Restraint Stress Disrupted Intestinal Homeostasis via 5-HT/HTR7/Wnt/β-Catenin/NF-kB Signaling
Abstract
1. Introduction
2. Results
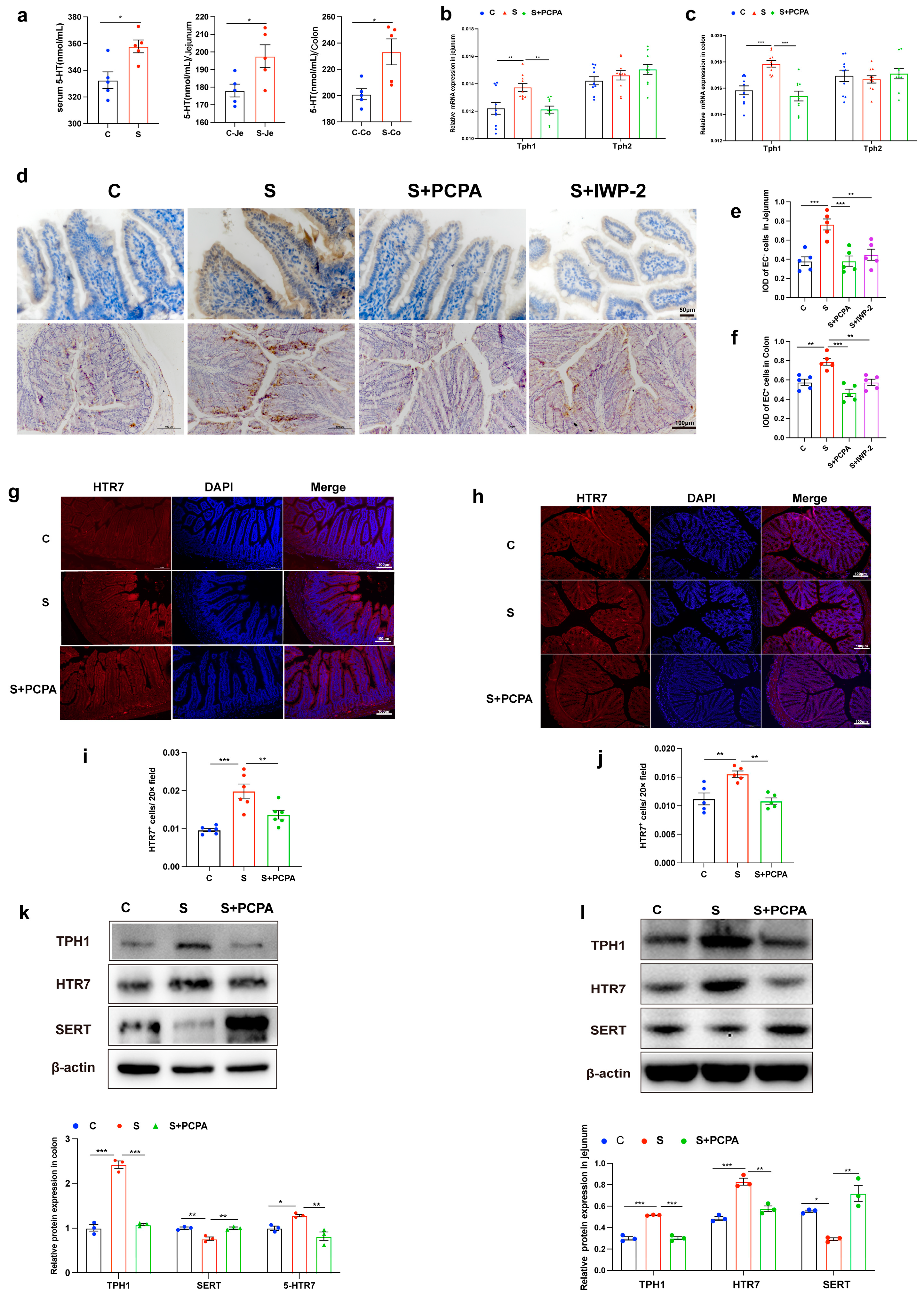
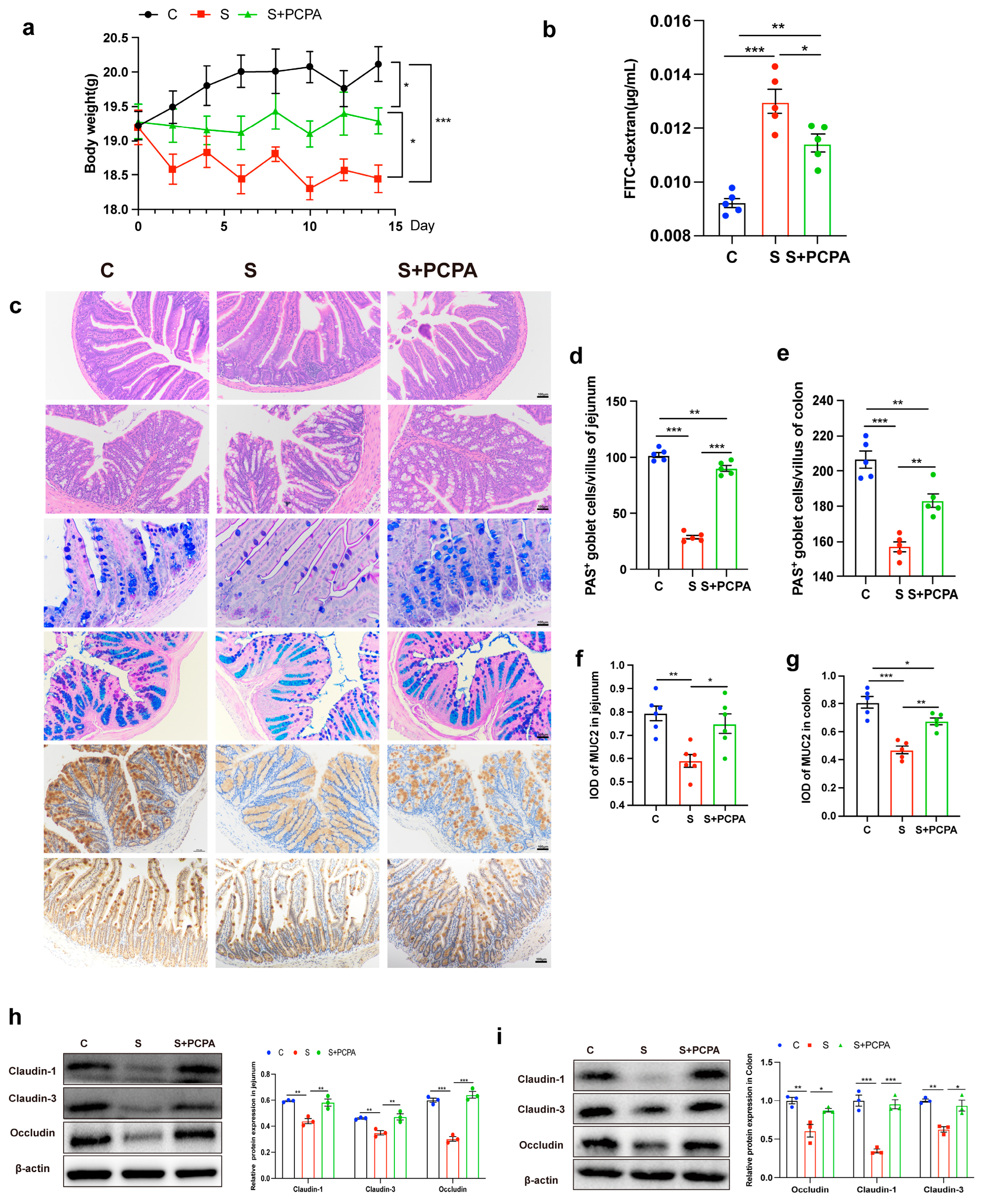
2.1. Restraint Stress Induced the Increase in 5-HT Level, Leading to the Damage of Intestinal Barrier
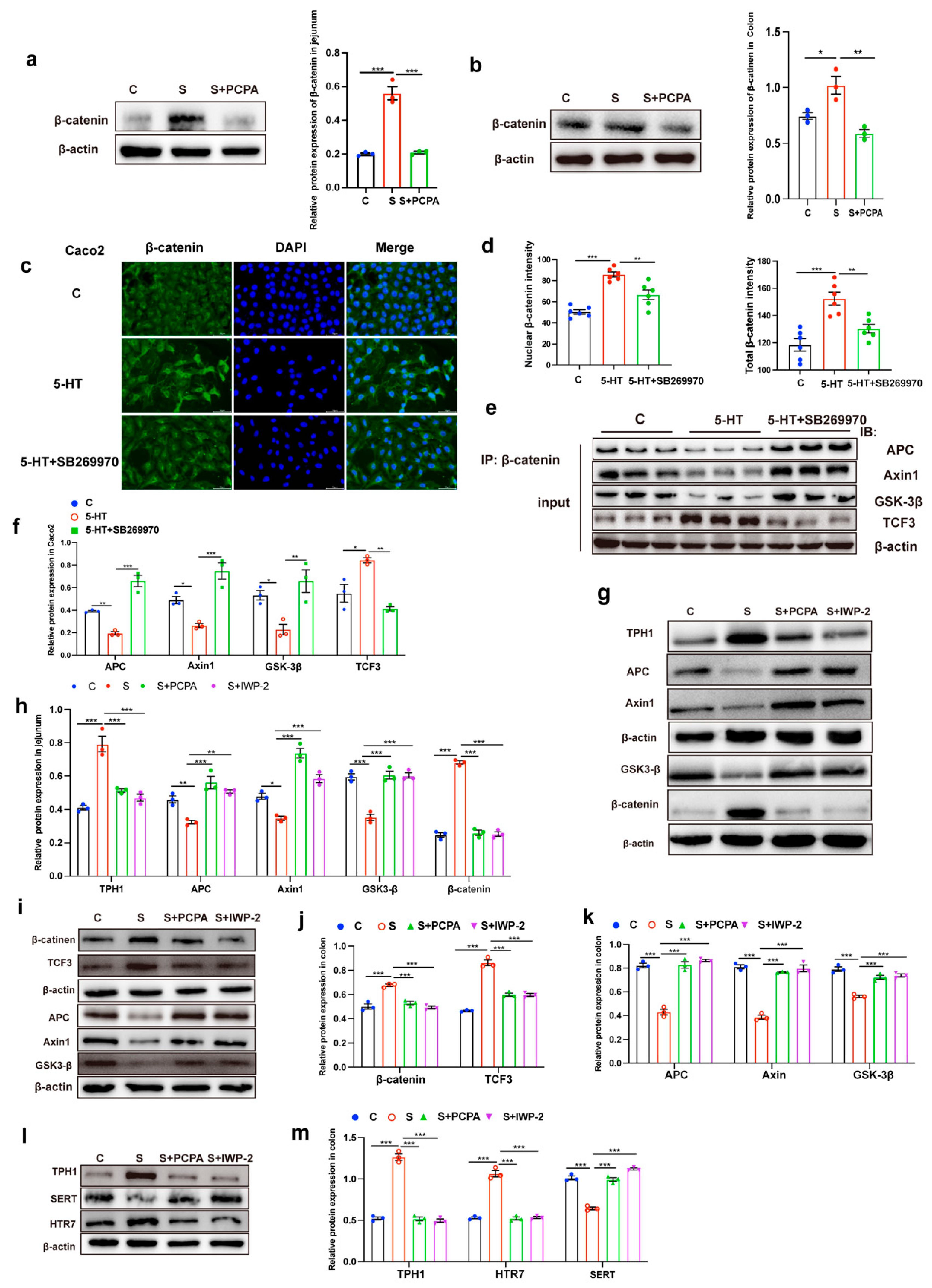
2.2. 5-HT Engagement Triggered Wnt/β-Catenin Signaling Activation
2.3. Intestinal Inflammation Induced by 5-HT May Be Due to the Interaction Between Wnt/β-Catenin Signal and NF-KB Signal
3. Discussion
4. Materials and Methods
4.1. Animal Treatments
4.2. Ethics Approval and Consent to Participate
4.3. Intestinal Permeability
4.4. RNA Isolation and RT–qPCR Analysis
4.5. Cell Treatment
4.6. Cellular Immunofluorescence Analysis
4.7. Histological Analysis
4.8. Western Blotting
4.9. Measurement of Serotonin Concentration in Intestinal Tissues
4.10. Coimmunoprecipitation Assay for Protein–Protein Binding Interactions
4.11. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrews, J.M.; Holtmann, G. IBD: Stress causes flares of IBD—How much evidence is enough? Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Sgambato, D.; Miranda, A.; Ranaldo, R.; Federico, A.; Romano, M. The Role of Stress in Inflammatory Bowel Diseases. Curr. Pharm. Des. 2017, 23, 3997–4002. [Google Scholar] [CrossRef]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.Y.; Cheng, C.W.; Tang, X.D.; Bian, Z.X. Impact of psychological stress on irritable bowel syndrome. World J. Gastroenterol. 2014, 20, 14126–14131. [Google Scholar] [CrossRef] [PubMed]
- Mawdsley, J.E.; Rampton, D.S. Psychological stress in IBD: New insights into pathogenic and therapeutic implications. Gut 2005, 54, 1481–1491. [Google Scholar] [CrossRef]
- Goodhand, J.R.; Wahed, M.; Mawdsley, J.E.; Farmer, A.D.; Aziz, Q.; Rampton, D.S. Mood disorders in inflammatory bowel disease: Relation to diagnosis, disease activity, perceived stress, and other factors. Inflamm. Bowel Dis. 2012, 18, 2301–2309. [Google Scholar] [CrossRef]
