Cell-Autonomous Immunity: From Cytosolic Sensing to Self-Defense
Abstract
1. Introduction
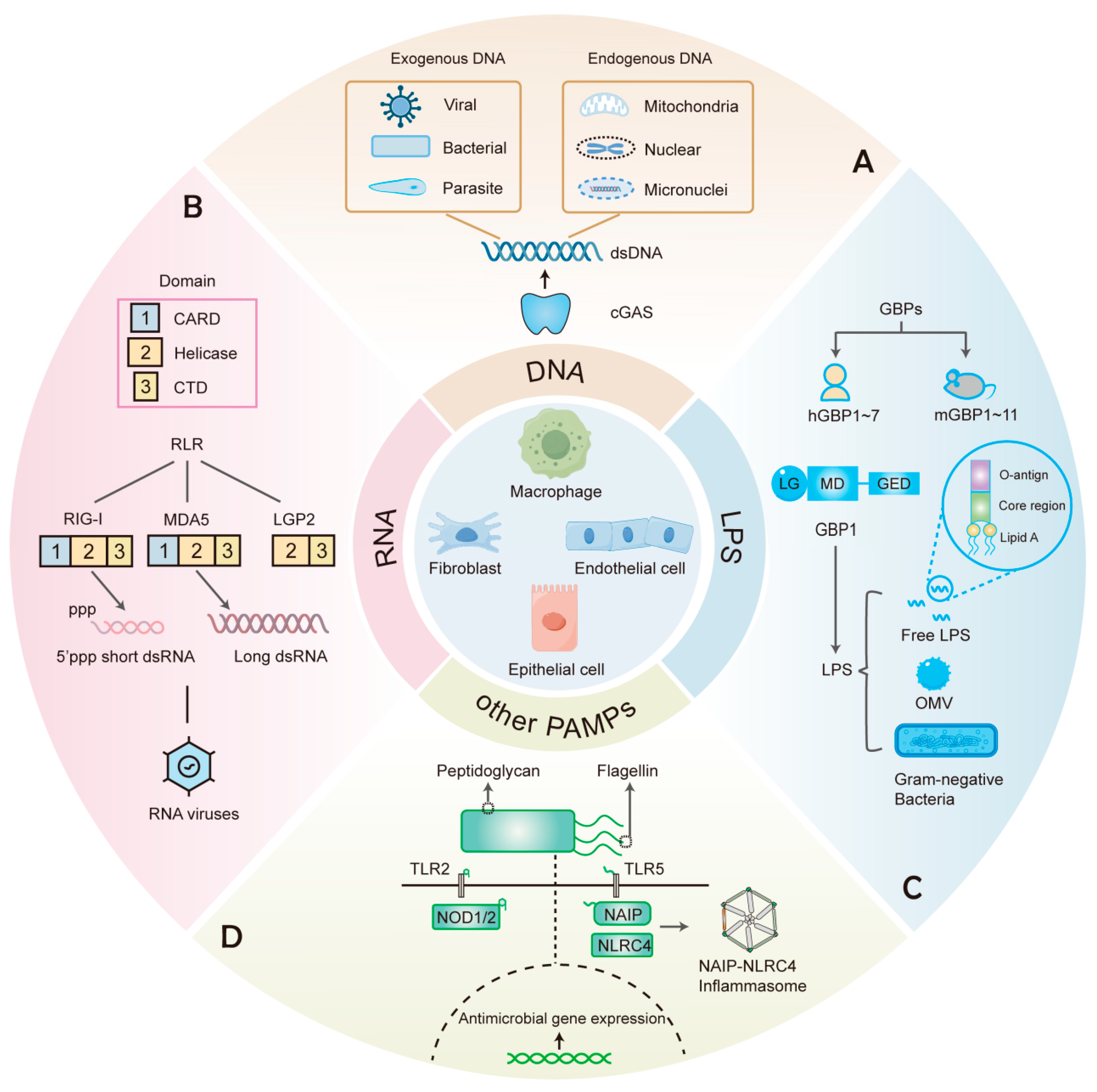
2. Detection of Cytosolic DNA
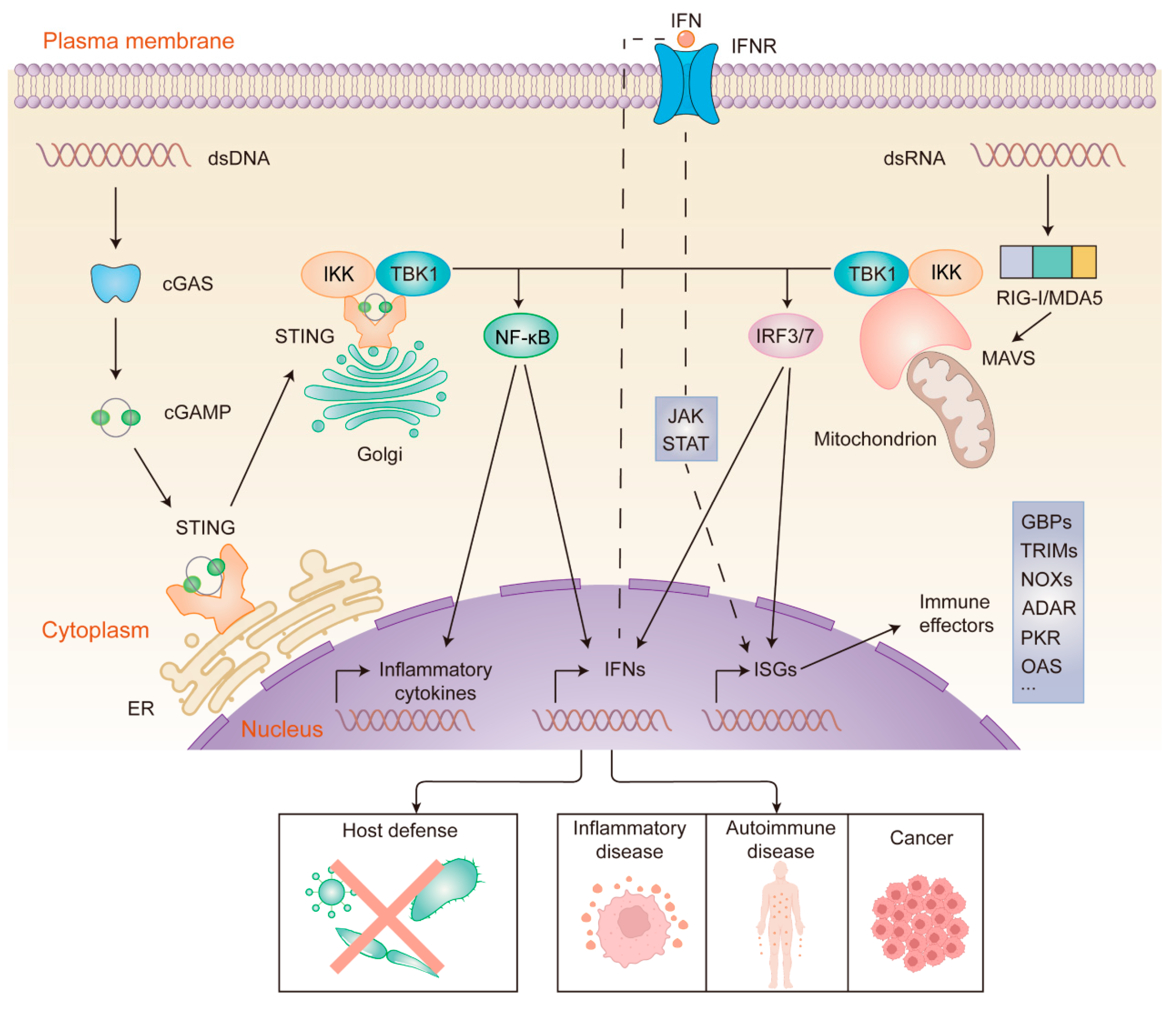
2.1. DNA-Sensing and the cGAS-STING Pathway
2.2. Regulation of the cGAS-STING Pathway
3. The Cytosolic RNA Sensors
3.1. RLRs
3.2. Other RNA Sensors
4. GBPs: Cytosolic Sensors for LPS
4.1. Overview of IFN-Induced GBPs
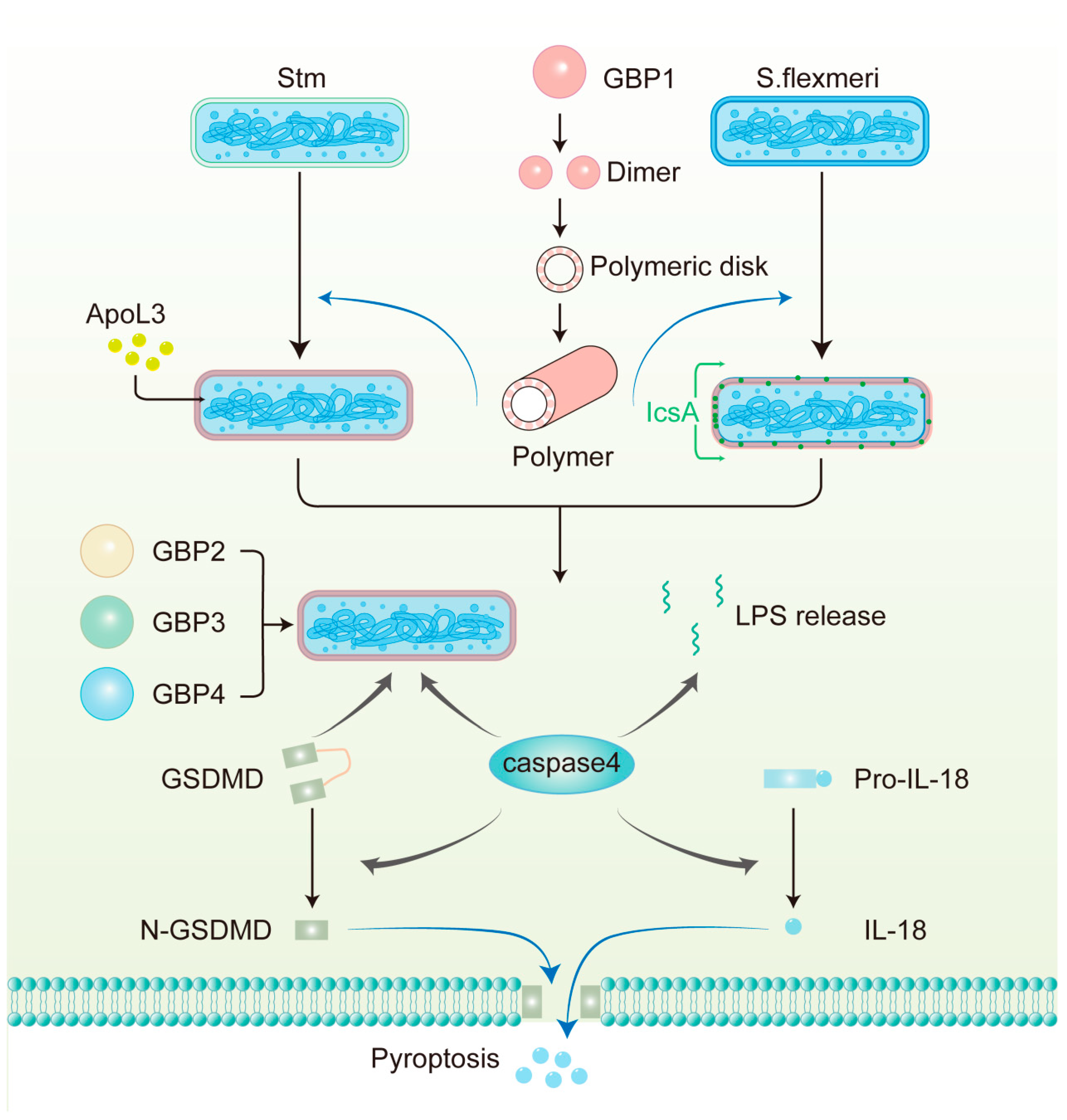
4.2. LPS Recognition and Assembly of GBP1 Defense Complex
4.3. GBPs Recognize Pathogens Residing Within Vacuoles
4.4. Host Defense Responses Downstream of GBPs Activation
5. Recognition of Other Pathogen Components
6. Cell-Autonomous Immunity: Implications for Diseases
6.1. Autoinflammatory and Autoimmune Diseases
6.2. Cancers
6.3. Clinical Translation and Challenges
7. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Jenner, E. History of the Inoculation of the Cow-Pox: Further Observations on the Variolæ Vaccinæ, or Cow-Pox. Med. Phys. J. 1799, 1, 313–318. [Google Scholar] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Gaudet, R.G.; Bradfield, C.J.; MacMicking, J.D. Evolution of Cell-Autonomous Effector Mechanisms in Macrophages versus Non-Immune Cells. Microbiol. Spectr. 2016, 4, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Randow, F.; MacMicking, J.D.; James, L.C. Cellular self-defense: How cell-autonomous immunity protects against pathogens. Science 2013, 340, 701–706. [Google Scholar] [CrossRef]
- Doron, S.; Melamed, S.; Ofir, G.; Leavitt, A.; Lopatina, A.; Keren, M.; Amitai, G.; Sorek, R. Systematic discovery of antiphage defense systems in the microbial pangenome. Science 2018, 359, eaar4120. [Google Scholar] [CrossRef] [PubMed]
- Martineau, C.N.; Kirienko, N.V.; Pujol, N. Innate immunity in C. elegans. Curr. Top. Dev. Biol. 2021, 144, 309–351. [Google Scholar] [CrossRef]
- Yano, T.; Mita, S.; Ohmori, H.; Oshima, Y.; Fujimoto, Y.; Ueda, R.; Takada, H.; Goldman, W.E.; Fukase, K.; Silverman, N.; et al. Autophagic control of listeria through intracellular innate immune recognition in drosophila. Nat. Immunol. 2008, 9, 908–916. [Google Scholar] [CrossRef]
- McGettigan, J.; McLennan, R.K.; Broderick, K.E.; Kean, L.; Allan, A.K.; Cabrero, P.; Regulski, M.R.; Pollock, V.P.; Gould, G.W.; Davies, S.A.; et al. Insect renal tubules constitute a cell-autonomous immune system that protects the organism against bacterial infection. Insect Biochem. Mol. Biol. 2005, 35, 741–754. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, A.; Wang, Z.; Choi, M.K.; Yanai, H.; Negishi, H.; Ban, T.; Lu, Y.; Miyagishi, M.; Kodama, T.; Honda, K.; et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 2007, 448, 501–505. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, Q.; Florence, J.M.; Jung, B.G.; Kurdowska, A.K.; Samten, B.; Idell, S.; Tang, H. Influenza Virus Infection Induces ZBP1 Expression and Necroptosis in Mouse Lungs. Front. Cell Infect. Microbiol. 2019, 9, 286. [Google Scholar] [CrossRef]
- Ablasser, A.; Bauernfeind, F.; Hartmann, G.; Latz, E.; Fitzgerald, K.A.; Hornung, V. RIG-I-dependent sensing of poly(dA:dT) through the induction of an RNA polymerase III-transcribed RNA intermediate. Nat. Immunol. 2009, 10, 1065–1072. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef]
- Gray, E.E.; Winship, D.; Snyder, J.M.; Child, S.J.; Geballe, A.P.; Stetson, D.B. The AIM2-like Receptors Are Dispensable for the Interferon Response to Intracellular DNA. Immunity 2016, 45, 255–266. [Google Scholar] [CrossRef]
- Li, G.; Zhao, X.; Zheng, Z.; Zhang, H.; Wu, Y.; Shen, Y.; Chen, Q. cGAS-STING pathway mediates activation of dendritic cell sensing of immunogenic tumors. Cell Mol. Life Sci. 2024, 81, 149. [Google Scholar] [CrossRef]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 2013, 339, 786–791. [Google Scholar] [CrossRef]
- Neufeldt, C.J.; Cerikan, B.; Cortese, M.; Frankish, J.; Lee, J.Y.; Plociennikowska, A.; Heigwer, F.; Prasad, V.; Joecks, S.; Burkart, S.S.; et al. SARS-CoV-2 infection induces a pro-inflammatory cytokine response through cGAS-STING and NF-κB. Commun. Biol. 2022, 5, 45. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, B.; Bao, M.; Lu, N.; Kim, T.; Liu, Y.J. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat. Immunol. 2011, 12, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Parvatiyar, K.; Zhang, Z.; Teles, R.M.; Ouyang, S.; Jiang, Y.; Iyer, S.S.; Zaver, S.A.; Schenk, M.; Zeng, S.; Zhong, W.; et al. The helicase DDX41 recognizes the bacterial secondary messengers cyclic di-GMP and cyclic di-AMP to activate a type I interferon immune response. Nat. Immunol. 2012, 13, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Unterholzner, L.; Keating, S.E.; Baran, M.; Horan, K.A.; Jensen, S.B.; Sharma, S.; Sirois, C.M.; Jin, T.; Latz, E.; Xiao, T.S.; et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010, 11, 997–1004. [Google Scholar] [CrossRef]
- Kerur, N.; Veettil, M.V.; Sharma-Walia, N.; Bottero, V.; Sadagopan, S.; Otageri, P.; Chandran, B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 2011, 9, 363–375. [Google Scholar] [CrossRef]
- Xin, H.; Curry, J.; Johnstone, R.W.; Nickoloff, B.J.; Choubey, D. Role of IFI 16, a member of the interferon-inducible p200-protein family, in prostate epithelial cellular senescence. Oncogene 2003, 22, 4831–4840. [Google Scholar] [CrossRef]
- Corrales, L.; Woo, S.R.; Williams, J.B.; McWhirter, S.M.; Dubensky, T.W., Jr.; Gajewski, T.F. Antagonism of the STING Pathway via Activation of the AIM2 Inflammasome by Intracellular DNA. J. Immunol. 2016, 196, 3191–3198. [Google Scholar] [CrossRef]
- Ekchariyawat, P.; Hamel, R.; Bernard, E.; Wichit, S.; Surasombatpattana, P.; Talignani, L.; Thomas, F.; Choumet, V.; Yssel, H.; Desprès, P.; et al. Inflammasome signaling pathways exert antiviral effect against Chikungunya virus in human dermal fibroblasts. Infect. Genet. Evol. 2015, 32, 401–408. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Svolaki, I.P.; Evangelou, K.; Gorgoulis, V.G.; Manoussakis, M.N. Cell-autonomous epithelial activation of AIM2 (absent in melanoma-2) inflammasome by cytoplasmic DNA accumulations in primary Sjögren’s syndrome. J. Autoimmun. 2020, 108, 102381. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.H.; Macmillan, J.B.; Chen, Z.J. RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 2009, 138, 576–591. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kawabata, K.; Kouyama, E.; Ishii, K.J.; Katayama, K.; Suzuki, T.; Kurachi, S.; Sakurai, F.; Akira, S.; Mizuguchi, H. Induction of type I interferon by adenovirus-encoded small RNAs. Proc. Natl. Acad. Sci. USA 2010, 107, 17286–17291. [Google Scholar] [CrossRef]
