Liquid Biopsy in Pituitary Neuroendocrine Tumors—Potential Biomarkers for Diagnosis, Prognosis, and Therapy
Abstract
1. Introduction
2. Liquid Biopsy Potential Biomarkers in PitNETs
2.1. Circulating Tumor Cells (CTCs)
2.2. Circulating Tumor DNA (ctDNA)
2.2.1. Genetic Alterations
2.2.2. Epigenetic Profile
2.3. Cell-Free RNA (cfRNA)
2.3.1. MicroRNA (miRNA)
2.3.2. Long Non-Coding RNA (lncRNA)
2.3.3. Circular RNA (circRNA)
2.4. Exosomes
3. Liquid Biopsy in Patients with Suspected PitNETs or in the Postoperative Follow-Up
4. Conclusions
Funding
Conflicts of Interest
References
- Dai, C.; Kang, J.; Liu, X.; Yao, Y.; Wang, H.; Wang, R. How to Classify and Define Pituitary Tumors: Recent Advances and Current Controversies. Front. Endocrinol. 2021, 12, 604644. [Google Scholar] [CrossRef]
- Raverot, G.; Ilie, M.D.; Lasolle, H.; Amodru, V.; Trouillas, J.; Castinetti, F.; Brue, T. Aggressive Pituitary Tumours and Pituitary Carcinomas. Nat. Rev. Endocrinol. 2021, 17, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, X. Molecular Network Basis of Invasive Pituitary Adenoma: A Review. Front. Endocrinol. 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, J.; Roy, P.; Sturm, N.; Dantony, E.; Cortet-Rudelli, C.; Viennet, G.; Bonneville, J.F.; Assaker, R.; Auger, C.; Brue, T.; et al. A New Prognostic Clinicopathological Classification of Pituitary Adenomas: A Multicentric Case-Control Study of 410 Patients with 8 Years Post-Operative Follow-Up. Acta Neuropathol. 2013, 126, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Burman, P.; McCormack, A.; Heaney, A.; Petersenn, S.; Popovic, V.; Trouillas, J.; Dekkers, O.M.; The European Society of Endocrinology. European Society of Endocrinology Clinical Practice Guidelines for the Management of Aggressive Pituitary Tumours and Carcinomas. Eur. J. Endocrinol. 2018, 178, G1–G24. [Google Scholar] [CrossRef]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and Definitions for Research on Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef]
- Faltermeier, C.M.; Magill, S.T.; Blevins, L.S.; Aghi, M.K. Molecular Biology of Pituitary Adenomas. Neurosurg. Clin. N. Am. 2019, 30, 391–400. [Google Scholar] [CrossRef]
- Mercado, M.; Melgar, V.; Salame, L.; Cuenca, D. Clinically Non-Functioning Pituitary Adenomas: Pathogenic, Diagnostic and Therapeutic Aspects. Endocrinol. Diabetes Nutr. 2017, 64, 384–395. [Google Scholar] [CrossRef]
- Di Ieva, A.; Rotondo, F.; Syro, L.V.; Cusimano, M.D.; Kovacs, K. Aggressive Pituitary Adenomas--Diagnosis and Emerging Treatments. Nat. Rev. Endocrinol. 2014, 10, 423–435. [Google Scholar] [CrossRef]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef]
- Trouillas, J.; Jaffrain-Rea, M.L.; Vasiljevic, A.; Raverot, G.; Roncaroli, F.; Villa, C. How to Classify the Pituitary Neuroendocrine Tumors (PitNET)s in 2020. Cancers 2020, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Vasiljevic, A.; Jaffrain-Rea, M.L.; Ansorge, O.; Asioli, S.; Barresi, V.; Chinezu, L.; Gardiman, M.P.; Lania, A.; Lapshina, A.M.; et al. A Standardised Diagnostic Approach to Pituitary Neuroendocrine Tumours (PitNETs): A European Pituitary Pathology Group (EPPG) Proposal. Virchows Arch. 2019, 475, 687–692. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- Sisodiya, S.; Kasherwal, V.; Khan, A.; Roy, B.; Goel, A.; Kumar, S.; Arif, N.; Tanwar, P.; Hussain, S. Liquid Biopsies: Emerging Role and Clinical Applications in Solid Tumours. Transl. Oncol. 2023, 35, 101716. [Google Scholar] [CrossRef] [PubMed]