- Rengarajan, S.; Knoop, K.A.; Rengarajan, A.; Chai, J.N.; Grajales-Reyes, J.G.; Samineni, V.K.; Russler-Germain, E.V.; Ranganathan, P.; Fasano, A.; Sayuk, G.S.; et al. A Potential Role for Stress-Induced Microbial Alterations in IgA-Associated Irritable Bowel Syndrome with Diarrhea. Cell Rep. Med. 2020, 1, 100124. [Google Scholar] [CrossRef]
- Allen, J.M.; Mackos, A.R.; Jaggers, R.M.; Brewster, P.C.; Webb, M.; Lin, C.H.; Ladaika, C.; Davies, R.; White, P.; Loman, B.R.; et al. Psychological stress disrupts intestinal epithelial cell function and mucosal integrity through microbe and host-directed processes. Gut Microbes 2022, 14, 2035661. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Chen, C.C.; Gillilland, M., 3rd; Sun, X.; El-Zaatari, M.; Huffnagle, G.B.; Young, V.B.; Zhang, J.; Hong, S.C.; et al. Stress-induced corticotropin-releasing hormone-mediated NLRP6 inflammasome inhibition and transmissible enteritis in mice. Gastroenterology 2013, 144, 1478–1487.e8. [Google Scholar] [CrossRef]
- Cameron, H.L.; Perdue, M.H. Stress impairs murine intestinal barrier function: Improvement by glucagon-like peptide-2. J. Pharmacol. Exp. Ther. 2005, 314, 214–220. [Google Scholar] [CrossRef]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.; et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. USA 2018, 115, E2960–E2969. [Google Scholar] [CrossRef]
- Bailey, M.T.; Dowd, S.E.; Parry, N.M.; Galley, J.D.; Schauer, D.B.; Lyte, M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect. Immun. 2010, 78, 1509–1519. [Google Scholar] [CrossRef]
- Gershon, M.D.; Tack, J. The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterology 2007, 132, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Gershon, M.D. Review article: Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment. Pharmacol. Ther. 1999, 13 (Suppl. S2), 15–30. [Google Scholar] [CrossRef]
- Racké, K.; Reimann, A.; Schwörer, H.; Kilbinger, H. Regulation of 5-HT release from enterochromaffin cells. Behav. Brain Res. 1996, 73, 83–87. [Google Scholar] [CrossRef]
- Fitzpatrick, P.F. Tetrahydropterin-dependent amino acid hydroxylases. Annu. Rev. Biochem. 1999, 68, 355–381. [Google Scholar] [CrossRef] [PubMed]
