Local and Systemic Endothelial Damage in Patients with CEAP C2 Chronic Venous Insufficiency: Role of Mesoglycan
Abstract
:1. Introduction
2. Results
2.1. Materials, Population, and Treatment
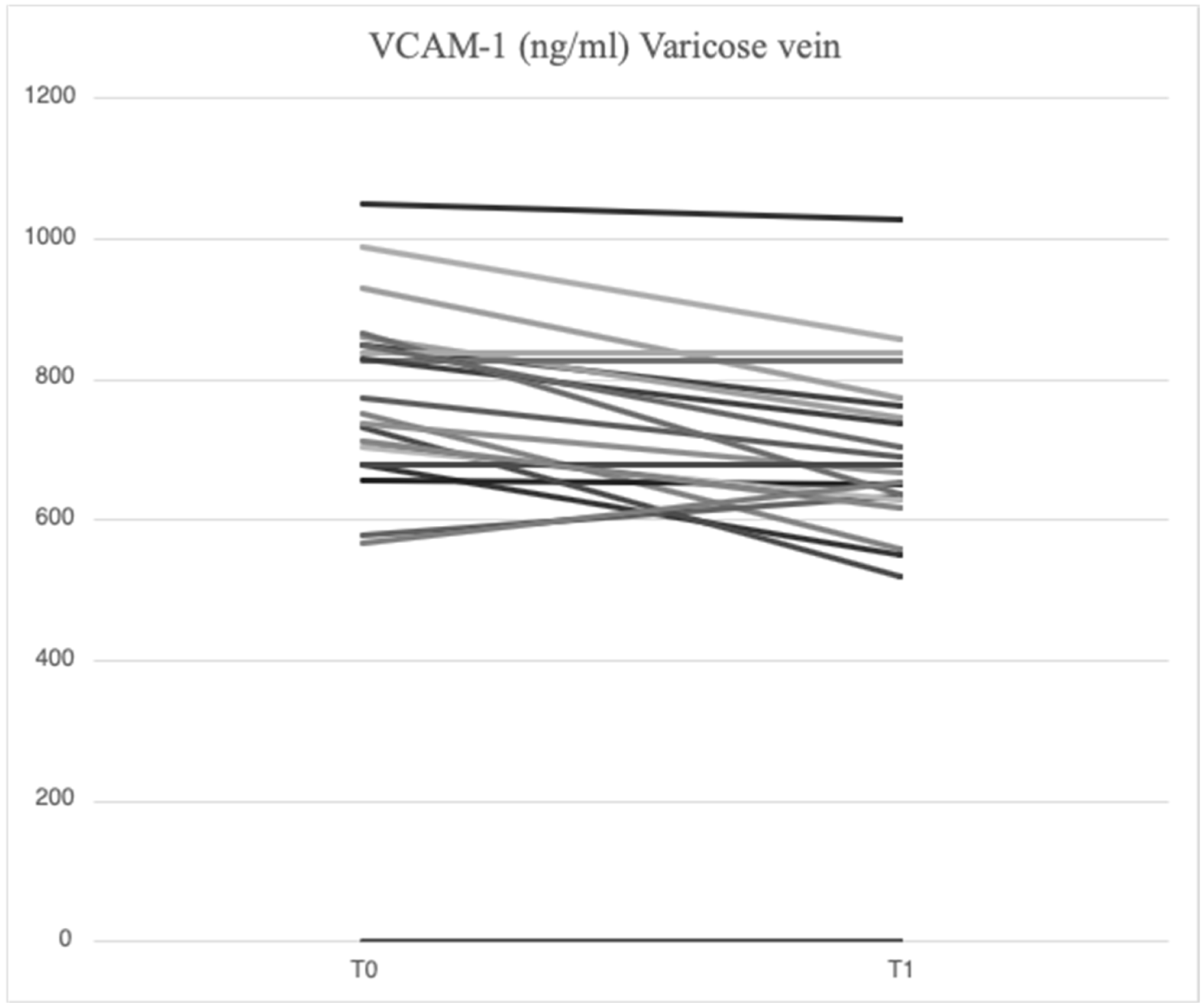
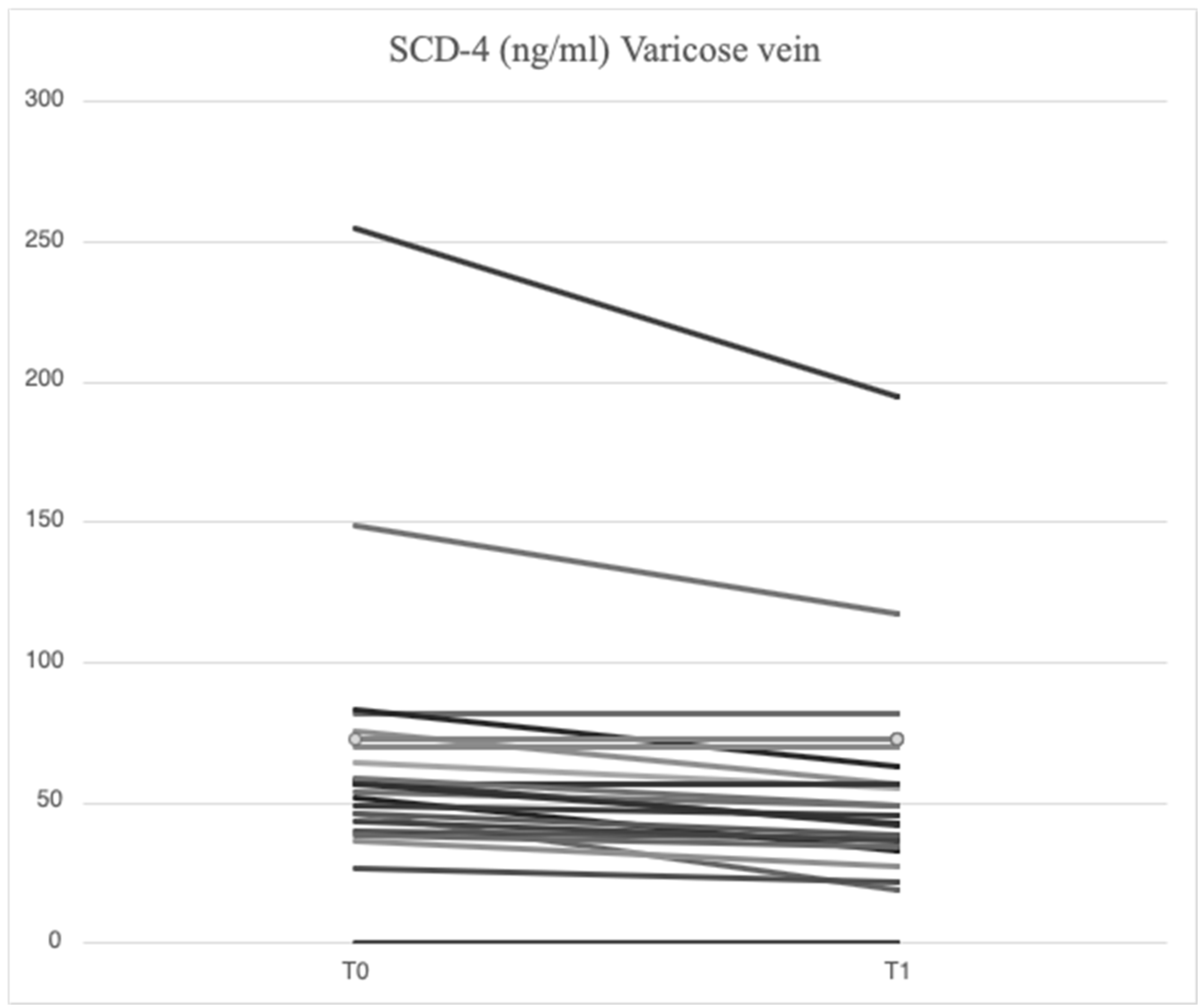
2.2. Serum Levels of Parameters at Time 0
2.3. Serum Levels of Parameters at Time 1
2.4. Signs and Symptoms at Time 0 and Time 1
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Patients
4.3. Assessments and Treatments
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fukaya, E.; Kolluri, R. Nonsurgical Management of Chronic Venous Insufficiency. N. Engl. J. Med. 2004, 391, 2350–2359. [Google Scholar] [CrossRef]
- Lurie, F.; Passman, M.; Meisner, M.; Dalsing, M.; Masuda, E.; Welch, H.; Bush, R.L.; Blebea, J.; Carpentier, P.H.; De Maeseneer, M.; et al. The 2020 update of the CEAP classification system and reporting standards. J. Vasc. Surg. Venous Lymphat. Disord. 2020, 8, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Mannello, F. Pathophysiology of chronic venous disease. Int. Angiol. 2014, 33, 212–221. [Google Scholar] [PubMed]
- Gwozdzinski, L.; Pieniazek, A.; Gwozdzinski, K. Factors Influencing Venous Remodeling in the Development of Varicose Veins of the Lower Limbs. Int. J. Mol. Sci. 2024, 25, 1560. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Shu, B.; Zhang, Y.; Wang, M. Endothelial Response to Pathophysiological Stress. Arter. Thromb. Vasc. Biol. 2019, 39, 11. [Google Scholar] [CrossRef]
- Serra, R.; Buffone, G.; Falcone, D.; Molinari, V.; Scaramuzzino, M.; Gallelli, L.; de Franciscis, S. Chronic venous leg ulcers are associated with high levels of metalloproteinases-9 and neutrophil gelatinase-associated lipocalin. Wound Repair Regen. 2013, 21, 395–401. [Google Scholar] [CrossRef]
- Grzela, T.; Niderla-Bielinska, J.; Litwiniuk, M.; White, R. The direct inhibition of MMP–2 and MMP–9 by an enzyme alginogel: A possible mechanism of healing support for venous leg ulcers. J. Wound Care 2014, 23, 278–285. [Google Scholar] [CrossRef]
- Matei, S.C.; Matei, M.; Anghel, F.M.; Carabenciov, E.; Murariu, M.S.; Olariu, S. Utility of routine laboratory tests in the assessment of chronic venous disease progression in female patients. Exp. Ther. Med. 2022, 24, 571. [Google Scholar] [CrossRef]