- Corvigno, S.; Johnson, A.M.; Wong, K.K.; Cho, M.S.; Afshar-Kharghan, V.; Menter, D.G.; Sood, A.K. Novel Markers for Liquid Biopsies in Cancer Management: Circulating Platelets and Extracellular Vesicles. Mol. Cancer Ther. 2022, 21, 1067–1075. [Google Scholar] [CrossRef]
- Kurma, K.; Eslami-S, Z.; Alix-Panabières, C.; Cayrefourcq, L. Liquid Biopsy: Paving a New Avenue for Cancer Research. Cell Adhes. Migr. 2024, 18, 1–26. [Google Scholar] [CrossRef]
- Hua, G.; Yanjiao, H.; Qian, L.; Jichao, W.; Yazhuo, Z. Detection of Circulating Tumor Cells in Patients with Pituitary Tumors. BMC Cancer 2018, 18, 336. [Google Scholar] [CrossRef]
- Gossing, W.; Frohme, M.; Radke, L. Biomarkers for Liquid Biopsies of Pituitary Neuroendocrine Tumors. Biomedicines 2020, 8, 148. [Google Scholar] [CrossRef] [PubMed]
- de Miranda, F.S.; Barauna, V.G.; Dos Santos, L.; Costa, G.; Vassallo, P.F.; Campos, L.C.G. Properties and Application of Cell-Free DNA as a Clinical Biomarker. Int. J. Mol. Sci. 2021, 22, 9110. [Google Scholar] [CrossRef]
- Aucamp, J.; Bronkhorst, A.J.; Badenhorst, C.P.; Pretorius, P.J. A Historical and Evolutionary Perspective on the Biological Significance of Circulating DNA and Extracellular Vesicles. Cell Mol. Life Sci. 2016, 73, 4355–4381. [Google Scholar] [CrossRef]
- Bronkhorst, A.J.; Ungerer, V.; Holdenrieder, S. The Emerging Role of Cell-Free DNA as a Molecular Marker for Cancer Management. Biomol. Detect. Quantif. 2019, 17, 100087. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor Biomarkers for Diagnosis, Prognosis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef]
- Megnis, K.; Peculis, R.; Rovite, V.; Laksa, P.; Niedra, H.; Balcere, I.; Caune, O.; Breiksa, A.; Nazarovs, J.; Stukens, J.; et al. Evaluation of the Possibility to Detect Circulating Tumor DNA From Pituitary Adenoma. Front. Endocrinol. 2019, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Fontanilles, M.; Duran-Peña, A.; Idbaih, A. Liquid Biopsy in Primary Brain Tumors: Looking for Stardust! Curr. Neurol. Neurosci. Rep. 2018, 18, 13. [Google Scholar] [CrossRef]
- Stejskal, P.; Goodarzi, H.; Srovnal, J.; Hajdúch, M.; van ’t Veer, L.J.; Magbanua, M.J.M. Circulating Tumor Nucleic Acids: Biology, Release Mechanisms, and Clinical Relevance. Mol. Cancer 2023, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Marrero-Rodríguez, D.; Taniguchi-Ponciano, K.; Kerbel, J.; Cano-Zaragoza, A.; Remba-Shapiro, I.; Silva-Román, G.; Vela-Patiño, S.; Andonegui-Elguera, S.; Valenzuela-Perez, A.; Mercado, M. The Hallmarks of Cancer… in Pituitary Tumors? Rev. Endocr. Metab. Disord. 2023, 24, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Yang, C.; Bao, X.; Wang, R. Genetic and Epigenetic Causes of Pituitary Adenomas. Front. Endocrinol. 2020, 11, 596554. [Google Scholar] [CrossRef]
- Spada, A.; Mantovani, G.; Lania, A.G.; Treppiedi, D.; Mangili, F.; Catalano, R.; Carosi, G.; Sala, E.; Peverelli, E. Pituitary Tumors: Genetic and Molecular Factors Underlying Pathogenesis and Clinical Behavior. Neuroendocrinology 2022, 112, 15–33. [Google Scholar] [CrossRef]