- Sikander, A.; Rana, S.V.; Prasad, K.K. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin. Chim. Acta 2009, 403, 47–55. [Google Scholar] [CrossRef]
- Dunlop, S.P.; Jenkins, D.; Neal, K.R.; Spiller, R.C. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 2003, 125, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Ghia, J.E.; Li, N.; Wang, H.; Collins, M.; Deng, Y.; El-Sharkawy, R.T.; Côté, F.; Mallet, J.; Khan, W.I. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology 2009, 137, 1649–1660. [Google Scholar] [CrossRef]
- Coates, M.D.; Mahoney, C.R.; Linden, D.R.; Sampson, J.E.; Chen, J.; Blaszyk, H.; Crowell, M.D.; Sharkey, K.A.; Gershon, M.D.; Mawe, G.M.; et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology 2004, 126, 1657–1664. [Google Scholar] [CrossRef]
- Li, N.; Ghia, J.E.; Wang, H.; McClemens, J.; Cote, F.; Suehiro, Y.; Mallet, J.; Khan, W.I. Serotonin activates dendritic cell function in the context of gut inflammation. Am. J. Pathol. 2011, 178, 662–671. [Google Scholar] [CrossRef]
- Freire-Garabal, M.; Núñez, M.J.; Balboa, J.; López-Delgado, P.; Gallego, R.; García-Caballero, T.; Fernández-Roel, M.D.; Brenlla, J.; Rey-Méndez, M. Serotonin upregulates the activity of phagocytosis through 5-HT1A receptors. Br. J. Pharmacol. 2003, 139, 457–463. [Google Scholar] [CrossRef]
- Zhu, P.; Lu, T.; Chen, Z.; Liu, B.; Fan, D.; Li, C.; Wu, J.; He, L.; Zhu, X.; Du, Y.; et al. 5-hydroxytryptamine produced by enteric serotonergic neurons initiates colorectal cancer stem cell self-renewal and tumorigenesis. Neuron 2022, 110, 2268–2282.e4. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Song, J.; Lee, H.; Sim, I.; Kwon, Y.V.; Jho, E.H.; Yoon, Y. LGK974 suppresses lipopolysaccharide-induced endotoxemia in mice by modulating the crosstalk between the Wnt/β-catenin and NF-κB pathways. Exp. Mol. Med. 2021, 53, 407–421. [Google Scholar] [CrossRef]
- Guan, X.; He, Y.; Wei, Z.; Shi, C.; Li, Y.; Zhao, R.; Pan, L.; Han, Y.; Hou, T.; Yang, J. Crosstalk between Wnt/β-catenin signaling and NF-κB signaling contributes to apical periodontitis. Int. Immunopharmacol. 2021, 98, 107843. [Google Scholar] [CrossRef] [PubMed]
