/lymphoma 2 (Bcl-2) and enhances autophagy and mitophagy. J. Biol. Chem. 2015, 290, 7269–7279. [Google Scholar] [CrossRef]
- Safari, F.; Dadvar, F. In vitro evaluation of autophagy and cell death induction in Panc1 pancreatic cancer by secretome of hAMSCs through downregulation of p-AKT/p-mTOR and upregulation of p-AMPK/ULK1 signal transduction pathways. Tissue Cell 2023, 84, 102160–102165. [Google Scholar] [CrossRef]
- Hason, M.; Bartunek, P. Zebrafish models of cancer-new insights on modeling human cancer in a non-mammalian vertebrate. Genes 2019, 10, 935. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendieta, I.; Leon-Pichardo, J.; Orizaga-Osti, G.; Juvera-Avalos, E.R.; Rangel-Chavez, U.; Delgado-Gonzalez, E.; Anguiano, B.; Aceves, C. Molecular Iodine Exhibited Differential Antiproliferative Actions in Progenitor and Stem Populations from Chemoresistant Cancer Cells. Int. J. Mol. Sci. 2025, 26, 4020. https://doi.org/10.3390/ijms26094020
Mendieta I, Leon-Pichardo J, Orizaga-Osti G, Juvera-Avalos ER, Rangel-Chavez U, Delgado-Gonzalez E, Anguiano B, Aceves C. Molecular Iodine Exhibited Differential Antiproliferative Actions in Progenitor and Stem Populations from Chemoresistant Cancer Cells. International Journal of Molecular Sciences. 2025; 26(9):4020. https://doi.org/10.3390/ijms26094020
Chicago/Turabian StyleMendieta, Irasema, Jazmin Leon-Pichardo, Gustavo Orizaga-Osti, Edgar R. Juvera-Avalos, Uriel Rangel-Chavez, Evangelina Delgado-Gonzalez, Brenda Anguiano, and Carmen Aceves. 2025. "Molecular Iodine Exhibited Differential Antiproliferative Actions in Progenitor and Stem Populations from Chemoresistant Cancer Cells" International Journal of Molecular Sciences 26, no. 9: 4020. https://doi.org/10.3390/ijms26094020
APA StyleMendieta, I., Leon-Pichardo, J., Orizaga-Osti, G., Juvera-Avalos, E. R., Rangel-Chavez, U., Delgado-Gonzalez, E., Anguiano, B., & Aceves, C. (2025). Molecular Iodine Exhibited Differential Antiproliferative Actions in Progenitor and Stem Populations from Chemoresistant Cancer Cells. International Journal of Molecular Sciences, 26(9), 4020. https://doi.org/10.3390/ijms26094020