- Vamvoukaki, R.; Chrysoulaki, M.; Betsi, G.; Xekouki, P. Pituitary Tumorigenesis-Implications for Management. Medicina 2023, 59, 812. [Google Scholar] [CrossRef]
- Peculis, R.; Niedra, H.; Rovite, V. Large Scale Molecular Studies of Pituitary Neuroendocrine Tumors: Novel Markers, Mechanisms and Translational Perspectives. Cancers 2021, 13, 1395. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, C.; Song, J.; Zhang, Y.; Chen, J.; Song, Z.; Shou, X.; Ma, Z.; Peng, H.; Jian, X.; et al. Germline Mutations in CDH23, Encoding Cadherin-Related 23, Are Associated with Both Familial and Sporadic Pituitary Adenomas. Am. J. Hum. Genet. 2017, 100, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, W.; Li, Z.; Wang, H.; Gao, H.; Zhang, Y. Impact of SLC20A1 on the Wnt/Β-catenin Signaling Pathway in Somatotroph Adenomas. Mol. Med. Rep. 2019, 20, 3276–3284. [Google Scholar] [CrossRef]
- Wei, D.; Yiyuan, C.; Qian, L.; Jianhua, L.; Yazhuo, Z.; Hua, G. The Absence of PRDM2 Involved the Tumorigenesis of Somatotroph Adenomas through Regulating C-Myc. Gene 2020, 737, 144456. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Moreno, E.; Vazquez-Borrego, M.C.; Dios, E.; Gros-Herguido, N.; Flores-Martinez, A.; Rivero-Cortés, E.; Madrazo-Atutxa, A.; Japón, M.A.; Luque, R.M.; Castaño, J.P.; et al. Association between Dopamine and Somatostatin Receptor Expression and Pharmacological Response to Somatostatin Analogues in Acromegaly. J. Cell Mol. Med. 2018, 22, 1640–1649. [Google Scholar] [CrossRef]
- Islam, M.T.; Chen, F.; Chen, H. The Oncogenic Role of Ubiquitin Specific Peptidase (USP8) and Its Signaling Pathways Targeting for Cancer Therapeutics. Arch. Biochem. Biophys. 2021, 701, 108811. [Google Scholar] [CrossRef] [PubMed]
- Castellnou, S.; Vasiljevic, A.; Lapras, V.; Raverot, V.; Alix, E.; Borson-Chazot, F.; Jouanneau, E.; Raverot, G.; Lasolle, H. SST5 Expression and USP8 Mutation in Functioning and Silent Corticotroph Pituitary Tumors. Endocr. Connect. 2020, 9, 243–253. [Google Scholar] [CrossRef]
- Chen, J.; Jian, X.; Deng, S.; Ma, Z.; Shou, X.; Shen, Y.; Zhang, Q.; Song, Z.; Li, Z.; Peng, H.; et al. Identification of Recurrent USP48 and BRAF Mutations in Cushing’s Disease. Nat. Commun. 2018, 9, 3171. [Google Scholar] [CrossRef]
- Perez-Rivas, L.G.; Simon, J.; Albani, A.; Tang, S.; Roeber, S.; Assié, G.; Deutschbein, T.; Fassnacht, M.; Gadelha, M.R.; Hermus, A.R.; et al. TP53 Mutations in Functional Corticotroph Tumors Are Linked to Invasion and Worse Clinical Outcome. Acta Neuropathol. Commun. 2022, 10, 139. [Google Scholar] [CrossRef]
- Li, C.; Xie, W.; Rosenblum, J.S.; Zhou, J.; Guo, J.; Miao, Y.; Shen, Y.; Wang, H.; Gong, L.; Li, M.; et al. Somatic SF3B1 Hotspot Mutation in Prolactinomas. Nat. Commun. 2020, 11, 2506. [Google Scholar] [CrossRef]
- Fedele, M.; Palmieri, D.; Fusco, A. HMGA2: A Pituitary Tumour Subtype-Specific Oncogene? Mol. Cell Endocrinol. 2010, 326, 19–24. [Google Scholar] [CrossRef]