- Malireddi, R.K.S.; Kesavardhana, S.; Kanneganti, T.D. ZBP1 and TAK1: Master Regulators of NLRP3 Inflammasome/Pyroptosis, Apoptosis, and Necroptosis (PAN-optosis). Front. Cell Infect. Microbiol. 2019, 9, 406. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Uematsu, S.; Matsui, K.; Tsujimura, T.; Takeda, K.; Fujita, T.; Takeuchi, O.; et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity 2005, 23, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Hornung, V.; Ellegast, J.; Kim, S.; Brzózka, K.; Jung, A.; Kato, H.; Poeck, H.; Akira, S.; Conzelmann, K.K.; Schlee, M.; et al. 5’-Triphosphate RNA is the ligand for RIG-I. Science 2006, 314, 994–997. [Google Scholar] [CrossRef]
- Wang, F.; Gao, X.; Barrett, J.W.; Shao, Q.; Bartee, E.; Mohamed, M.R.; Rahman, M.; Werden, S.; Irvine, T.; Cao, J.; et al. RIG-I mediates the co-induction of tumor necrosis factor and type I interferon elicited by myxoma virus in primary human macrophages. PLoS Pathog. 2008, 4, e1000099. [Google Scholar] [CrossRef]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef]
- Heutinck, K.M.; Rowshani, A.T.; Kassies, J.; Claessen, N.; van Donselaar-van der Pant, K.A.; Bemelman, F.J.; Eldering, E.; van Lier, R.A.; Florquin, S.; Ten Berge, I.J.; et al. Viral double-stranded RNA sensors induce antiviral, pro-inflammatory, and pro-apoptotic responses in human renal tubular epithelial cells. Kidney Int. 2012, 82, 664–675. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kato, H.; Fujita, T. Physiological functions of RIG-I-like receptors. Immunity 2024, 57, 731–751. [Google Scholar] [CrossRef]
- Harioudh, M.K.; Perez, J.; So, L.; Maheshwari, M.; Ebert, T.S.; Hornung, V.; Savan, R.; Rouf Banday, A.; Diamond, M.S.; Rathinam, V.A.; et al. The canonical antiviral protein oligoadenylate synthetase 1 elicits antibacterial functions by enhancing IRF1 translation. Immunity 2024, 57, 1812–1827.e1817. [Google Scholar] [CrossRef]
- Wickenhagen, A.; Sugrue, E.; Lytras, S.; Kuchi, S.; Noerenberg, M.; Turnbull, M.L.; Loney, C.; Herder, V.; Allan, J.; Jarmson, I.; et al. A prenylated dsRNA sensor protects against severe COVID-19. Science 2021, 374, eabj3624. [Google Scholar] [CrossRef]
- Magg, T.; Okano, T.; Koenig, L.M.; Boehmer, D.F.R.; Schwartz, S.L.; Inoue, K.; Heimall, J.; Licciardi, F.; Ley-Zaporozhan, J.; Ferdman, R.M.; et al. Heterozygous OAS1 gain-of-function variants cause an autoinflammatory immunodeficiency. Sci. Immunol. 2021, 6, eabf9564. [Google Scholar] [CrossRef] [PubMed]
- Magusali, N.; Graham, A.C.; Piers, T.M.; Panichnantakul, P.; Yaman, U.; Shoai, M.; Reynolds, R.H.; Botia, J.A.; Brookes, K.J.; Guetta-Baranes, T.; et al. A genetic link between risk for Alzheimer’s disease and severe COVID-19 outcomes via the OAS1 gene. Brain 2021, 144, 3727–3741. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Reuter, C.M.; Ruzhnikov, M.R.Z.; Beck, A.E.; Farrow, E.G.; Emrick, L.T.; Rosenfeld, J.A.; Mackenzie, K.M.; Robak, L.; Wheeler, M.T.; et al. De novo EIF2AK1 and EIF2AK2 Variants Are Associated with Developmental Delay, Leukoencephalopathy, and Neurologic Decompensation. Am. J. Hum. Genet. 2020, 106, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Gal-Ben-Ari, S.; Barrera, I.; Ehrlich, M.; Rosenblum, K. PKR: A Kinase to Remember. Front. Mol. Neurosci. 2018, 11, 480. [Google Scholar] [CrossRef]
- Sabbah, A.; Chang, T.H.; Harnack, R.; Frohlich, V.; Tominaga, K.; Dube, P.H.; Xiang, Y.; Bose, S. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009, 10, 1073–1080. [Google Scholar] [CrossRef]
- Bauernfried, S.; Scherr, M.J.; Pichlmair, A.; Duderstadt, K.E.; Hornung, V. Human NLRP1 is a sensor for double-stranded RNA. Science 2021, 371, eabd0811. [Google Scholar] [CrossRef]
- Laanesoo, A.; Mäe, M.; Remm, A.; Johnston, S.L.; Altraja, A.; Bochenek, G.; Jakiela, B.; Rebane, A. NLRP1 Is a Prominent Inflammasome Sensor Found in Bronchial Epithelial Cells in Asthma and Can Be Activated by Rhinovirus A16. Clin. Exp. Allergy 2025, 55, 239–246. [Google Scholar] [CrossRef]
- Shen, C.; Li, R.; Negro, R.; Cheng, J.; Vora, S.M.; Fu, T.M.; Wang, A.; He, K.; Andreeva, L.; Gao, P.; et al. Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell 2021, 184, 5759–5774.e5720. [Google Scholar] [CrossRef]
- Liu, T.; Liu, S.; Rui, X.; Cao, Y.; Hecker, J.; Guo, F.; Zhang, Y.; Gong, L.; Zhou, Y.; Yu, Y.; et al. Gasdermin B, an asthma-susceptibility gene, promotes MAVS-TBK1 signalling and airway inflammation. Eur. Respir. J. 2024, 63, 2301232. [Google Scholar] [CrossRef]
- Li, X.D.; Wu, J.; Gao, D.; Wang, H.; Sun, L.; Chen, Z.J. Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 2013, 341, 1390–1394. [Google Scholar] [CrossRef]
- Civril, F.; Deimling, T.; de Oliveira Mann, C.C.; Ablasser, A.; Moldt, M.; Witte, G.; Hornung, V.; Hopfner, K.P. Structural mechanism of cytosolic DNA sensing by cGAS. Nature 2013, 498, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Manzanillo, P.S.; Shiloh, M.U.; Portnoy, D.A.; Cox, J.S. Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 2012, 11, 469–480. [Google Scholar] [CrossRef]
- Wassermann, R.; Gulen, M.F.; Sala, C.; Perin, S.G.; Lou, Y.; Rybniker, J.; Schmid-Burgk, J.L.; Schmidt, T.; Hornung, V.; Cole, S.T.; et al. Mycobacterium tuberculosis Differentially Activates cGAS- and Inflammasome-Dependent Intracellular Immune Responses through ESX-1. Cell Host Microbe 2015, 17, 799–810. [Google Scholar] [CrossRef]
- Hansen, K.; Prabakaran, T.; Laustsen, A.; Jørgensen, S.E.; Rahbæk, S.H.; Jensen, S.B.; Nielsen, R.; Leber, J.H.; Decker, T.; Horan, K.A.; et al. Listeria monocytogenes induces IFNβ expression through an IFI16-, cGAS- and STING-dependent pathway. EMBO J. 2014, 33, 1654–1666. [Google Scholar] [CrossRef]
