Identification and Exploration of Pyroptosis-Related Genes in Macrophage Cells Reveal Necrotizing Enterocolitis Heterogeneity Through Single-Cell and Bulk-Sequencing
Abstract
1. Introduction
2. Results
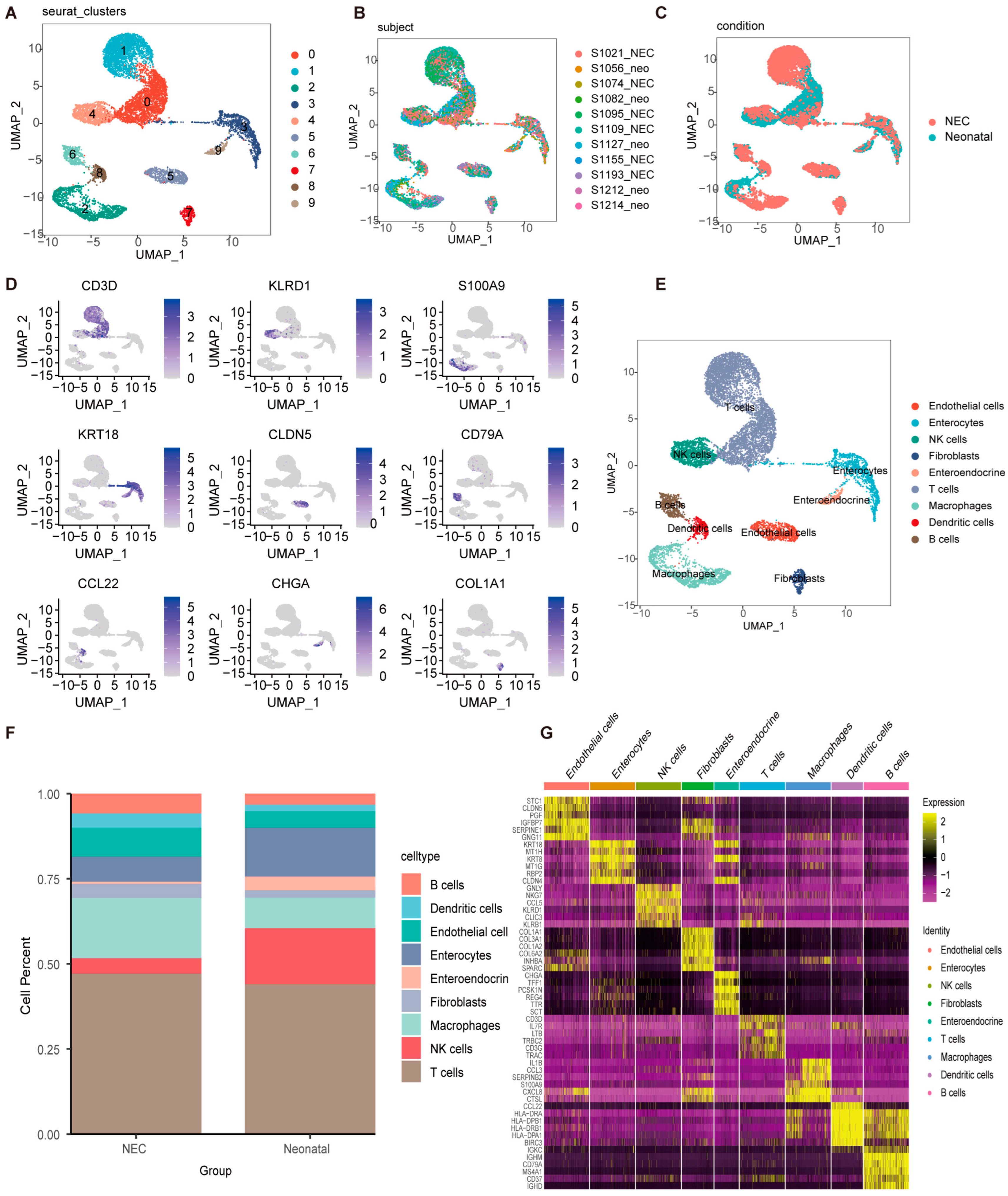
2.1. Clustering and Identification of Key Cell Subtypes at Single-Cell Resolution
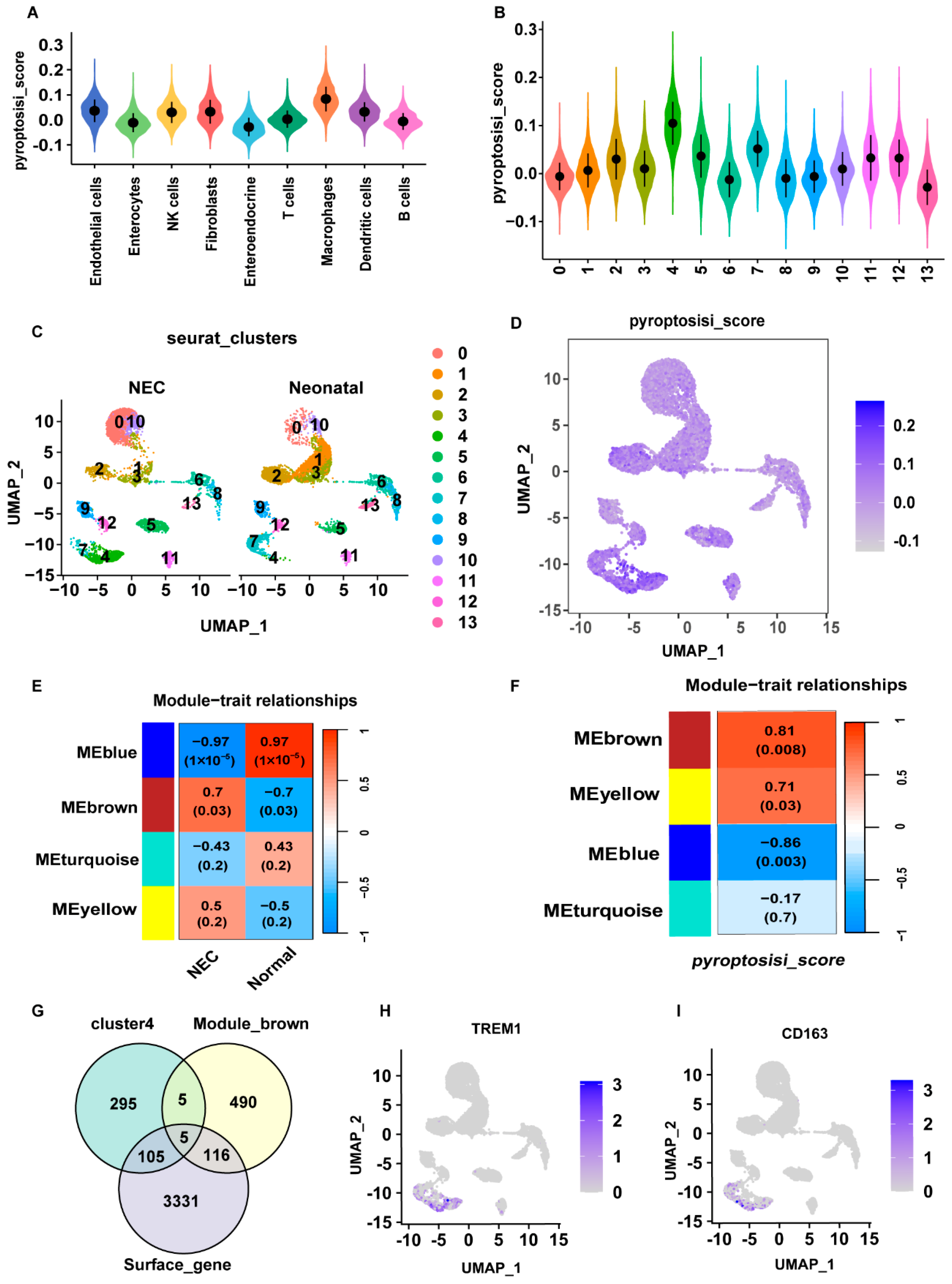
2.2. Identification of DE-PRGs in NEC
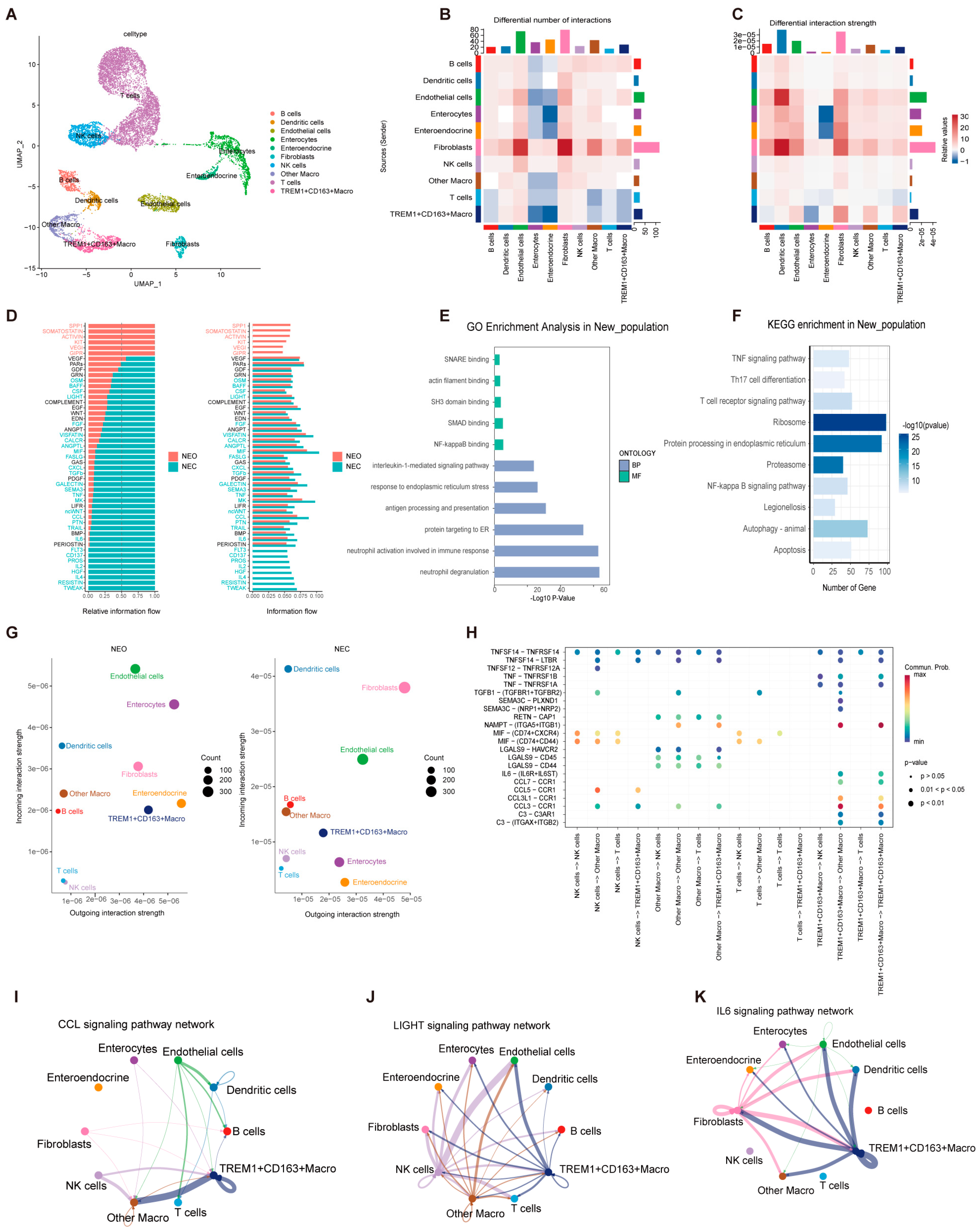
2.3. Interaction of Effector TREM1+CD163+ Macrophages with Other Cell Clusters
2.4. Exploring the Molecular Mechanism of Pyroptosis Based on Hub Genes
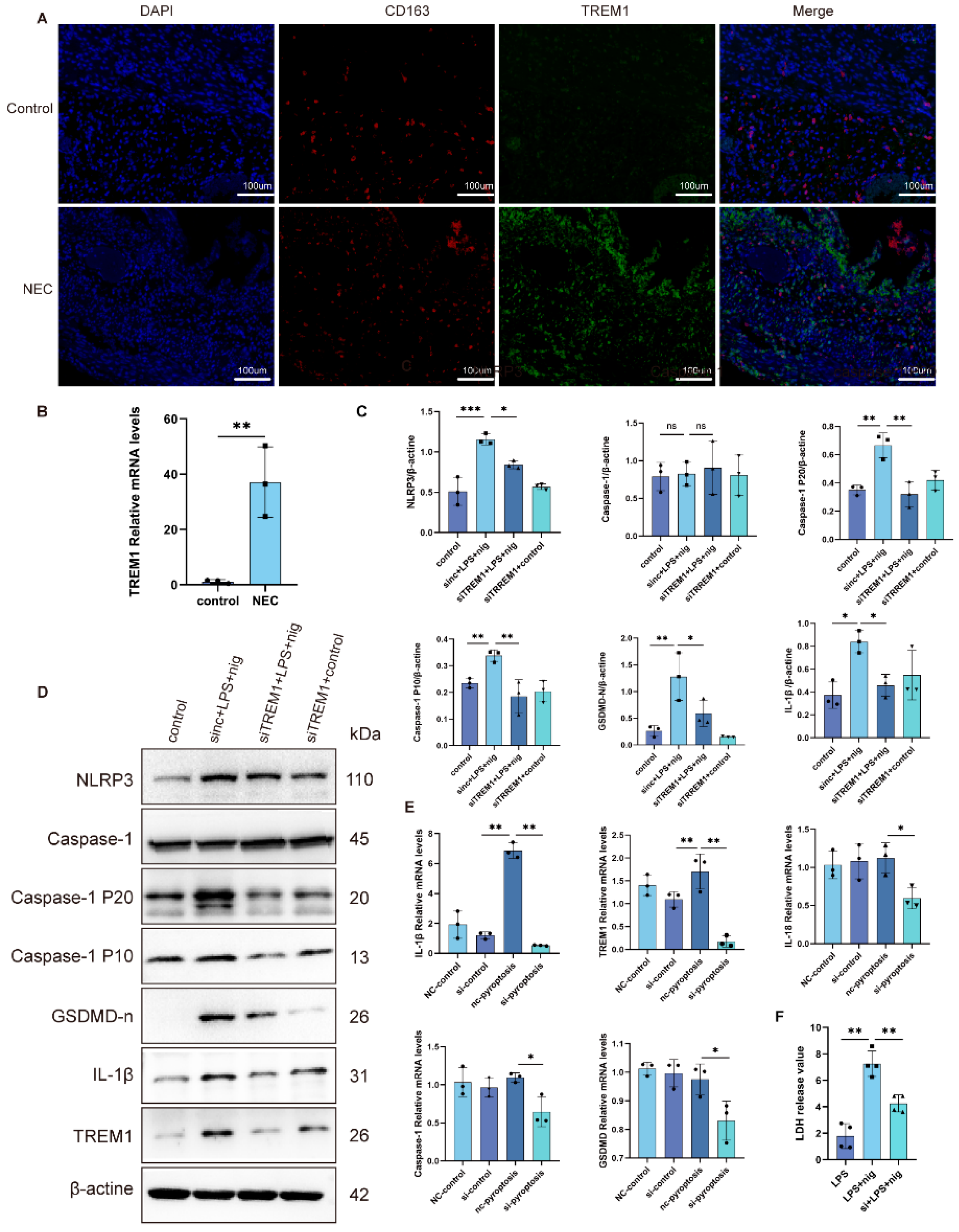
2.5. Analysis of Hub Genes in the Human Intestine
2.6. Inhibition of TREM1 in THP-1 Cells Alleviates Cell Pyroptosis
3. Discussion
4. Materials and Methods
4.1. Data Sources
4.2. Collection and Storage of the Intestinal Tissue
4.3. scRNA-seq Analysis
4.4. Functional Enrichment Analysis
4.5. Gene Set Variation Analysis
4.6. Bulk RNA-seq Data Validation
4.7. Cell–Cell Communication Analysis
4.8. scMetabolism Analysis
4.9. Weighted Gene Co-Expression Network Analysis
4.10. Immunofluorescence Experiments
4.11. Cell Model of Pyroptosis and TREM1 Small-Interfering RNA (siRNA) Transfection
4.12. Western Blot for Protein Expression
4.13. Reverse Transcription Quantitative PCR for mRNA Expression
4.14. Statistical Analysis
5. Concusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Charoentong, P.; Finotello, F.; Angelova, M.; Mayer, C.; Efremova, M.; Rieder, D.; Hackl, H.; Trajanoski, Z. Pan-cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep. 2017, 18, 248–262. [Google Scholar] [CrossRef]