- Portovedo, S.; Gaido, N.; de Almeida Nunes, B.; Nascimento, A.G.; Rocha, A.; Magalhães, M.; Nascimento, G.C.; Pires de Carvalho, D.; Soares, P.; Takiya, C.; et al. Differential Expression of HMGA1 and HMGA2 in Pituitary Neuroendocrine Tumors. Mol. Cell Endocrinol. 2019, 490, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Murat, C.B.; Braga, P.B.; Fortes, M.A.; Bronstein, M.D.; Corrêa-Giannella, M.L.; Giorgi, R.R. Mutation and Genomic Amplification of the PIK3CA Proto-Oncogene in Pituitary Adenomas. Braz. J. Med. Biol. Res. 2012, 45, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jiang, X.; Shen, Y.; Li, M.; Ma, H.; Xing, M.; Lu, Y. Frequent Mutations and Amplifications of the PIK3CA Gene in Pituitary Tumors. Endocr. Relat. Cancer 2009, 16, 301–310. [Google Scholar] [CrossRef]
- Lasolle, H.; Elsensohn, M.H.; Wierinckx, A.; Alix, E.; Bonnefille, C.; Vasiljevic, A.; Cortet, C.; Decoudier, B.; Sturm, N.; Gaillard, S.; et al. Chromosomal Instability in the Prediction of Pituitary Neuroendocrine Tumors Prognosis. Acta Neuropathol. Commun. 2020, 8, 190. [Google Scholar] [CrossRef]
- Møller, M.W.; Nortvig, M.J.; Andersen, M.S.; Poulsen, F.R. DNA Methylation in Pituitary Adenomas: A Scoping Review. Int. J. Mol. Sci. 2025, 26, 531. [Google Scholar] [CrossRef]
- Herrgott, G.A.; Asmaro, K.P.; Wells, M.; Sabedot, T.S.; Malta, T.M.; Mosella, M.S.; Nelson, K.; Scarpace, L.; Barnholtz-Sloan, J.S.; Sloan, A.E.; et al. Detection of Tumor-Specific DNA Methylation Markers in the Blood of Patients with Pituitary Neuroendocrine Tumors. Neuro Oncol. 2022, 24, 1126–1139. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.S.; Wang, E.L.; Xu, W.F.; Yamada, S.; Yoshimoto, K.; Qian, Z.R.; Shi, L.; Liu, L.L.; Li, X.H. Overexpression of DNA (Cytosine-5)-Methyltransferase 1 (DNMT1) And DNA (Cytosine-5)-Methyltransferase 3A (DNMT3A) Is Associated with Aggressive Behavior and Hypermethylation of Tumor Suppressor Genes in Human Pituitary Adenomas. Med. Sci. Monit. 2018, 24, 4841–4850. [Google Scholar] [CrossRef]
- Revill, K.; Dudley, K.J.; Clayton, R.N.; McNicol, A.M.; Farrell, W.E. Loss of Neuronatin Expression Is Associated with Promoter Hypermethylation in Pituitary Adenoma. Endocr. Relat. Cancer 2009, 16, 537–548. [Google Scholar] [CrossRef]
- Qian, Z.R.; Sano, T.; Yoshimoto, K.; Asa, S.L.; Yamada, S.; Mizusawa, N.; Kudo, E. Tumor-Specific Downregulation and Methylation of the CDH13 (H-Cadherin) and CDH1 (E-Cadherin) Genes Correlate with Aggressiveness of Human Pituitary Adenomas. Mod. Pathol. 2007, 20, 1269–1277. [Google Scholar] [CrossRef]
- Simpson, D.J.; Clayton, R.N.; Farrell, W.E. Preferential Loss of Death Associated Protein Kinase Expression in Invasive Pituitary Tumours Is Associated with Either CpG Island Methylation or Homozygous Deletion. Oncogene 2002, 21, 1217–1224. [Google Scholar] [CrossRef]
- Jotanovic, J.; Boldt, H.B.; Burton, M.; Andersen, M.S.; Bengtsson, D.; Bontell, T.O.; Ekman, B.; Engström, B.E.; Feldt-Rasmussen, U.; Heck, A.; et al. Genome-Wide Methylation Profiling Differentiates Benign from Aggressive and Metastatic Pituitary Neuroendocrine Tumors. Acta Neuropathol. 2024, 148, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, H.; Danila, D.C.; Johnson, S.R.; Zhou, Y.; Swearingen, B.; Klibanski, A. Loss of Expression of GADD45 Gamma, a Growth Inhibitory Gene, in Human Pituitary Adenomas: Implications for Tumorigenesis. J. Clin. Endocrinol. Metab. 2002, 87, 1262–1267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Righi, A.; Jin, L.; Zhang, S.; Stilling, G.; Scheithauer, B.W.; Kovacs, K.; Lloyd, R.V. Identification and Consequences of Galectin-3 Expression in Pituitary Tumors. Mol. Cell Endocrinol. 2010, 326, 8–14. [Google Scholar] [CrossRef]