- Hahn, W.O.; Butler, N.S.; Lindner, S.E.; Akilesh, H.M.; Sather, D.N.; Kappe, S.H.; Hamerman, J.A.; Gale, M., Jr.; Liles, W.C.; Pepper, M. cGAS-mediated control of blood-stage malaria promotes Plasmodium-specific germinal center responses. JCI Insight 2018, 3, e94142. [Google Scholar] [CrossRef]
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Cao, Y.; Dang, Y.; Ge, B. The multifaceted functions of cGAS. J. Mol. Cell Biol. 2022, 14, mjac031. [Google Scholar] [CrossRef]
- Sun, B.; Sundström, K.B.; Chew, J.J.; Bist, P.; Gan, E.S.; Tan, H.C.; Goh, K.C.; Chawla, T.; Tang, C.K.; Ooi, E.E. Dengue virus activates cGAS through the release of mitochondrial DNA. Sci. Rep. 2017, 7, 3594. [Google Scholar] [CrossRef]
- Domizio, J.D.; Gulen, M.F.; Saidoune, F.; Thacker, V.V.; Yatim, A.; Sharma, K.; Nass, T.; Guenova, E.; Schaller, M.; Conrad, C.; et al. The cGAS-STING pathway drives type I IFN immunopathology in COVID-19. Nature 2022, 603, 145–151. [Google Scholar] [CrossRef]
- Mackenzie, K.J.; Carroll, P.; Martin, C.A.; Murina, O.; Fluteau, A.; Simpson, D.J.; Olova, N.; Sutcliffe, H.; Rainger, J.K.; Leitch, A.; et al. cGAS surveillance of micronuclei links genome instability to innate immunity. Nature 2017, 548, 461–465. [Google Scholar] [CrossRef]
- Gao, P.; Ascano, M.; Wu, Y.; Barchet, W.; Gaffney, B.L.; Zillinger, T.; Serganov, A.A.; Liu, Y.; Jones, R.A.; Hartmann, G.; et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell 2013, 153, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef] [PubMed]
- Burdette, D.L.; Monroe, K.M.; Sotelo-Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011, 478, 515–518. [Google Scholar] [CrossRef]
- Lio, C.W.; McDonald, B.; Takahashi, M.; Dhanwani, R.; Sharma, N.; Huang, J.; Pham, E.; Benedict, C.A.; Sharma, S. cGAS-STING Signaling Regulates Initial Innate Control of Cytomegalovirus Infection. J. Virol. 2016, 90, 7789–7797. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Yang, H.; Li, T.; Tan, X.; Shi, P.; Li, M.; Du, F.; Chen, Z.J. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature 2019, 567, 262–266. [Google Scholar] [CrossRef]
- Watson, R.O.; Manzanillo, P.S.; Cox, J.S.; Extracellular, M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 2012, 150, 803–815. [Google Scholar] [CrossRef]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Ramshorn, K.; Pinci, F.; Zuber, S.; O’Duill, F.; Schmid-Burgk, J.L.; Hoss, F.; Buhmann, R.; et al. The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell 2017, 171, 1110–1124.e1118. [Google Scholar] [CrossRef]
- Seo, G.J.; Kim, C.; Shin, W.J.; Sklan, E.H.; Eoh, H.; Jung, J.U. TRIM56-mediated monoubiquitination of cGAS for cytosolic DNA sensing. Nat. Commun. 2018, 9, 613. [Google Scholar] [CrossRef]
- Tsuchida, T.; Zou, J.; Saitoh, T.; Kumar, H.; Abe, T.; Matsuura, Y.; Kawai, T.; Akira, S. The ubiquitin ligase TRIM56 regulates innate immune responses to intracellular double-stranded DNA. Immunity 2010, 33, 765–776. [Google Scholar] [CrossRef]
- Liang, Q.; Seo, G.J.; Choi, Y.J.; Kwak, M.J.; Ge, J.; Rodgers, M.A.; Shi, M.; Leslie, B.J.; Hopfner, K.P.; Ha, T.; et al. Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe 2014, 15, 228–238. [Google Scholar] [CrossRef]
- Field, A.K.; Tytell, A.A.; Lampson, G.P.; Hilleman, M.R. Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc. Natl. Acad. Sci. USA 1967, 58, 1004–1010. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Tanner, N.K.; Linder, P. DExD/H box RNA helicases: From generic motors to specific dissociation functions. Mol. Cell 2001, 8, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Chow, K.T.; Gale, M., Jr.; Loo, Y.M. RIG-I and Other RNA Sensors in Antiviral Immunity. Annu. Rev. Immunol. 2018, 36, 667–694. [Google Scholar] [CrossRef]
- Deddouche, S.; Matt, N.; Budd, A.; Mueller, S.; Kemp, C.; Galiana-Arnoux, D.; Dostert, C.; Antoniewski, C.; Hoffmann, J.A.; Imler, J.L. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in drosophila. Nat. Immunol. 2008, 9, 1425–1432. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kikuchi, M.; Matsumoto, K.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Foy, E.; Loo, Y.M.; Gale, M., Jr.; Akira, S.; et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005, 175, 2851–2858. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Gack, M.U. RIG-I-like receptors: Their regulation and roles in RNA sensing. Nat. Rev. Immunol. 2020, 20, 537–551. [Google Scholar] [CrossRef]
- Seth, R.B.; Sun, L.; Ea, C.K.; Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef]
- Zevini, A.; Olagnier, D.; Hiscott, J. Crosstalk between Cytoplasmic RIG-I and STING Sensing Pathways. Trends Immunol. 2017, 38, 194–205. [Google Scholar] [CrossRef]
- Ye, S.; Liang, Y.; Chang, Y.; Lai, B.; Zhong, J. Dengue Virus Replicative-Form dsRNA Is Recognized by Both RIG-I and MDA5 to Activate Innate Immunity. J. Med. Virol. 2025, 97, e70194. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, M.; Koshiba, T.; Ichinohe, T. Influenza A virus M2 protein triggers mitochondrial DNA-mediated antiviral immune responses. Nat. Commun. 2019, 10, 4624. [Google Scholar] [CrossRef] [PubMed]
- Piersma, S.J.; Poursine-Laurent, J.; Yang, L.; Barber, G.N.; Parikh, B.A.; Yokoyama, W.M. Virus infection is controlled by hematopoietic and stromal cell sensing of murine cytomegalovirus through STING. Elife 2020, 9, e56882. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, H.; Gad, H.H.; Eskildsen-Larsen, S.; Despres, P.; Hartmann, R. The oligoadenylate synthetase family: An ancient protein family with multiple antiviral activities. J. Interferon Cytokine Res. 2011, 31, 41–47. [Google Scholar] [CrossRef]
- Yang, T.; Wang, G.; Zhang, M.; Hu, X.; Li, Q.; Yun, F.; Xing, Y.; Song, X.; Zhang, H.; Hu, G.; et al. Triggering endogenous Z-RNA sensing for anti-tumor therapy through ZBP1-dependent necroptosis. Cell Rep. 2023, 42, 113377. [Google Scholar] [CrossRef]
- DeAntoneo, C.; Herbert, A.; Balachandran, S. Z-form nucleic acid-binding protein 1 (ZBP1) as a sensor of viral and cellular Z-RNAs: Walking the razor’s edge. Curr. Opin. Immunol. 2023, 83, 102347. [Google Scholar] [CrossRef]