- Karahan, O.; Yavuz, C.; Kankilic, N.; Demirtas, S.; Tezcan, O.; Caliskan, A.; Mavitas, B. Simple blood tests as predictive markers of disease severity and clinical condition in patients with venous insufficiency. Blood Coagul. Fibrinolysis 2016, 27, 684–690. [Google Scholar] [CrossRef]
- Diab, L.; Al Katta, S.; Oueini, N.; Hawi, J.; Chrabieh, A.; Dosh, L.; Jurjus, R.; Leone, A.; Jurjus, A. Syndecan-1: A key player in health and disease. Immunogenetics 2024, 17, 9. [Google Scholar] [CrossRef]
- Foote, C.A.; Soares, R.N.; Ramirez-Perez, F.I.; Ghiarone, T.; Aroor, A.; Manrique-Acevedo, C.; Padilla, J.; Martinez-Lemus, L. Endothelial Glycocalyx. Compr. Physiol. 2022, 12, 3781–3811. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.; Kim, C.; Kokenyesi, R.; Scott Adzick, N.; Bernfield, M. Syndecans-1 and -4 Are Induced During Wound Repair of Neonatal but Not Fetal Skin. J. Investig. Dermatol. 1996, 107, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Nagy, N.; Németh, I.B.; Szabad, G.; Szolnoky, G.; Belsõ, N.; Bata-Csörgõ, Z.; Dobozy, A.; Kemény, L.; Széll, M. The altered expression of syndecan 4 in the uninvolved skin of venous leg ulcer patients may predispose to venous leg ulcer. Wound Repair Regen. 2008, 16, 495–502. [Google Scholar] [CrossRef]
- Echtermeyer, F.; Streit, M.; Wilcox-Adelman, S.; Saoncella, S.; Denhez, F.; Detmar, M.; Goetinck, P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J. Clin. Investig. 2001, 107, R9–R14. [Google Scholar] [CrossRef]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A High Admission Syndecan-1 Level, A Marker of Endothelial Glycocalyx Degradation, Is Associated With Inflammation, Protein C Depletion, Fibrinolysis, and Increased Mortality in Trauma Patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef]
- Nelson, A.; Berkestedt, I.; Bodelsson, M. Circulating glycosaminoglycan species in septic shock: Glycosaminoglycans during septic shock. Acta Anaesthesiol. Scand. 2014, 58, 36–43. [Google Scholar] [CrossRef]
- Liu, J.X.; Yan, Z.P.; Zhang, Y.Y.; Wu, J.; Liu, X.H.; Zeng, Y. Hemodynamic shear stress regulates the transcriptional expression of heparan sulfate proteoglycans in human umbilical vein endothelial cell. Cell. Mol. Biol. 2016, 62, 28–34. [Google Scholar]