- Qian, Z.R.; Sano, T.; Yoshimoto, K.; Yamada, S.; Ishizuka, A.; Mizusawa, N.; Horiguchi, H.; Hirokawa, M.; Asa, S.L. Inactivation of RASSF1A Tumor Suppressor Gene by Aberrant Promoter Hypermethylation in Human Pituitary Adenomas. Lab. Investig. 2005, 85, 464–473. [Google Scholar] [CrossRef]
- Araki, T.; Tone, Y.; Yamamoto, M.; Kameda, H.; Ben-Shlomo, A.; Yamada, S.; Takeshita, A.; Kawakami, Y.; Tone, M.; Melmed, S. Two Distinctive POMC Promoters Modify Gene Expression in Cushing Disease. J. Clin. Endocrinol. Metab. 2021, 106, e3346–e3363. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S. Epigenetic Control in Pituitary Tumors. Endocr. J. 2008, 55, 951–957. [Google Scholar] [CrossRef]
- Seemann, N.; Kuhn, D.; Wrocklage, C.; Keyvani, K.; Hackl, W.; Buchfelder, M.; Fahlbusch, R.; Paulus, W. CDKN2A/P16 Inactivation Is Related to Pituitary Adenoma Type and Size. J. Pathol. 2001, 193, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Pease, M.; Ling, C.; Mack, W.J.; Wang, K.; Zada, G. The Role of Epigenetic Modification in Tumorigenesis and Progression of Pituitary Adenomas: A Systematic Review of the Literature. PLoS ONE 2013, 8, e82619. [Google Scholar] [CrossRef]
- Feng, J.; Hong, L.; Wu, Y.; Li, C.; Wan, H.; Li, G.; Sun, Y.; Yu, S.; Chittiboina, P.; Montgomery, B.; et al. Identification of a Subtype-Specific ENC1 Gene Related to Invasiveness in Human Pituitary Null Cell Adenoma and Oncocytomas. J. Neurooncol. 2014, 119, 307–315. [Google Scholar] [CrossRef]
- Cheng, S.; Li, C.; Xie, W.; Miao, Y.; Guo, J.; Wang, J.; Zhang, Y. Integrated Analysis of DNA Methylation and mRNA Expression Profiles to Identify Key Genes Involved in the Regrowth of Clinically Non-Functioning Pituitary Adenoma. Aging 2020, 12, 2408–2427. [Google Scholar] [CrossRef]
- Crick, F. Central Dogma of Molecular Biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Poliseno, L.; Lanza, M.; Pandolfi, P.P. Coding, or Non-Coding, That Is the Question. Cell Res. 2024, 34, 609–629. [Google Scholar] [CrossRef]
- Wu, W.; Cao, L.; Jia, Y.; Xiao, Y.; Zhang, X.; Gui, S. Emerging Roles of miRNA, lncRNA, circRNA, and Their Cross-Talk in Pituitary Adenoma. Cells 2022, 11, 2920. [Google Scholar] [CrossRef]
- Donati, S.; Aurilia, C.; Palmini, G.; Miglietta, F.; Falsetti, I.; Iantomasi, T.; Brandi, M.L. MicroRNAs as Potential Biomarkers in Pituitary Adenomas. Noncoding RNA 2021, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Zumwalt, T.J.; Goel, A. Non-Coding RNAs and Potential Therapeutic Targeting in Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sun, C.; Zhao, Y.; Wang, Q.; Guo, J.; Ye, B.; Yu, G. Overview of MicroRNAs as Diagnostic and Prognostic Biomarkers for High-Incidence Cancers in 2021. Int. J. Mol. Sci. 2022, 23, 11389. [Google Scholar] [CrossRef]
- El-Daly, S.M.; Gouhar, S.A.; Abd Elmageed, Z.Y. Circulating microRNAs as Reliable Tumor Biomarkers: Opportunities and Challenges Facing Clinical Application. J. Pharmacol. Exp. Ther. 2023, 384, 35–51. [Google Scholar] [CrossRef]