- Rice, G.I.; Kasher, P.R.; Forte, G.M.; Mannion, N.M.; Greenwood, S.M.; Szynkiewicz, M.; Dickerson, J.E.; Bhaskar, S.S.; Zampini, M.; Briggs, T.A.; et al. Mutations in ADAR1 cause Aicardi-Goutières syndrome associated with a type I interferon signature. Nat. Genet. 2012, 44, 1243–1248. [Google Scholar] [CrossRef]
- Zhang, T.; Yin, C.; Fedorov, A.; Qiao, L.; Bao, H.; Beknazarov, N.; Wang, S.; Gautam, A.; Williams, R.M.; Crawford, J.C.; et al. ADAR1 masks the cancer immunotherapeutic promise of ZBP1-driven necroptosis. Nature 2022, 606, 594–602. [Google Scholar] [CrossRef]
- Kirkby, M.; Enosi Tuipulotu, D.; Feng, S.; Lo Pilato, J.; Man, S.M. Guanylate-binding proteins: Mechanisms of pattern recognition and antimicrobial functions. Trends Biochem. Sci. 2023, 48, 883–893. [Google Scholar] [CrossRef]
- Wandel, M.P.; Kim, B.H.; Park, E.S.; Boyle, K.B.; Nayak, K.; Lagrange, B.; Herod, A.; Henry, T.; Zilbauer, M.; Rohde, J.; et al. Guanylate-binding proteins convert cytosolic bacteria into caspase-4 signaling platforms. Nat. Immunol. 2020, 21, 880–891. [Google Scholar] [CrossRef]
- Santos, J.C.; Boucher, D.; Schneider, L.K.; Demarco, B.; Dilucca, M.; Shkarina, K.; Heilig, R.; Chen, K.W.; Lim, R.Y.H.; Broz, P. Human GBP1 binds LPS to initiate assembly of a caspase-4 activating platform on cytosolic bacteria. Nat. Commun. 2020, 11, 3276. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Cuevas, Y.; Clough, B.; Frickel, E.M. Human guanylate-binding proteins in intracellular pathogen detection, destruction, and host cell death induction. Curr. Opin. Immunol. 2023, 84, 102373. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Meng, Q.; Maminska, A.; MacMicking, J.D. Cell-autonomous immunity by IFN-induced GBPs in animals and plants. Curr. Opin. Immunol. 2019, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Zamyatina, A.; Heine, H. Lipopolysaccharide Recognition in the Crossroads of TLR4 and Caspase-4/11 Mediated Inflammatory Pathways. Front. Immunol. 2020, 11, 585146. [Google Scholar] [CrossRef]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Pilla, D.M.; Hagar, J.A.; Haldar, A.K.; Mason, A.K.; Degrandi, D.; Pfeffer, K.; Ernst, R.K.; Yamamoto, M.; Miao, E.A.; Coers, J. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl. Acad. Sci. USA 2014, 111, 6046–6051. [Google Scholar] [CrossRef]
- Santos, J.C.; Dick, M.S.; Lagrange, B.; Degrandi, D.; Pfeffer, K.; Yamamoto, M.; Meunier, E.; Pelczar, P.; Henry, T.; Broz, P. LPS targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J. 2018, 37, e98089. [Google Scholar] [CrossRef]
- Cui, W.; Braun, E.; Wang, W.; Tang, J.; Zheng, Y.; Slater, B.; Li, N.; Chen, C.; Liu, Q.; Wang, B.; et al. Structural basis for GTP-induced dimerization and antiviral function of guanylate-binding proteins. Proc. Natl. Acad. Sci. USA 2021, 118, e2022269118. [Google Scholar] [CrossRef]
- Weismehl, M.; Chu, X.; Kutsch, M.; Lauterjung, P.; Herrmann, C.; Kudryashev, M.; Daumke, O. Structural insights into the activation mechanism of antimicrobial GBP1. EMBO J. 2024, 43, 615–636. [Google Scholar] [CrossRef]
- Kohler, K.M.; Kutsch, M.; Piro, A.S.; Wallace, G.D.; Coers, J.; Barber, M.F. A Rapidly Evolving Polybasic Motif Modulates Bacterial Detection by Guanylate Binding Proteins. mBio 2020, 11, e00340-20. [Google Scholar] [CrossRef]
- Kutsch, M.; Sistemich, L.; Lesser, C.F.; Goldberg, M.B.; Herrmann, C.; Coers, J. Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J. 2020, 39, e104926. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Bradfield, C.J.; Maminska, A.; Park, E.S.; Kim, B.H.; Kumar, P.; Huang, S.; Kim, M.; Zhang, Y.; Bewersdorf, J.; et al. Native architecture of a human GBP1 defense complex for cell-autonomous immunity to infection. Science 2024, 383, eabm9903. [Google Scholar] [CrossRef] [PubMed]
- Goers, L.; Kim, K.; Stedman, T.C.; Canning, P.J.; Mou, X.; Ernst, N.H.; Coers, J.; Lesser, C.F. Shigella IpaH9.8 limits GBP1-dependent LPS release from intracytosolic bacteria to suppress caspase-4 activation. Proc. Natl. Acad. Sci. USA 2023, 120, e2218469120. [Google Scholar] [CrossRef]
- Sasai, M.; Yamamoto, M. Innate, adaptive, and cell-autonomous immunity against Toxoplasma gondii infection. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- Haldar, A.K.; Foltz, C.; Finethy, R.; Piro, A.S.; Feeley, E.M.; Pilla-Moffett, D.M.; Komatsu, M.; Frickel, E.M.; Coers, J. Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc. Natl. Acad. Sci. USA 2015, 112, E5628–E5637. [Google Scholar] [CrossRef]
- Selleck, E.M.; Orchard, R.C.; Lassen, K.G.; Beatty, W.L.; Xavier, R.J.; Levine, B.; Virgin, H.W.; Sibley, L.D. A Noncanonical Autophagy Pathway Restricts Toxoplasma gondii Growth in a Strain-Specific Manner in IFN-γ-Activated Human Cells. mBio 2015, 6, e01157-15. [Google Scholar] [CrossRef]
- Rudolph, M.; Carsten, A.; Kulnik, S.; Aepfelbacher, M.; Wolters, M. Live imaging of Yersinia translocon formation and immune recognition in host cells. PLoS Pathog. 2022, 18, e1010251. [Google Scholar] [CrossRef]
- Li, L.; Dickinson, M.S.; Coers, J.; Miao, E.A. Pyroptosis in defense against intracellular bacteria. Semin. Immunol. 2023, 69, 101805. [Google Scholar] [CrossRef]
- Feng, S.; Man, S.M. Captain GBP1: Inflammasomes assemble, pyroptotic endgame. Nat. Immunol. 2020, 21, 829–830. [Google Scholar] [CrossRef]
- Shi, X.; Sun, Q.; Hou, Y.; Zeng, H.; Cao, Y.; Dong, M.; Ding, J.; Shao, F. Recognition and maturation of IL-18 by caspase-4 noncanonical inflammasome. Nature 2023, 624, 442–450. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Ruan, J.; Pan, Y.; Magupalli, V.G.; Wu, H.; Lieberman, J. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 2016, 535, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Xavier, A.; Al-Zeer, M.A.; Meyer, T.F.; Daumke, O. hGBP1 Coordinates Chlamydia Restriction and Inflammasome Activation through Sequential GTP Hydrolysis. Cell Rep. 2020, 31, 107667. [Google Scholar] [CrossRef] [PubMed]