- Nemoto, T.; Minami, Y.; Yamaoka-Tojo, M.; Kato, A.; Katsura, A.; Sato, T.; Muramatsu, Y.; Kakizaki, R.; Fujiyoshi, K.; Hashimoto, T.; et al. Endothelial glycocalyx and severity and vulnerability of coronary plaque in patients with coronary artery disease. Atherosclerosis 2020, 302, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qi, Z.; Sun, C.; Zhang, Y.; Gong, L.; Li, Y.; Dong, H.; Jia, W.; Zhong, L.; Yang, J. Circulating glycocalyx shedding products as biomarkers for evaluating prognosis of patients with out-of-hospital cardiac arrest after return of spontaneous circulation. Sci. Rep. 2024, 14, 17582. [Google Scholar] [CrossRef]
- Klobucar, I.; Klobucar, L.; Lechleitner, M.; Pregartner, G.; Frank, S.; Degoricija, V. Association of inflammatory markers and ultrasonographic indicators of endothelial (dys)function in humans. Cardiovasc. Res. 2024, 120, cvae088.159. [Google Scholar] [CrossRef]
- Hemdahl, A.L.; Gabrielsen, A.; Zhu, C.; Eriksson, P.; Hedin, U.; Kastrup, J.; Thorén, P.; Hansson, G.K. Expression of Neutrophil Gelatinase–Associated Lipocalin in Atherosclerosis and Myocardial Infarction. Arter. Thromb. Vasc. Biol. 2006, 6, 136–142. [Google Scholar] [CrossRef]
- Prandoni, P. Links between arterial and venous disease. J. Intern. Med. 2007, 262, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.L.; Huang, Y.L.; Lee, M.C.; Hu, S.; Hsiao, Y.C.; Chang, S.W.; Chang, C.J.; Chen, P.C. Association of Varicose Veins With Incident Venous Thromboembolism and Peripheral Artery Disease. JAMA 2018, 319, 807. [Google Scholar] [CrossRef]
- Prochaska, J.H.; Arnold, N.; Falcke, A.; Kopp, S.; Schulz, A.; Buch, G.; Moll, S.; Panova-Noeva, M.; Jünger, C.; Eggebrecht, L.; et al. Chronic venous insufficiency, cardiovascular disease, and mortality: A population study. Eur. Heart J. 2021, 42, 4157–4165. [Google Scholar] [CrossRef] [PubMed]
- Huwait, E.; Mobashir, M. Potential and Therapeutic Roles of Diosmin in Human Diseases. Biomedicines 2022, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kakkos, S.K.; Allaert, F.A. Efficacy of Ruscus extract, HMC and vitamin C, constituents of Cyclo 3 fort®, on improving individual venous symptoms and edema: A systematic review and meta-analysis of randomized double-blind placebo-controlled trials. Int. Angiol. 2017, 36, 93–106. [Google Scholar] [CrossRef]
- Matei, S.C.; Matei, M.; Anghel, F.M.; Derban, M.D.; Olariu, A.; Olariu, S. Impact of statin treatment on patients diagnosed with chronic venous disease. Morphological analysis of the venous wall and clinical implications. Phlebology 2022, 37, 188–195. [Google Scholar] [CrossRef]