- Shaler, C.R.; Parco, A.A.; Elhenawy, W.; Dourka, J.; Jury, J.; Verdu, E.F.; Coombes, B.K. Psychological stress impairs IL22-driven protective gut mucosal immunity against colonising pathobionts. Nat. Commun. 2021, 12, 6664. [Google Scholar] [CrossRef]
- Brindley, R.L.; Bauer, M.B.; Blakely, R.D.; Currie, K.P.M. An interplay between the serotonin transporter (SERT) and 5-HT receptors controls stimulus-secretion coupling in sympathoadrenal chromaffin cells. Neuropharmacology 2016, 110, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Barker, N.; van Es, J.H.; Kuipers, J.; Kujala, P.; van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Mackos, A.R.; Galley, J.D.; Eubank, T.D.; Easterling, R.S.; Parry, N.M.; Fox, J.G.; Lyte, M.; Bailey, M.T. Social stress-enhanced severity of Citrobacter rodentium-induced colitis is CCL2-dependent and attenuated by probiotic Lactobacillus reuteri. Mucosal Immunol. 2016, 9, 515–526. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Xie, R.; Wang, B.; Jiang, K.; Cao, H. Stress Triggers Flare of Inflammatory Bowel Disease in Children and Adults. Front. Pediatr. 2019, 7, 432. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Wang, H.; Denou, E.; Ghia, J.E.; Rossi, L.; Fontes, M.E.; Bernier, S.P.; Shajib, M.S.; Banskota, S.; Collins, S.M.; et al. Modulation of Gut Microbiota Composition by Serotonin Signaling Influences Intestinal Immune Response and Susceptibility to Colitis. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 709–728. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, B.; Verne, G.N. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 2009, 146, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Vicario, M.; Ramos, L.; Lobo, B.; Mosquera, J.L.; Alonso, C.; Sánchez, A.; Guilarte, M.; Antolín, M.; de Torres, I.; et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am. J. Gastroenterol. 2012, 107, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.; Nyström, E.E.; Johansson, M.E.; Hansson, G.C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.V.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef]