- Meunier, E.; Wallet, P.; Dreier, R.F.; Costanzo, S.; Anton, L.; Rühl, S.; Dussurgey, S.; Dick, M.S.; Kistner, A.; Rigard, M.; et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 2015, 16, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Fisch, D.; Clough, B.; Domart, M.C.; Encheva, V.; Bando, H.; Snijders, A.P.; Collinson, L.M.; Yamamoto, M.; Shenoy, A.R.; Frickel, E.M. Human GBP1 Differentially Targets Salmonella and Toxoplasma to License Recognition of Microbial Ligands and Caspase-Mediated Death. Cell Rep. 2020, 32, 108008. [Google Scholar] [CrossRef]
- Wandel, M.P.; Pathe, C.; Werner, E.I.; Ellison, C.J.; Boyle, K.B.; von der Malsburg, A.; Rohde, J.; Randow, F. GBPs Inhibit Motility of Shigella flexneri but Are Targeted for Degradation by the Bacterial Ubiquitin Ligase IpaH9.8. Cell Host Microbe 2017, 22, 507–518.e5. [Google Scholar] [CrossRef]
- Gaudet, R.G.; Zhu, S.; Halder, A.; Kim, B.H.; Bradfield, C.J.; Huang, S.; Xu, D.; Mamiñska, A.; Nguyen, T.N.; Lazarou, M.; et al. A human apolipoprotein L with detergent-like activity kills intracellular pathogens. Science 2021, 373, eabf8113. [Google Scholar] [CrossRef]
- Constant, D.A.; Nice, T.J.; Rauch, I. Innate immune sensing by epithelial barriers. Curr. Opin. Immunol. 2021, 73, 1–8. [Google Scholar] [CrossRef]
- Sundaram, B.; Tweedell, R.E.; Prasanth Kumar, S.; Kanneganti, T.D. The NLR family of innate immune and cell death sensors. Immunity 2024, 57, 674–699. [Google Scholar] [CrossRef]
- Uehara, A.; Fujimoto, Y.; Fukase, K.; Takada, H. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol. Immunol. 2007, 44, 3100–3111. [Google Scholar] [CrossRef]
- Ernst, C.; Andreassen, P.R.; Giger, G.H.; Nguyen, B.D.; Gäbelein, C.G.; Guillaume-Gentil, O.; Fattinger, S.A.; Sellin, M.E.; Hardt, W.D.; Vorholt, J.A. Direct Salmonella injection into enteroid cells allows the study of host-pathogen interactions in the cytosol with high spatiotemporal resolution. PLoS Biol. 2024, 22, e3002597. [Google Scholar] [CrossRef]
- Gao, D.; Li, T.; Li, X.D.; Chen, X.; Li, Q.Z.; Wight-Carter, M.; Chen, Z.J. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl. Acad. Sci. USA 2015, 112, E5699–E5705. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jesus, A.A.; Marrero, B.; Yang, D.; Ramsey, S.E.; Sanchez, G.A.M.; Tenbrock, K.; Wittkowski, H.; Jones, O.Y.; Kuehn, H.S.; et al. Activated STING in a vascular and pulmonary syndrome. N. Engl. J. Med. 2014, 371, 507–518. [Google Scholar] [CrossRef]
- Jeremiah, N.; Neven, B.; Gentili, M.; Callebaut, I.; Maschalidi, S.; Stolzenberg, M.C.; Goudin, N.; Frémond, M.L.; Nitschke, P.; Molina, T.J.; et al. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J. Clin. Investig. 2014, 124, 5516–5520. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Durcan, L.; Karr, R.M.; Briggs, T.A.; Rice, G.I.; Teal, T.H.; Woodward, J.J.; Elkon, K.B. Expression of Cyclic GMP-AMP Synthase in Patients with Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017, 69, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Singh, A.K.; Ouseph, M.M.; Ahmed, S. Regulation of Synovial Inflammation and Tissue Destruction by Guanylate Binding Protein 5 in Synovial Fibroblasts From Patients with Rheumatoid Arthritis and Rats with Adjuvant-Induced Arthritis. Arthritis Rheumatol. 2021, 73, 943–954. [Google Scholar] [CrossRef]
- Wang, J.; Li, R.; Lin, H.; Qiu, Q.; Lao, M.; Zeng, S.; Wang, C.; Xu, S.; Zou, Y.; Shi, M.; et al. Accumulation of cytosolic dsDNA contributes to fibroblast-like synoviocytes-mediated rheumatoid arthritis synovial inflammation. Int. Immunopharmacol. 2019, 76, 105791. [Google Scholar] [CrossRef]
- Rutsch, F.; MacDougall, M.; Lu, C.; Buers, I.; Mamaeva, O.; Nitschke, Y.; Rice, G.I.; Erlandsen, H.; Kehl, H.G.; Thiele, H.; et al. A specific IFIH1 gain-of-function mutation causes Singleton-Merten syndrome. Am. J. Hum. Genet. 2015, 96, 275–282. [Google Scholar] [CrossRef]
- Dann, A.; Poeck, H.; Croxford, A.L.; Gaupp, S.; Kierdorf, K.; Knust, M.; Pfeifer, D.; Maihoefer, C.; Endres, S.; Kalinke, U.; et al. Cytosolic RIG-I-like helicases act as negative regulators of sterile inflammation in the CNS. Nat. Neurosci. 2011, 15, 98–106. [Google Scholar] [CrossRef]
- Hundeshagen, A.; Hecker, M.; Paap, B.K.; Angerstein, C.; Kandulski, O.; Fatum, C.; Hartmann, C.; Koczan, D.; Thiesen, H.J.; Zettl, U.K. Elevated type I interferon-like activity in a subset of multiple sclerosis patients: Molecular basis and clinical relevance. J. Neuroinflammation 2012, 9, 140. [Google Scholar] [CrossRef]
- Zurawek, M.; Fichna, M.; Fichna, P.; Skowronska, B.; Dzikiewicz-Krawczyk, A.; Januszkiewicz, D.; Nowak, J. Cumulative effect of IFIH1 variants and increased gene expression associated with type 1 diabetes. Diabetes Res. Clin. Pract. 2015, 107, 259–266. [Google Scholar] [CrossRef]
- Ferreira, R.C.; Guo, H.; Coulson, R.M.; Smyth, D.J.; Pekalski, M.L.; Burren, O.S.; Cutler, A.J.; Doecke, J.D.; Flint, S.; McKinney, E.F.; et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes 2014, 63, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xue, X.; Tang, W.; Su, L.; Zhang, L.; Zhang, Y. Cytosolic DNA–Mediated STING-Dependent Inflammation Contributes to the Progression of Psoriasis. J. Investig. Dermatol. 2022, 142, 898–906.e894. [Google Scholar] [CrossRef] [PubMed]
- König, N.; Fiehn, C.; Wolf, C.; Schuster, M.; Cura Costa, E.; Tüngler, V.; Alvarez, H.A.; Chara, O.; Engel, K.; Goldbach-Mansky, R.; et al. Familial chilblain lupus due to a gain-of-function mutation in STING. Ann. Rheum. Dis. 2017, 76, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Cananzi, M.; Wohler, E.; Marzollo, A.; Colavito, D.; You, J.; Jing, H.; Bresolin, S.; Gaio, P.; Martin, R.; Mescoli, C.; et al. IFIH1 loss-of-function variants contribute to very early-onset inflammatory bowel disease. Hum. Genet. 2021, 140, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Schnoor, M.; Betanzos, A.; Weber, D.A.; Parkos, C.A. Guanylate-binding protein-1 is expressed at tight junctions of intestinal epithelial cells in response to interferon-gamma and regulates barrier function through effects on apoptosis. Mucosal Immunol. 2009, 2, 33–42. [Google Scholar] [CrossRef]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Aspects Med. 2020, 76, 100924. [Google Scholar] [CrossRef]