- Huang, X.; Ye, C.; Zhao, X.; Tong, Y.; Lin, W.; Huang, Q.; Zheng, Y.; Wang, J.; Zhang, A.; Mo, Y. TRIM45 aggravates microglia pyroptosis via Atg5/NLRP3 axis in septic encephalopathy. J. Neuroinflammation 2023, 20, 284. [Google Scholar] [CrossRef]
- Hackam, D.J.; Sodhi, C.P. Bench to bedside-new insights into the pathogenesis of necrotizing enterocolitis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 468–479. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, Pyroptosis, and Necroptosis-Oh My! The Many Ways a Cell Can Die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef]
- Yan, M.; Li, W.; Wei, R.; Li, S.; Liu, Y.; Huang, Y.; Zhang, Y.; Lu, Z.; Lu, Q. Identification of pyroptosis-related genes and potential drugs in diabetic nephropathy. J. Transl. Med. 2023, 21, 490. [Google Scholar] [CrossRef]
- Liu, X.; Xia, S.; Zhang, Z.; Wu, H.; Lieberman, J. Channelling inflammation: Gasdermins in physiology and disease. Nat. Rev. Drug Discov. 2021, 20, 384–405. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J.; Zhao, X.; Wu, X.; Zou, H.; Qin, Z.; Cao, J. Overexpressed FOXO3 improves inflammatory status in mice by affecting NLRP3-mediated cell coronation in necrotizing colitis mice. Biomed. Pharmacother. 2020, 125, 109867. [Google Scholar] [CrossRef]
- Xiong, X.; Bao, Z.; Mi, Y.; Wang, X.; Zhu, J. Melatonin Alleviates Neonatal Necrotizing Enterocolitis by Repressing the Activation of the NLRP3 Inflammasome. Gastroenterol. Res. Pract. 2022, 2022, 6920577. [Google Scholar] [CrossRef]