- Ortiz-Masiá, D.; Cosín-Roger, J.; Calatayud, S.; Hernández, C.; Alós, R.; Hinojosa, J.; Apostolova, N.; Alvarez, A.; Barrachina, M.D. Hypoxic macrophages impair autophagy in epithelial cells through Wnt1: Relevance in IBD. Mucosal Immunol. 2014, 7, 929–938. [Google Scholar] [CrossRef]
- Xing, Y.; Chen, X.; Cao, Y.; Huang, J.; Xie, X.; Wei, Y. Expression of Wnt and Notch signaling pathways in inflammatory bowel disease treated with mesenchymal stem cell transplantation: Evaluation in a rat model. Stem Cell Res. Ther. 2015, 6, 101. [Google Scholar] [CrossRef]
- Claessen, M.M.; Schipper, M.E.; Oldenburg, B.; Siersema, P.D.; Offerhaus, G.J.; Vleggaar, F.P. WNT-pathway activation in IBD-associated colorectal carcinogenesis: Potential biomarkers for colonic surveillance. Cell Oncol. 2010, 32, 303–310. [Google Scholar] [CrossRef]
- Serafino, A.; Moroni, N.; Zonfrillo, M.; Andreola, F.; Mercuri, L.; Nicotera, G.; Nunziata, J.; Ricci, R.; Antinori, A.; Rasi, G.; et al. WNT-pathway components as predictive markers useful for diagnosis, prevention and therapy in inflammatory bowel disease and sporadic colorectal cancer. Oncotarget 2014, 5, 978–992. [Google Scholar] [CrossRef]
- Wong, H.L.X.; Qin, H.Y.; Tsang, S.W.; Zuo, X.; Che, S.; Chow, C.F.W.; Li, X.; Xiao, H.T.; Zhao, L.; Huang, T.; et al. Early life stress disrupts intestinal homeostasis via NGF-TrkA signaling. Nat. Commun. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Pereira, C.P.; Bachli, E.B.; Schoedon, G. The wnt pathway: A macrophage effector molecule that triggers inflammation. Curr. Atheroscler. Rep. 2009, 11, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Moparthi, L.; Koch, S. Wnt signaling in intestinal inflammation. Differentiation 2019, 108, 24–32. [Google Scholar] [CrossRef]