- Petrasek, J.; Iracheta-Vellve, A.; Csak, T.; Satishchandran, A.; Kodys, K.; Kurt-Jones, E.A.; Fitzgerald, K.A.; Szabo, G. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl. Acad. Sci. USA 2013, 110, 16544–16549. [Google Scholar] [CrossRef]
- Chen, R.; Du, J.; Zhu, H.; Ling, Q. The role of cGAS-STING signalling in liver diseases. JHEP Rep. 2021, 3, 100324. [Google Scholar] [CrossRef]
- Luo, X.; Li, H.; Ma, L.; Zhou, J.; Guo, X.; Woo, S.L.; Pei, Y.; Knight, L.R.; Deveau, M.; Chen, Y.; et al. Expression of STING Is Increased in Liver Tissues From Patients with NAFLD and Promotes Macrophage-Mediated Hepatic Inflammation and Fibrosis in Mice. Gastroenterology 2018, 155, 1971–1984.e1974. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Zeng, Z. Advances in cGAS-STING Signaling Pathway and Diseases. Front. Cell Dev. Biol. 2022, 10, 800393. [Google Scholar] [CrossRef]
- Kim, B.H.; Shenoy, A.R.; Kumar, P.; Das, R.; Tiwari, S.; MacMicking, J.D. A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science 2011, 332, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.C.; Cai, H.; Li, T.; Franco, L.H.; Li, X.D.; Nair, V.R.; Scharn, C.R.; Stamm, C.E.; Levine, B.; Chen, Z.J.; et al. Cyclic GMP-AMP Synthase Is an Innate Immune DNA Sensor for Mycobacterium tuberculosis. Cell Host Microbe 2015, 17, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Mesner, D.; Reuschl, A.K.; Whelan, M.V.X.; Bronzovich, T.; Haider, T.; Thorne, L.G.; Ragazzini, R.; Bonfanti, P.; Towers, G.J.; Jolly, C. SARS-CoV-2 evolution influences GBP and IFITM sensitivity. Proc. Natl. Acad. Sci. USA 2023, 120, e2212577120. [Google Scholar] [CrossRef]
- Lee, S.; Zhang, Y.; Newhams, M.; Novak, T.; Thomas, P.G.; Mourani, P.M.; Hall, M.W.; Loftis, L.L.; Cvijanovich, N.Z.; Tarquinio, K.M.; et al. DDX58 Is Associated with Susceptibility to Severe Influenza Virus Infection in Children and Adolescents. J. Infect. Dis. 2022, 226, 2030–2036. [Google Scholar] [CrossRef]
- Gallego-Marin, C.; Schrum, J.E.; Andrade, W.A.; Shaffer, S.A.; Giraldo, L.F.; Lasso, A.M.; Kurt-Jones, E.A.; Fitzgerald, K.A.; Golenbock, D.T. Cyclic GMP-AMP Synthase Is the Cytosolic Sensor of Plasmodium falciparum Genomic DNA and Activates Type I IFN in Malaria. J. Immunol. 2018, 200, 768–774. [Google Scholar] [CrossRef]
- Yamamoto, M.; Okuyama, M.; Ma, J.S.; Kimura, T.; Kamiyama, N.; Saiga, H.; Ohshima, J.; Sasai, M.; Kayama, H.; Okamoto, T.; et al. A cluster of interferon-γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity 2012, 37, 302–313. [Google Scholar] [CrossRef]
- Zhu, Q.; Man, S.M.; Gurung, P.; Liu, Z.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.D. Cutting edge: STING mediates protection against colorectal tumorigenesis by governing the magnitude of intestinal inflammation. J. Immunol. 2014, 193, 4779–4782. [Google Scholar] [CrossRef]
- Low, J.T.; Chandramohan, V.; Bowie, M.L.; Brown, M.C.; Waitkus, M.S.; Briley, A.; Stevenson, K.; Fuller, R.; Reitman, Z.J.; Muscat, A.M.; et al. Epigenetic STING silencing is developmentally conserved in gliomas and can be rescued by methyltransferase inhibition. Cancer Cell 2022, 40, 439–440. [Google Scholar] [CrossRef]
- Li, M.; Mukasa, A.; Inda, M.M.; Zhang, J.; Chin, L.; Cavenee, W.; Furnari, F. Guanylate binding protein 1 is a novel effector of EGFR-driven invasion in glioblastoma. J. Exp. Med. 2011, 208, 2657–2673. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Yang, Q.; Wan, L.; Zhao, J.; Wu, Y.; Wang, J.; Yang, Y.; Niu, M.; Liu, H.; et al. Increased Drp1 promotes autophagy and ESCC progression by mtDNA stress mediated cGAS-STING pathway. J. Exp. Clin. Cancer Res. 2022, 41, 76. [Google Scholar] [CrossRef]
- Hong, C.; Schubert, M.; Tijhuis, A.E.; Requesens, M.; Roorda, M.; van den Brink, A.; Ruiz, L.A.; Bakker, P.L.; van der Sluis, T.; Pieters, W.; et al. cGAS-STING drives the IL-6-dependent survival of chromosomally instable cancers. Nature 2022, 607, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y.; Wu, W.; Wang, F.; Liu, X.; Shui, G.; Nie, C. Guanylate-binding protein 2 regulates Drp1-mediated mitochondrial fission to suppress breast cancer cell invasion. Cell Death Dis. 2017, 8, e3151. [Google Scholar] [CrossRef] [PubMed]
- Funabiki, M.; Kato, H.; Miyachi, Y.; Toki, H.; Motegi, H.; Inoue, M.; Minowa, O.; Yoshida, A.; Deguchi, K.; Sato, H.; et al. Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity 2014, 40, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Sepsis-induced immunosuppression: From cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013, 13, 862–874. [Google Scholar] [CrossRef]
- Huang, M.; Zhan, C.; Yang, B.; Lu, Y.; Yang, X.; Hou, J. Guanylate-binding protein 5-mediated cell-autonomous immunity suppresses inflammation in dental pulpitis: An in vitro study. Int. Endod. J. 2024, 57, 208–218. [Google Scholar] [CrossRef]
- Ma, X.Y.; Chen, M.M.; Meng, L.H. Second messenger 2’3’-cyclic GMP-AMP (2’3’-cGAMP): The cell autonomous and non-autonomous roles in cancer progression. Acta Pharmacol. Sin. 2024, 45, 890–899. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, P.; Wang, L.; Li, W.; Chen, B.; Liu, Y.; Wang, H.; Zhao, S.; Ye, L.; He, Y.; et al. cGAS-STING, an important pathway in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 81. [Google Scholar] [CrossRef]
- Tang, C.H.; Zundell, J.A.; Ranatunga, S.; Lin, C.; Nefedova, Y.; Del Valle, J.R.; Hu, C.C. Agonist-Mediated Activation of STING Induces Apoptosis in Malignant B Cells. Cancer Res. 2016, 76, 2137–2152. [Google Scholar] [CrossRef]
- Gulen, M.F.; Koch, U.; Haag, S.M.; Schuler, F.; Apetoh, L.; Villunger, A.; Radtke, F.; Ablasser, A. Signalling strength determines proapoptotic functions of STING. Nat. Commun. 2017, 8, 427. [Google Scholar] [CrossRef]
- Xun, J.; Zhang, Z.; Lv, B.; Lu, D.; Yang, H.; Shang, G.; Tan, J.X. A conserved ion channel function of STING mediates noncanonical autophagy and cell death. EMBO Rep. 2024, 25, 544–569. [Google Scholar] [CrossRef]