- Chen, J.; Chen, T.; Zhou, J.; Zhao, X.; Sheng, Q.; Lv, Z. MiR-146a-5p Mimic Inhibits NLRP3 Inflammasome Downstream Inflammatory Factors and CLIC4 in Neonatal Necrotizing Enterocolitis. Front. Cell Dev. Biol. 2020, 8, 594143. [Google Scholar] [CrossRef]
- Egozi, A.; Olaloye, O.; Werner, L.; Silva, T.; McCourt, B.; Pierce, R.W.; An, X.; Wang, F.; Chen, K.; Pober, J.S.; et al. Single-cell atlas of the human neonatal small intestine affected by necrotizing enterocolitis. PLoS Biol. 2023, 21, e3002124. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Y.; Yang, L.; Aikebaier, M.; Shan, S.; Zha, Q.; Yang, K. Identification of pyroptosis-associated genes with diagnostic value in calcific aortic valve disease. Front. Cardiovasc. Med. 2024, 11, 1340199. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Yu, Q.; Xiao, J.; Zhao, H. TREM-1 aggravates chronic obstructive pulmonary disease development via activation NLRP3 inflammasome-mediated pyroptosis. Inflamm. Res. 2021, 70, 971–980. [Google Scholar] [CrossRef]
- Riviere, J.E.; Coppoc, G.L.; Hinsman, E.J.; Carlton, W.W.; Traver, D.S. Species dependent gentamicin pharmacokinetics and nephrotoxicity in the young horse. Fundam. Appl. Toxicol. 1983, 3, 448–457. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, L.; Liu, S.; Huang, S.; Lin, Q.; Zeng, M.; Huang, H.; Sun, X.; Chen, H.; Huang, J.; et al. The role of TREM-1 in septic myocardial pyroptosis and septic cardiomyopathy in vitro and in vivo. J. Cell Physiol. 2024, 239, e31445. [Google Scholar] [CrossRef]