- Ma, B.; Hottiger, M.O. Crosstalk between Wnt/β-Catenin and NF-κB Signaling Pathway during Inflammation. Front. Immunol. 2016, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- George, S.J. Wnt pathway: A new role in regulation of inflammation. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Ha, J.H.; Chung, S.I.; Yoon, Y. Β-catenin regulates NF-κB activity and inflammatory cytokine expression in bronchial epithelial cells treated with lipopolysaccharide. Int. J. Mol. Med. 2014, 34, 632–638. [Google Scholar] [CrossRef]
- Lin, J.C.; Chang, R.L.; Chen, Y.F.; Yang, J.J.; Baskaran, R.; Chung, L.C.; Chen, R.J.; Day, C.H.; Vijaya Padma, V.; Huang, C.Y. β-Catenin overexpression causes an increase in inflammatory cytokines and NF-κB activation in cardiomyocytes. Cell. Mol. Biol. 2016, 63, 17–22. [Google Scholar] [CrossRef]
- Yang, D.; Sun, Y.; Wen, P.; Chen, Y.; Cao, J.; Sun, X.; Dong, Y. Chronic Stress-induced Serotonin Impairs Intestinal Epithelial Cell Mitochondrial Biogenesis via the AMPK-PGC-1α Axis. Int. J. Biol. Sci. 2024, 20, 4476–4495. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Y.; Hou, Y.; Cao, S.; Chen, Z.; Zhang, Y.; Cai, X.; Yan, Q.; Li, Z.; Yuan, Y.; et al. Psychological stress-induced microbial metabolite indole-3-acetate disrupts intestinal cell lineage commitment. Cell Metab. 2024, 36, 466–483.e467. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Richards, M.C.; Wakabayashi, C.; Kunugi, H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 112–119. [Google Scholar] [CrossRef] [PubMed]

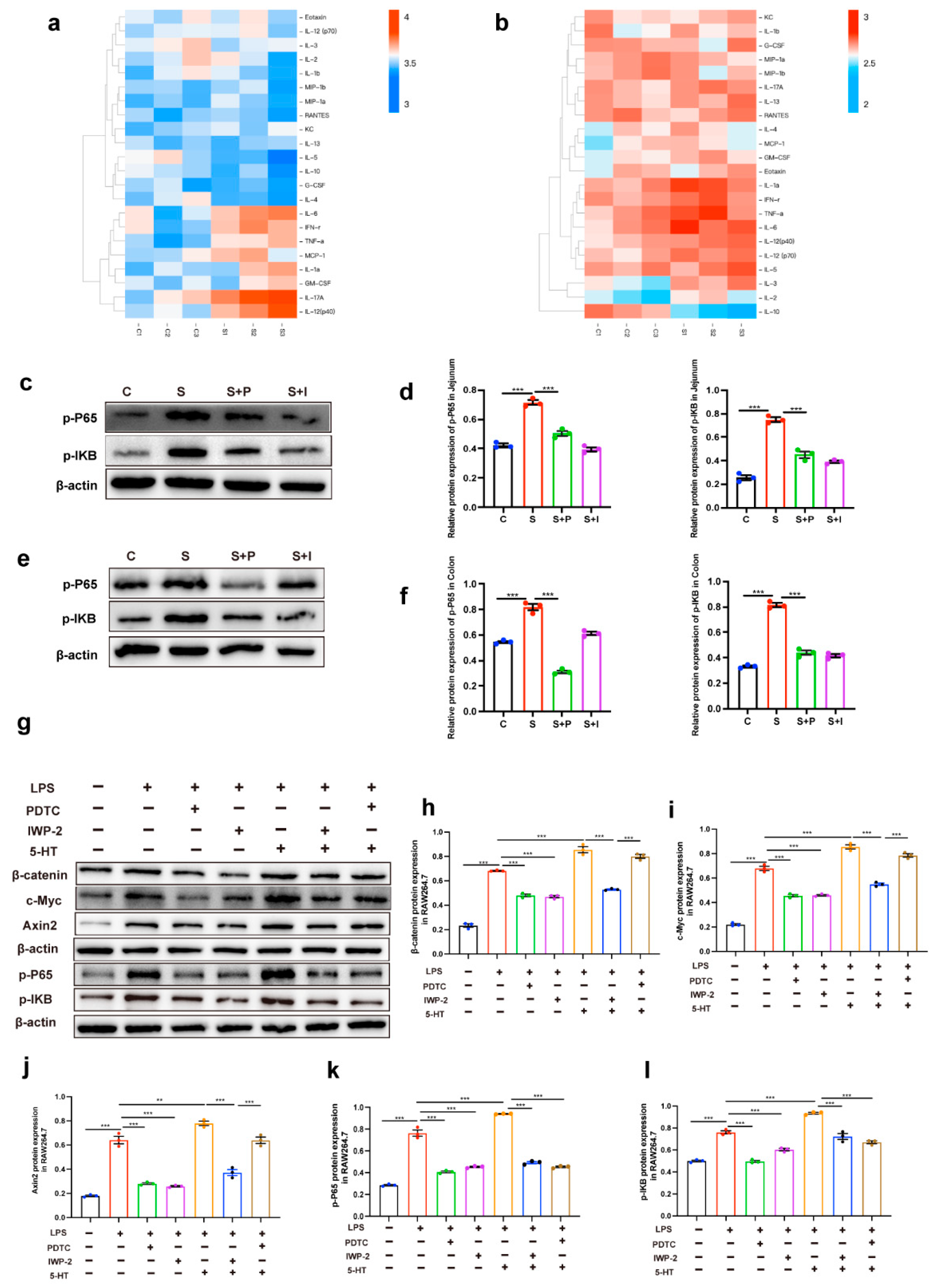
| Cytokines | C | S | p Value |
|---|---|---|---|
| IL-1α | 11.92 ± 0.5486 | 14.19 ± 1.237 | 0.1694 |
| IL-1β | 12.57 ± 0.8827 | 11.72 ± 0.6573 | 0.4830 |
| IL-2 | 13.15 ± 0.8552 | 12.38 ± 0.9782 | 0.5866 |
| IL-3 | 13.80 ± 0.5379 | 12.62 ± 0.3701 | 0.1469 |
| IL-6 | 12.45 ± 0.9461 | 16.11 ± 0.7175 | 0.0369 * |
| IL-12(p40) | 12.42 ± 0.4264 | 16.85 ± 1.049 | 0.0173 * |
| IL-12 (p70) | 12.60 ± 0.3567 | 12.74 ± 0.6593 | 0.8590 |
| IL-17A | 13.29 ± 0.9735 | 18.25 ± 0.6980 | 0.0144 * |
| Eotaxin | 12.92 ± 0.5740 | 12.73 ± 0.6233 | 0.8304 |
| GM-CSF | 12.36 ± 0.5398 | 13.34 ± 1.024 | 0.4443 |
| IFN-γ | 12.14 ± 0.9250 | 15.65 ± 0.6022 | 0.0336 * |
| KC | 11.68 ± 0.4232 | 12.55 ± 0.5199 | 0.2660 |