- Eschrich, J.; Meyer, R.; Kuk, H.; Wagner, A.H.; Noppeney, T.; Debus, S.; Hecker, M.; Korff, T. Varicose Remodeling of Veins Is Suppressed by 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitors. J. Am. Heart Assoc. 2016, 23, e002405. [Google Scholar] [CrossRef]
- Masola, V.; Zaza, G.; Onisto, M.; Lupo, A.; Gambaro, G. Glycosaminoglycans, proteoglycans and sulodexide and the endothelium: Biological roles and pharmacological effects. Int. Angiol. 2014, 33, 243–254. [Google Scholar]
- Carroll, B.J.; Piazza, G.; Goldhaber, S.Z. Sulodexide in venous disease. J. Thromb. Haemost. 2019, 17, 31–38. [Google Scholar] [CrossRef]
- Andreozzi, G.M. Effectiveness of mesoglycan in patients with previous deep venous thrombosis and chronic venous insufficiency. Minerva Cardioangiol. 2007, 55, 741–753. [Google Scholar]
- Arosio, E.; Ferrari, G.; Santoro, L.; Gianese, F.; Coccheri, S. A Placebo-controlled, Double-blind Study of Mesoglycan in the Treatment of Chronic Venous Ulcers. Eur. J. Vasc. Endovasc. Surg. 2001, 22, 365–372. [Google Scholar] [CrossRef]
- Giuseppe, D.; Angela, D.; Romano, D.; Pamela, M. Evaluation of the Effects of Mesoglycan on Some Markers of Endothelial Damage and Walking Distance in Diabetic Patients with Peripheral Arterial Disease. Int. J. Mol. Sci. 2017, 18, 572. [Google Scholar] [CrossRef] [PubMed]
- Yasim, A.; Kilinc, M.; Aral, M.; Oksuz, H.; Kabalci, M.; Eroglu, E.; Imrek, S. Serum concentration of procoagulant, endothelial and oxidative stress markers in early primary varicose veins. Phlebol. J. Venous Dis. 2008, 23, 15–20. [Google Scholar] [CrossRef]
- Castro-Ferreira, R.; Cardoso, R.; Leite-Moreira, A.; Mansilha, A. The Role of Endothelial Dysfunction and Inflammation in Chronic Venous Disease. Ann. Vasc. Surg. 2018, 46, 380–393. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, A.; Jasińska-Sumińska, K.; Bręborowicz, A.; Kowalska, K.; Zabel, M.; Wysocka, T.; Khalil, R.A.; Raffetto, J.D.; Urbanek, T. Changes of the serum properties and its effect on the endothelial cells restoration in patients with chronic venous disease treated with sulodexide. J. Vasc. Surg. Venous Lymphat. Disord. 2024, 12, 101941. [Google Scholar] [CrossRef] [PubMed]
- Mansilha, A.; Sousa, J. Pathophysiological Mechanisms of Chronic Venous Disease and Implications for Venoactive Drug Therapy. Int. J. Mol. Sci. 2018, 19, 1669. [Google Scholar] [CrossRef]