- Alvarado-Vazquez, P.A.; Bernal, L.; Paige, C.A.; Grosick, R.L.; Moracho Vilrriales, C.; Ferreira, D.W.; Ulecia-Moron, C.; Romero-Sandoval, E.A. Macrophage-specific nanotechnology-driven CD163 overexpression in human macrophages results in an M2 phenotype under inflammatory conditions. Immunobiology 2017, 222, 900–912. [Google Scholar] [CrossRef]
- Halpern, M.D.; Gupta, A.; Zaghloul, N.; Thulasingam, S.; Calton, C.M.; Camp, S.M.; Garcia, J.G.N.; Ahmed, M. Extracellular Nicotinamide Phosphoribosyltransferase Is a Therapeutic Target in Experimental Necrotizing Enterocolitis. Biomedicines 2024, 12, 970. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, K.; Ding, X.; Mo, D.; Guo, H.; Chen, B.; Xia, B.; Ye, C.; Chen, G.; Guo, C. NAMPT inhibition relieves intestinal inflammation by regulating macrophage activation in experimental necrotizing enterocolitis. Biomed. Pharmacother. 2023, 165, 115012. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, W.; Li, Y.; Chen, K.; Li, H.; Tang, H.; Yin, Y.; Song, Z.; Chen, D. CCL3 aggravates intestinal damage in NEC by promoting macrophage chemotaxis and M1 macrophage polarization. Pediatr. Res. 2023, 94, 119–128. [Google Scholar] [CrossRef]
- Yuan, X.; Xiong, Z.; Liu, W.; Li, Y.; Li, H.; Zhang, X.; Yin, Y.; Xu, P.; Cao, J.; Chen, D.; et al. Novel Therapeutic Targeting of CCL3-CCR4 Axis Mediated Apoptotic Intesitnal Injury in Necrotizing Enterocolitis. Front. Immunol. 2022, 13, 859398. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Miller, M.; Fu, Y.X. Immunoregulation by tumor necrosis factor superfamily member LIGHT. Immunol. Rev. 2009, 229, 232–243. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rane, M. Interleukin-6 Signaling and Anti-Interleukin-6 Therapeutics in Cardiovascular Disease. Circ. Res. 2021, 128, 1728–1746. [Google Scholar] [CrossRef]