- Bakhoum, S.F.; Ngo, B.; Laughney, A.M.; Cavallo, J.A.; Murphy, C.J.; Ly, P.; Shah, P.; Sriram, R.K.; Watkins, T.B.K.; Taunk, N.K.; et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature 2018, 553, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Fang, S.; Fang, J.; Lin, K.; Nie, H.; Xiong, P.; Qiu, P.; Zhao, Q.; Wang, H.; Wang, F. Guanylate-binding proteins signature predicts favorable prognosis, immune-hot microenvironment, and immunotherapy response in hepatocellular carcinoma. Cancer Med. 2023, 12, 17504–17521. [Google Scholar] [CrossRef] [PubMed]
- Britzen-Laurent, N.; Lipnik, K.; Ocker, M.; Naschberger, E.; Schellerer, V.S.; Croner, R.S.; Vieth, M.; Waldner, M.; Steinberg, P.; Hohenadl, C.; et al. GBP-1 acts as a tumor suppressor in colorectal cancer cells. Carcinogenesis 2013, 34, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Tailor, D.; Garcia-Marques, F.J.; Bermudez, A.; Pitteri, S.J.; Malhotra, S.V. Guanylate-binding protein 1 modulates proteasomal machinery in ovarian cancer. iScience 2023, 26, 108292. [Google Scholar] [CrossRef]
- Wu, Z.H.; Cai, F.; Zhong, Y. Comprehensive Analysis of the Expression and Prognosis for GBPs in Head and neck squamous cell carcinoma. Sci. Rep. 2020, 10, 6085. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Sweis, R.F.; Hodi, F.S.; Messersmith, W.A.; Andtbacka, R.H.I.; Ingham, M.; Lewis, N.; Chen, X.; Pelletier, M.; Chen, X.; et al. Phase I Dose-Escalation Trial of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients with Advanced/Metastatic Solid Tumors or Lymphomas. Clin. Cancer Res. 2022, 28, 677–688. [Google Scholar] [CrossRef]
- Chang, W.; Altman, M.D.; Lesburg, C.A.; Perera, S.A.; Piesvaux, J.A.; Schroeder, G.K.; Wyss, D.F.; Cemerski, S.; Chen, Y.; DiNunzio, E.; et al. Discovery of MK-1454: A Potent Cyclic Dinucleotide Stimulator of Interferon Genes Agonist for the Treatment of Cancer. J. Med. Chem. 2022, 65, 5675–5689. [Google Scholar] [CrossRef]
- Carideo Cunniff, E.; Sato, Y.; Mai, D.; Appleman, V.A.; Iwasaki, S.; Kolev, V.; Matsuda, A.; Shi, J.; Mochizuki, M.; Yoshikawa, M.; et al. TAK-676: A Novel Stimulator of Interferon Genes (STING) Agonist Promoting Durable IFN-dependent Antitumor Immunity in Preclinical Studies. Cancer Res. Commun. 2022, 2, 489–502. [Google Scholar] [CrossRef]
- Gogoi, H.; Mansouri, S.; Jin, L. The Age of Cyclic Dinucleotide Vaccine Adjuvants. Vaccines 2020, 8, 453. [Google Scholar] [CrossRef]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef]
- Haag, S.M.; Gulen, M.F.; Reymond, L.; Gibelin, A.; Abrami, L.; Decout, A.; Heymann, M.; van der Goot, F.G.; Turcatti, G.; Behrendt, R.; et al. Targeting STING with covalent small-molecule inhibitors. Nature 2018, 559, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, C.; Kaundal, R.K. The role of cGAS-STING signaling in ischemic stroke: From immune response to therapeutic targeting. Drug Discov. Today 2023, 28, 103792. [Google Scholar] [CrossRef] [PubMed]
- Vasiyani, H.; Wadhwa, B. STING activation and overcoming the challenges associated with STING agonists using ADC (antibody-drug conjugate) and other delivery systems. Cell Signal 2025, 128, 111647. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; James, S.J.; Png, C.W.; Cui, C.; Li, H.; Li, L.; Chia, W.N.; Min, N.; Li, W.; Claser, C.; et al. DUSP4 modulates RIG-I- and STING-mediated IRF3-type I IFN response. Cell Death Differ. 2024, 31, 280–291. [Google Scholar] [CrossRef]
- Corrales, L.; Glickman, L.H.; McWhirter, S.M.; Kanne, D.B.; Sivick, K.E.; Katibah, G.E.; Woo, S.R.; Lemmens, E.; Banda, T.; Leong, J.J.; et al. Direct Activation of STING in the Tumor Microenvironment Leads to Potent and Systemic Tumor Regression and Immunity. Cell Rep. 2015, 11, 1018–1030. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Tannahill, G.M.; Curtis, A.M.; Adamik, J.; Palsson-McDermott, E.M.; McGettrick, A.F.; Goel, G.; Frezza, C.; Bernard, N.J.; Kelly, B.; Foley, N.H.; et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 2013, 496, 238–242. [Google Scholar] [CrossRef]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef]
- Netea, M.G.; Joosten, L.A.; Latz, E.; Mills, K.H.; Natoli, G.; Stunnenberg, H.G.; O’Neill, L.A.; Xavier, R.J. Trained immunity: A program of innate immune memory in health and disease. Science 2016, 352, aaf1098. [Google Scholar] [CrossRef]
- Baumeister, W. Cryo-electron tomography: A long journey to the inner space of cells. Cell 2022, 185, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
| Sensor | Cell Types | Ligand | Signaling Pathways | Downstream Responses | References |
|---|---|---|---|---|---|
| cGAS | Macrophage, dendritic cell, fibroblast, epithelial cell | dsDNA | STING-TBK1-IRF3 and IKK-NF-κB pathways | Type I IFN, inflammatory cytokine synthesis | [18,19,20] |
| DDX41 | Macrophage, dendritic cell | dsDNA | STING-TBK1-IRF3 and IKK-NF-κB pathways | Type I IFN, inflammatory cytokines synthesis | [21,22] |
| IFI16 | Macrophage, fibroblast, endothelial cell, epithelial cell | dsDNA | STING-TBK1-IRF3 and IKK-NF-κB pathways, inflammasome activation | Type I IFN, inflammatory cytokines synthesis, pyroptosis | [23,24,25] |
| AIM2 | Macrophage, monocyte, dendritic cell, fibroblast, epithelial cell | dsDNA | Inflammasome activation | Pyroptosis, cytokine secretion | [16,26,27,28] |
| RNA Pol III | Macrophage, dendritic cell | B-form dsDNA | MAVS-TBK1-IRF3 and IKK-NF-κB pathways | Type I IFN, inflammatory cytokines synthesis | [29,30] |
| DAI/ZBP1 | Macrophage, dendritic cell, fibroblast, epithelial cell | Z-DNA or Z-RNA | TBK1-IRF3 and IKK-NF-κB pathways, RIPK3-MLKL activation, NLRP3 inflammasome activation | Type I IFN, inflammatory cytokine synthesis, PANoptosis | [13,14,31] |
| RIG-I | Macrophage, dendritic cell, fibroblast, epithelial cell | 5′-triphosphate (ppp) short dsRNA | MAVS-TBK1-IRF3 and IKK-NF-κB pathways | Type I IFN, inflammatory cytokines synthesis | [32,33,34,35] |
| MDA5 | Macrophage, dendritic cell, fibroblast, epithelial cell | long dsRNA | MAVS-TBK1-IRF3 and IKK-NF-κB pathways | Type I IFN, inflammatory cytokines synthesis | [36,37,38] |
| OAS1 | Macrophage, monocyte, epithelial cell, microglia | dsRNA | RNase L activation | RNA degradation | [39,40,41,42] |
| PKR | Macrophage, monocyte, fibroblast, epithelial cell | dsRNA | eIF2α phosphorylation, NF-κB activation | Protein synthesis inhibition, apoptosis, inflammatory cytokine synthesis | [43,44] |