- Urbanek, T.; Zbigniew, K.; Begier-Krasińska, B.; Baum, E.; Bręborowicz, A. Sulodexide suppresses inflammation in patients with chronic venous insufficiency. Int. Angiol. Dec. 2015, 34, 589–596. [Google Scholar]
- Baucom, M.R.; Weissman, N.; Price, A.D.; England, L.; Schuster, R.M.; Pritts, T.A.; Goodman, M.D. Syndecan-1 as the Effect or Effector of the Endothelial Inflammatory Response? J. Surg. Res. 2024, 295, 611–618. [Google Scholar] [CrossRef]
- Giuliani, A.; Ramini, D.; Sbriscia, M.; Crocco, P.; Tiano, L.; Rippo, M.R.; Bonfigli, A.R.; Rose, G.; De Luca, M.; Olivieri, F.; et al. Syndecan 4 is a marker of endothelial inflammation in pathological aging and predicts long-term cardiovascular outcomes in type 2 diabetes. Diabetol. Metab. Syndr. 2024, 20, 03. [Google Scholar] [CrossRef]
- Manon-Jensen, T.; Multhaupt, H.A.B.; Couchman, J.R. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013, 280, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Y.; Zhi, L.; Yu, L.; Hu, X.; Shen, Y.; Du, W. SDC4 protein action and related key genes in nonhealing diabetic foot ulcers based on bioinformatics analysis and machine learning. Int. J. Biol. Macromol. 2024, 283, 137789. [Google Scholar] [CrossRef] [PubMed]
- Balistreri, C.R.; Monastero, R. Syndecans in Alzheimer’s disease: Pathogenetic mechanisms and potential therapeutic targets. Neural Regen. Res. 2025, 1, 2594–2595. [Google Scholar] [CrossRef]
- Pessolano, E.; Belvedere, R.; Novizio, N.; Filippelli, A.; Perretti, M.; Whiteford, J.; Petrella, A. Mesoglycan connects Syndecan-4 and VEGFR2 through Annexin A1 and formyl peptide receptors to promote angiogenesis in vitro. FEBS J. 2021, 288, 6428–6446. [Google Scholar] [CrossRef]
- Vuong, T.T.; Reine, T.M.; Sudworth, A.; Jenssen, T.G.; Kolset, S.O. Syndecan-4 Is a Major Syndecan in Primary Human Endothelial Cells In Vitro, Modulated by Inflammatory Stimuli and Involved in Wound Healing. J. Histochem. Cytochem. 2015, 63, 280–292. [Google Scholar] [CrossRef]
- Lattimer, C.R.; Kalodiki, E.; Geroulakos, G.; Hoppensteadt, D.; Fareed, J. Are Inflammatory Biomarkers Increased in Varicose Vein Blood? Clin. Appl. Thromb. 2016, 22, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 26, 12906. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Álvarez-Mon, M.A.; Chaowen, C.; Ruiz-Grande, F.; Pekarek, L.; Monserrat, J.; Asúnsolo, A.; García-Honduvilla, N.; et al. Understanding Chronic Venous Disease: A Critical Overview of Its Pathophysiology and Medical Management. J. Clin. Med. 2021, 22, 3239. [Google Scholar] [CrossRef]