- Audrain, M.; Haure-Mirande, J.V.; Mleczko, J.; Wang, M.; Griffin, J.K.; St George-Hyslop, P.H.; Fraser, P.; Zhang, B.; Gandy, S.; Ehrlich, M.E. Reactive or transgenic increase in microglial TYROBP reveals a TREM2-independent TYROBP-APOE link in wild-type and Alzheimer’s-related mice. Alzheimers Dement. 2021, 17, 149–163. [Google Scholar] [CrossRef]
- Ormsby, T.; Schlecker, E.; Ferdin, J.; Tessarz, A.S.; Angelisová, P.; Köprülü, A.D.; Borte, M.; Warnatz, K.; Schulze, I.; Ellmeier, W.; et al. Btk is a positive regulator in the TREM-1/DAP12 signaling pathway. Blood 2011, 118, 936–945. [Google Scholar] [CrossRef]
- Colonna, M. TREMs in the immune system and beyond. Nat. Rev. Immunol. 2003, 3, 445–453. [Google Scholar] [CrossRef]
- Lanier, L.L. DAP10- and DAP12-associated receptors in innate immunity. Immunol. Rev. 2009, 227, 150–160. [Google Scholar] [CrossRef]
- Filippova, N.; Hromov, R.; Shi, J.; King, P.H.; Nabors, L.B. Pilot Screening of TREM1 Inhibitors in Cell-Based Reporter Assays Reflecting TREM1/DAP12 Receptor Assembly and Functionality. ACS Chem. Neurosci. 2025, 16, 52–65. [Google Scholar] [CrossRef]
- Chen, X.; Eksioglu, E.A.; Carter, J.D.; Fortenbery, N.; Donatelli, S.S.; Zhou, J.; Liu, J.; Yang, L.; Gilvary, D.; Djeu, J.; et al. Inactivation of DAP12 in PMN inhibits TREM1-mediated activation in rheumatoid arthritis. PLoS ONE 2015, 10, e0115116. [Google Scholar] [CrossRef]