| MCP-1 | 12.55 ± 0.5398 | 14.53 ± 0.8864 | 0.1288 |
| MIP-1a | 11.20 ± 0.2905 | 11.76 ± 0.6532 | 0.4797 |
| MIP-1b | 11.64 ± 0.3495 | 11.68 ± 0.6964 | 0.9618 |
| RANTES | 11.44 ± 0.4923 | 11.31 ± 0.5398 | 0.8694 |
| TNF-α | 11.25 ± 0.4965 | 14.39 ± 0.4431 | 0.0092 ** |
| IL-13 | 11.72 ± 0.4328 | 11.56 ± 0.3449 | 0.7822 |
| G-CSF | 11.67 ± 0.6958 | 10.86 ± 0.2745 | 0.3347 |
| IL-4 | 12.63 ± 0.7342 | 10.72 ± 0.2379 | 0.0686 |
| IL-5 | 13.15 ± 0.6566 | 10.40 ± 0.5798 | 0.0350 * |
| IL-10 | 12.46 ± 0.3397 | 10.88 ± 0.5998 | 0.0834 |
| Cytokines | C | S | p Value |
|---|---|---|---|
| IL-1α | 10.50 ± 0.5220 | 13.26 ± 0.6310 | 0.0281 * |
| IL-1β | 9.416 ± 1.193 | 9.748 ± 0.6097 | 0.8166 |
| IL-2 | 6.884 ± 0.5568 | 8.704 ± 0.5932 | 0.0889 |
| IL-3 | 7.918 ± 0.5836 | 10.80 ± 0.5604 | 0.0235 * |
| IL-6 | 10.22 ± 0.6225 | 12.91 ± 0.7110 | 0.0466 * |
| IL-12(p40) | 9.820 ± 0.5456 | 11.95 ± 0.1158 | 0.0188 * |
| IL-12 (p70) | 9.715 ± 0.5167 | 11.56 ± 0.3348 | 0.0399 * |
| IL-17A | 9.931 ± 0.6067 | 11.21 ± 0.3143 | 0.1340 |
| Eotaxin | 9.387 ± 0.7543 | 9.657 ± 0.8402 | 0.8228 |
| GM-CSF | 8.559 ± 0.3482 | 9.962 ± 0.5812 | 0.1072 |
| IFN-γ | 10.16 ± 0.3184 | 12.02 ± 0.4696 | 0.0303 * |
| KC | 9.754 ± 0.6356 | 9.787 ± 0.5508 | 0.9709 |
| MCP-1 | 8.506 ± 0.8345 | 8.927 ± 0.5487 | 0.6950 |
| MIP-1a | 10.82 ± 0.5459 | 9.910 ± 0.5770 | 0.3155 |
| MIP-1b | 10.80 ± 0.5945 | 9.560 ± 0.8817 | 0.3072 |
| RANTES | 10.82 ± 0.6647 | 10.53 ± 0.7689 | 0.7914 |
| TNF-α | 10.40 ± 0.5129 | 12.89 ± 0.5803 | 0.0321 * |
| IL-13 | 9.940 ± 0.5399 | 10.92 ± 0.6066 | 0.2927 |
| G-CSF | 10.59 ± 0.3494 | 9.887 ± 1.094 | 0.5732 |
| IL-4 | 8.710 ± 0.6097 | 9.252 ± 0.8162 | 0.6231 |
| IL-5 | 10.77 ± 0.5018 | 11.45 ± 0.8444 | 0.5284 |
| IL-10 | 10.85 ± 0.5929 | 5.920 ± 0.5837 | 0.0041 ** |
| Gene Name | Forword Sequences (5′-3′) | Reverse Sequences (5′-3′) |
|---|---|---|
| β-catenin | TGACACCTCCCAAGTCCTTT | TTGCATACTGCCCGTCAAT |
| Axin2 | GAGAGTGAGCGGCAGAGC | CGGCTGACTCGTTCTCCT |
| Cd44 | TCGATTTGAATGTAACCTGCCG | TCGATTTGAATGGATGTAACCTGCA |
| Myc | ATGCCCCTCAACGTGAACTTC | CGCAACATAGGATGGAGAGCA |