- O’Rourke, N.; Meens-Miller, E.; Jeffrey, M.; Saleem, L.; Green-Johnson, J.; Dogra, S. Short bouts of walking attenuates the response of IL-8 to prolonged sitting in healthy adults. Eur. J. Appl. Physiol. 2023, 123, 1271–1281. [Google Scholar] [CrossRef]
- Said, E.A.; Al Reesi, I.; Al Shizawi, N. Defining IL-6 levels in healthy individuals: A meta-analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef]
- Dogra, S.; Wolf, M.; Jeffrey, M.P.; Foley, R.C.A.; Logan-Sprenger, H.; Jones-Taggart, H.; Green-Johnson, J.M. Disrupting prolonged sitting reduces IL-8 and lower leg swell in active young adults. BMC Sports Sci. Med. Rehabil. 2019, 18, 23. [Google Scholar] [CrossRef] [PubMed]
- Beidler, S.K.; Douillet, C.D.; Berndt, D.F.; Keagy, B.A.; Rich, P.B.; Marston, W.A. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J. Vasc. Surg. 2009, 49, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, J.; Przywara, S.; Iłżecka, J.; Iłżecki, M. The influence of compression therapy on the level of inflammatory biomarkers in patients with chronic venous disease. Acta Angiol. 2021, 1, 32–36. [Google Scholar] [CrossRef]
- Jacob, M.P.; Cazaubon, M.; Scemama, A.; Prié, D.; Blanchet, F.; Guillin, M.C.; Michel, J.B. Plasma matrix metalloproteinase-9 as a marker of blood stasis in varicose veins. Circulation 2002, 30, 535–538. [Google Scholar] [CrossRef]
- Weyl, A.; Vanscheidt, W.; Weiss, J.M.; Peschen, M.; Schopf, E.; Simon, J. Expression of the adhesion molecules ICAM-1, VCAM-1, and E-selectin and their ligands VLA-4 and LFA-1 in chronic venous leg ulcers. J. Am. Acad. Dermatol. 1996, 34, 418–423. [Google Scholar] [CrossRef]
- Kucukguven, A.; Khalil, R.A. Matrix metalloproteinases as potential targets in the venous dilation associated with varicose veins. Curr. Drug Targets 2013, 14, 287–324. [Google Scholar] [PubMed]
- Van Meeteren, L.A.; Ten Dijke, P. Regulation of endothelial cell plasticity by TGF-β. Cell Tissue Res. 2012, 347, 177–186. [Google Scholar] [CrossRef]
- Ku, M.J.; Maeng, Y.H.; Chang, J.W.; Song, J.K.; Kim, Y.R. Stasis and Inflammation in Varicose Vein Development: An Interleukin-Mediated Process from Intima to Media. J. Vasc. Res. 2024, 61, 244–251. [Google Scholar] [CrossRef]