| NOD2 | Macrophage, fibroblast, epithelial cell | ssRNA | MAVS-TBK1-IRF3 | Type I IFN, inflammatory cytokines synthesis | [45] |
| NLRP1 | Epithelial cell, keratinocyte | dsRNA | Inflammasome activation | Pyroptosis, cytokine secretion | [46,47] |
| NLRP6 | Epithelial cell, hepatocyte | dsRNA | Inflammasome activation | Pyroptosis, cytokine secretion | [48] |
| GSDMB | Airway epithelium | dsRNA | MAVS-TBK1 pathway | IFN and ISG expression | [49] |
| Type of Disease | Specific Disease | Mechanism of Disease | References |
|---|---|---|---|
| Autoimmune diseases | Aicardi-Goutières syndrome (AGS) | Mutations in ADAR1 and three prime repair exonuclease 1 (TREX1) lead to hyperactivation of nucleic acid sensors. | [87,121] |
| STING-associated vasculopathy with onset in infancy (SAVI) | Gain-of-function mutations in the TMEM173 gene encoding STING | [122,123] | |
| Systemic lupus erythematosus (SLE) | Increased cGAS and cGAMP expression | [124] | |
| Rheumatoid arthritis (RA) | Knockdown of GBP5 in RA synovial fibroblasts exacerbates inflammation and tissue destruction. | [125] | |
| Elevated levels of cytosolic dsDNA promote inflammatory responses through activation of the cGAS-STING pathway. | [126] | ||
| Singleton-Merten syndrome (SMS) | A gain-of-function IFIH1 mutation causes SMS. | [127] | |
| Experimental autoimmune encephalomyelitis (EAE) | The loss of the MAVS gene exacerbates the severity of EVE. | [128] | |
| Multiple sclerosis (MS) | RIG-I and IFIH1 are expressed strongly in patients. | [129] | |
| Type 1 diabetes | Cumulative effect of IFIH1 variants and increased IFIH1 gene expression | [130,131] | |
| Psoriasis | STING deficiency reduces psoriatic symptoms and inflammation in mouse models of psoriasis. | [132] | |
| Familial chilblain lupus | Gain-of-function mutations in STING | [133] | |
| Inflammatory diseases | Inflammatory bowel disease (IBD) | Loss-of-function mutations in IFIH1 | [134] |
| GBP1 may protect against inflammatory cytokine-induced epithelial apoptosis and the consequent loss of barrier function. | [135] | ||
| Sepsis | Noncanonical inflammasome contributes to sepsis when it fails to clear the infection and a sustained inflammatory response develops. | [136] | |
| Alcoholic liver disease (ALD) | Ethanol induces ER stress and triggers the interaction between IRF3 and STING. | [137] | |
| Non-alcoholic fatty liver disease (NAFLD) | cGAS-STING signaling is involved in the development of NAFLD by DNA-mediated type I IFN production. | [138] | |
| Levels of STING are increased in liver tissues from patients with NAFLD. | [139] | ||
| Acute kidney injury (AKI) | Abnormal cytosolic mtDNA can be sensed by cGAS, resulting in STING-dependent inflammation and renal injury. | [140] | |
| Infectious diseases | Mycobacterium infection | GBP1−/− mice have difficulty killing Mycobacterium bovis BCG through cell-autonomous effects, resulting in an increased bacterial burden. | [141] |
| cGAS-deficient mice show increased susceptibility to Mtb infection. | [142] | ||
| L. monocytogenes infection | The knockdown of IFI16, cGAS, or STING shows reduced induction of IFN expression. | [54] | |
| GBP1−/− mice are susceptible to orogastric infection. | [141] | ||
| F. novicida infection | F. novicida infection could trigger the cGAS-STING-NLRP3 inflammasome axis in CASP4 × TRIF-deficient human monocytes. | [67] | |
| GBP2-deficient mice are unable to control F. novicida infection. | [113] | ||
| SARS-CoV-2 infection | GBP2 and GBP5 inhibit cleavage of the SARS-CoV-2 spike and reduce viral infections. | [143] | |
| SARS-CoV-2 infection triggers cGAS-STING signaling in endothelial cells via the release of mtDNA, resulting in cell death and type I IFN production. | [59] | ||
| HSV infection | cGAS-deficient mice are more susceptible to lethal infection with HSV-1. | [50] | |
| CMV infection | Type I IFN expression is essentially abolished in STING-deficient endothelial cells after CMV infection. | [64] | |
| Influenza virus infection | Loss-of-function mutations in DDX58, which encodes the RIG-I receptor, correlate with high susceptibility to respiratory infections caused by the influenza virus. | [144] | |
| Plasmodium infection | Genomic DNA from Plasmodium falciparum may access the cytosol due to phagosomal destabilization and activate type I IFN in Malaria. | [145] | |
| T. gondii infection | Gbpchr3-deficient mice exhibit increased susceptibility to T. gondii infection. | [146] | |
| Cancers | Colorectal cancer | The absence of STING leads to excessive colon inflammation during the early stages of tumor development, and STING-deficient mice are highly susceptible to colorectal cancer. | [147] |
| Glioma | Hypermethylation of the STING promoter mediates STING silencing in glioblastoma, contributing to immune suppression. | [148] | |
| GBP1 promotes EGFR-mediated MMP1 expression, thereby enhancing glioma cell invasion. | [149] | ||
| Esophageal squamous cell carcinoma (ESCC) | The mtDNA stress activates the cGAS-STING pathway, promoting autophagy and ESCC progression. | [150] | |
| Breast cancer | The cGAS-STING drives the IL-6-dependent survival of triple-negative breast cancer. | [151] | |
| GBP2 inhibits Drp1-mediated mitochondrial fission to suppress breast cancer invasion. | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, D.; Zhang, B.; Wang, Z.; Mi, Y. Cell-Autonomous Immunity: From Cytosolic Sensing to Self-Defense. Int. J. Mol. Sci. 2025, 26, 4025. https://doi.org/10.3390/ijms26094025
Han D, Zhang B, Wang Z, Mi Y. Cell-Autonomous Immunity: From Cytosolic Sensing to Self-Defense. International Journal of Molecular Sciences. 2025; 26(9):4025. https://doi.org/10.3390/ijms26094025
Chicago/Turabian StyleHan, Danlin, Bozheng Zhang, Zhe Wang, and Yang Mi. 2025. "Cell-Autonomous Immunity: From Cytosolic Sensing to Self-Defense" International Journal of Molecular Sciences 26, no. 9: 4025. https://doi.org/10.3390/ijms26094025
APA StyleHan, D., Zhang, B., Wang, Z., & Mi, Y. (2025). Cell-Autonomous Immunity: From Cytosolic Sensing to Self-Defense. International Journal of Molecular Sciences, 26(9), 4025. https://doi.org/10.3390/ijms26094025