| Lgr5 | CCTACTCGAAGACTTACCCAGT | GCATTGGGGTGAATGATAGCA |
| Sox9 | CTGGAGGCTGCTGAACGAGAG | CGGCGGACCCTGAGATTGC |
| Olfm4 | CAGCCACTTTCCAATTTCACTG | GCTGGACATACTCCTTCACCTTA |
| Htr7 | TGCGGGGAGCAGATCAACTA | GACAAAGCACACCGAGATCAC |
| Htr4 | GGCTATATCAATTCGGGGTTGAA | GTGTATGGGCAATTTCTCCAGTT |
| Htr3 | CCTGGCTAACTACAAGAAGGGG | TGCAGAAACTCATCAGTCCAGTA |
| Htr2 | TAATGCAATTAGGTGACGACTCG | GCAGGAGAGGTTGGTTCTGTTT |
| Tph1 | AACTTTCACACTTCAGATTCACC | ATAGGCCGTCTCTGAGGAAC |
| Tph2 | GGTTGTCCTTGGATTCTGCTG | GCCTGGATTCGATATGAAGCAT |
| Slc6a4 | TATCCAATGGGTACTCCGCAG | CCGTTCCCCTTGGTGAATCT |
| Muc2 | AGGGCTCGGAACTCCAGAAA | CCAGGGAATCGGTAGACATCG |
| Tff3 | TTGCTGGGTCCTCTGGGATAG | TACACTGCTCCGATGTGACAG |
| Klf3 | GAAGCCCAACAAATATGGGGT | GGACGGGAACTTCAGAGAGG |
| Muc2 | AGGGCTCGGAACTCCAGAAA | CCAGGGAATCGGTAGACATCG |
| Tff3 | TTGCTGGGTCCTCTGGGATAG | TACACTGCTCCGATGTGACAG |
| Klf3 | GAAGCCCAACAAATATGGGGT | GGACGGGAACTTCAGAGAGG |
| Tff3 | TTGCTGGGTCCTCTGGGATAG | TACACTGCTCCGATGTGACAG |
| Klf3 | GAAGCCCAACAAATATGGGGT | GGACGGGAACTTCAGAGAGG |
| Gapdh | AGCTTGTCATCAACGGGAAG | TTTGATGTTAGTGGGGTCTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Wang, Z.; Chen, Y.; Dong, Y. Restraint Stress Disrupted Intestinal Homeostasis via 5-HT/HTR7/Wnt/β-Catenin/NF-kB Signaling. Int. J. Mol. Sci. 2025, 26, 4021. https://doi.org/10.3390/ijms26094021
Yu J, Wang Z, Chen Y, Dong Y. Restraint Stress Disrupted Intestinal Homeostasis via 5-HT/HTR7/Wnt/β-Catenin/NF-kB Signaling. International Journal of Molecular Sciences. 2025; 26(9):4021. https://doi.org/10.3390/ijms26094021
Chicago/Turabian StyleYu, Jiayu, Zixu Wang, Yaoxing Chen, and Yulan Dong. 2025. "Restraint Stress Disrupted Intestinal Homeostasis via 5-HT/HTR7/Wnt/β-Catenin/NF-kB Signaling" International Journal of Molecular Sciences 26, no. 9: 4021. https://doi.org/10.3390/ijms26094021
APA StyleYu, J., Wang, Z., Chen, Y., & Dong, Y. (2025). Restraint Stress Disrupted Intestinal Homeostasis via 5-HT/HTR7/Wnt/β-Catenin/NF-kB Signaling. International Journal of Molecular Sciences, 26(9), 4021. https://doi.org/10.3390/ijms26094021