| Characteristics of the Patients | |
|---|---|
| No. | 23 |
| Median age (IQR)—yr | 60 (23–74) |
| Female sex—No. (%) | 18 (78.2%) |
| Caucasian—No. (%) | 22 (95.6%) |
| Median height (IQR)—cm | 165 (158–192) |
| Median weight (IQR)—kg | 68 (52–102) |
| Body mass index > 25 kg/m2—No. (%) | 9 (39.1%) |
| Inheritance—No. (%) | 19 (82.6%) |
| Pregnancies—No. (%) | 13 (72.2%) |
| Sedentary lifestyle—No. (%) | 15 (65.2%) |
| Smoking habit—No. (%) | 6 (26%) |
| Population (No. = 23) | |||
|---|---|---|---|
| T0 | T1 | P | |
| Heaviness—No. (%) | 18 (78.2%) | 11 (47.8%) | 0.023 |
| Edema—No. (%) | 15 (65.2%) | 8 (34.7%) | 0.023 |
| Cramps—No. (%) | 13 (56.5%) | 3 (13%) | 0.004 |
| Itching—No. (%) | 12 (52.1%) | 6 (26%) | 0.023 |
| Pain—No. (%) | 12 (52.1%) | 7 (30.4%) | 0.041 |
| Heat—No. (%) | 9 (39.1%) | 4 (17.3%) | 0.041 |
| Paresthesia—No. (%) | 8 (34.7%) | 2 (8.6%) | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoliquido, A.; Carnuccio, C.; Santoro, L.; Di Giorgio, A.; D'Alessandro, A.; Ponziani, F.R.; Angelini, F.; Izzo, M.; Nesci, A. Local and Systemic Endothelial Damage in Patients with CEAP C2 Chronic Venous Insufficiency: Role of Mesoglycan. Int. J. Mol. Sci. 2025, 26, 4046. https://doi.org/10.3390/ijms26094046
Santoliquido A, Carnuccio C, Santoro L, Di Giorgio A, D'Alessandro A, Ponziani FR, Angelini F, Izzo M, Nesci A. Local and Systemic Endothelial Damage in Patients with CEAP C2 Chronic Venous Insufficiency: Role of Mesoglycan. International Journal of Molecular Sciences. 2025; 26(9):4046. https://doi.org/10.3390/ijms26094046
Chicago/Turabian StyleSantoliquido, Angelo, Claudia Carnuccio, Luca Santoro, Angela Di Giorgio, Alessia D'Alessandro, Francesca Romana Ponziani, Flavia Angelini, Marcello Izzo, and Antonio Nesci. 2025. "Local and Systemic Endothelial Damage in Patients with CEAP C2 Chronic Venous Insufficiency: Role of Mesoglycan" International Journal of Molecular Sciences 26, no. 9: 4046. https://doi.org/10.3390/ijms26094046
APA StyleSantoliquido, A., Carnuccio, C., Santoro, L., Di Giorgio, A., D'Alessandro, A., Ponziani, F. R., Angelini, F., Izzo, M., & Nesci, A. (2025). Local and Systemic Endothelial Damage in Patients with CEAP C2 Chronic Venous Insufficiency: Role of Mesoglycan. International Journal of Molecular Sciences, 26(9), 4046. https://doi.org/10.3390/ijms26094046