- Yu, J.; Mu, X.; Guan, C.; Wang, Y.; Li, H. Tyrobp deficiency blocks NLRP3-mediated inflammation and pyroptosis to alleviate myocardial ischemia-reperfusion injury through regulating Syk. Tissue Cell 2024, 91, 102555. [Google Scholar] [CrossRef]
- Stewart, C.J.; Embleton, N.D.; Marrs, E.C.; Smith, D.P.; Nelson, A.; Abdulkadir, B.; Skeath, T.; Petrosino, J.F.; Perry, J.D.; Berrington, J.E.; et al. Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome 2016, 4, 67. [Google Scholar] [CrossRef]
- Wang, S.; Liu, R.; Yu, Q.; Dong, L.; Bi, Y.; Liu, G. Metabolic reprogramming of macrophages during infections and cancer. Cancer Lett. 2019, 452, 14–22. [Google Scholar] [CrossRef]
- Sun, S.; Fan, Z.; Liu, X.; Wang, L.; Ge, Z. Microglia TREM1-mediated neuroinflammation contributes to central sensitization via the NF-kappaB pathway in a chronic migraine model. J. Headache Pain. 2024, 25, 3. [Google Scholar] [CrossRef]
- Yang, Z.; Pan, X.; Wu, X.; Lin, Q.; Chen, Y.; Cai, S.; Zhang, Y.; Mai, Z.; Ahmad, N.; Ma, D.; et al. TREM-1 induces pyroptosis in cardiomyocytes by activating NLRP3 inflammasome through the SMC4/NEMO pathway. FEBS J. 2023, 290, 1549–1562. [Google Scholar] [CrossRef]
- Liang, Y.B.; Song, P.P.; Zhu, Y.H.; Xu, J.M.; Zhu, P.Z.; Liu, R.R.; Zhang, Y.S. TREM-1-targeting LP17 attenuates cerebral ischemia-induced neuronal injury by inhibiting oxidative stress and pyroptosis. Biochem. Biophys. Res. Commun. 2020, 529, 554–561. [Google Scholar] [CrossRef]
- Li, F.; Qin, N.; Yu, Y.; Dong, R.; Li, X.; Gong, S.; Zeng, Z.; Huang, L.; Yang, H. TREM-1 inhibition or ondansetron administration ameliorates NLRP3 inflammasome and pyroptosis in traumatic brain injury-induced acute lung injury. Arch. Med. Sci. 2024, 20, 984–996. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Li, Y.; Xu, P.; Zou, P.; Sheng, S.; Xiao, R.; Xu, P.; Chen, Y.; Du, Y.; Ma, L.; et al. Identification and Exploration of Pyroptosis-Related Genes in Macrophage Cells Reveal Necrotizing Enterocolitis Heterogeneity Through Single-Cell and Bulk-Sequencing. Int. J. Mol. Sci. 2025, 26, 4036. https://doi.org/10.3390/ijms26094036
Zhang P, Li Y, Xu P, Zou P, Sheng S, Xiao R, Xu P, Chen Y, Du Y, Ma L, et al. Identification and Exploration of Pyroptosis-Related Genes in Macrophage Cells Reveal Necrotizing Enterocolitis Heterogeneity Through Single-Cell and Bulk-Sequencing. International Journal of Molecular Sciences. 2025; 26(9):4036. https://doi.org/10.3390/ijms26094036
Chicago/Turabian StyleZhang, Peipei, Ying Li, Panpan Xu, Peicen Zou, Sihan Sheng, Ruiqi Xiao, Pu Xu, Ying Chen, Yue Du, Lishuang Ma, and et al. 2025. "Identification and Exploration of Pyroptosis-Related Genes in Macrophage Cells Reveal Necrotizing Enterocolitis Heterogeneity Through Single-Cell and Bulk-Sequencing" International Journal of Molecular Sciences 26, no. 9: 4036. https://doi.org/10.3390/ijms26094036
APA StyleZhang, P., Li, Y., Xu, P., Zou, P., Sheng, S., Xiao, R., Xu, P., Chen, Y., Du, Y., Ma, L., & Wang, Y. (2025). Identification and Exploration of Pyroptosis-Related Genes in Macrophage Cells Reveal Necrotizing Enterocolitis Heterogeneity Through Single-Cell and Bulk-Sequencing. International Journal of Molecular Sciences, 26(9), 4036. https://doi.org/10.3390/ijms26094036