- Clayton, R.N.; Farrell, W.E. Pituitary Tumour Clonality Revisited. Front. Horm. Res. 2004, 32, 186–204. [Google Scholar] [CrossRef]
- Bottoni, A.; Piccin, D.; Tagliati, F.; Luchin, A.; Zatelli, M.C.; degli Uberti, E.C. miR-15a and miR-16-1 down-Regulation in Pituitary Adenomas. J. Cell Physiol. 2005, 204, 280–285. [Google Scholar] [CrossRef]
- Alnaaim, S.A.; Al-Kuraishy, H.M.; Zailaie, M.M.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E. The Potential Link between Acromegaly and Risk of Acute Ischemic Stroke in Patients with Pituitary Adenoma: A New Perspective. Acta Neurol. Belg. 2024, 124, 755–766. [Google Scholar] [CrossRef]
- Xiong, Y.; Tang, Y.; Fan, F.; Zeng, Y.; Li, C.; Zhou, G.; Hu, Z.; Zhang, L.; Liu, Z. Exosomal Hsa-miR-21-5p Derived from Growth Hormone-Secreting Pituitary Adenoma Promotes Abnormal Bone Formation in Acromegaly. Transl. Res. 2020, 215, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Belaya, Z.; Grebennikova, T.; Melnichenko, G.; Nikitin, A.; Solodovnikov, A.; Brovkina, O.; Grigoriev, A.; Rozhinskaya, L.; Lutsenko, A.; Dedov, I. Effects of Active Acromegaly on Bone mRNA and microRNA Expression Patterns. Eur. J. Endocrinol. 2018, 178, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, H.; Hekimler Öztürk, K.; Torus, B. Circulating miR-29c-3p Is Downregulated in Patients with Acromegaly. Turk. J. Med. Sci. 2021, 51, 2081–2086. [Google Scholar] [CrossRef]
- Zhao, S.; Li, J.; Feng, J.; Li, Z.; Liu, Q.; Lv, P.; Wang, F.; Gao, H.; Zhang, Y. Identification of Serum miRNA-423-5p Expression Signature in Somatotroph Adenomas. Int. J. Endocrinol. 2019, 2019, 8516858. [Google Scholar] [CrossRef]
- Bogner, E.M.; Daly, A.F.; Gulde, S.; Karhu, A.; Irmler, M.; Beckers, J.; Mohr, H.; Beckers, A.; Pellegata, N.S. miR-34a Is Upregulated in AIP-Mutated Somatotropinomas and Promotes Octreotide Resistance. Int. J. Cancer 2020, 147, 3523–3538. [Google Scholar] [CrossRef]
- Daly, A.F.; Tichomirowa, M.A.; Petrossians, P.; Heliövaara, E.; Jaffrain-Rea, M.L.; Barlier, A.; Naves, L.A.; Ebeling, T.; Karhu, A.; Raappana, A.; et al. Clinical Characteristics and Therapeutic Responses in Patients with Germ-Line AIP Mutations and Pituitary Adenomas: An International Collaborative Study. J. Clin. Endocrinol. Metab. 2010, 95, E373–E383. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Jing, G.; Jichao, W.; Xiaohui, L.; Fang, Q.; Hua, G.; Yazhou, M.; Zhang, Y. MiR-137’s Tumor Suppression on Prolactinomas by Targeting MITF and Modulating Wnt Signaling Pathway. J. Clin. Endocrinol. Metab. 2019, 104, 6391–6402. [Google Scholar] [CrossRef]
- Zhao, P.; Cheng, J.; Li, B.; Nie, D.; Li, C.; Gui, S.; Wang, H.; Zhang, Y. Up-Regulation of the Expressions of MiR-149-5p and MiR-99a-3p in Exosome Inhibits the Progress of Pituitary Adenomas. Cell Biol. Toxicol. 2021, 37, 633–651. [Google Scholar] [CrossRef]
- Rad, S.G.; Orang, F.N.; Shadbad, M.A. MicroRNA Networks in Prolactinoma Tumorigenesis: A Scoping Review. Cancer Cell Int. 2024, 24, 418. [Google Scholar] [CrossRef]
- Wu, Z.; Zheng, Y.; Xie, W.; Li, Q.; Zhang, Y.; Ren, B.; Cai, L.; Cheng, Y.; Tang, H.; Su, Z.; et al. The Long Noncoding RNA-H19/miRNA-93a/ATG7 Axis Regulates the Sensitivity of Pituitary Adenomas to Dopamine Agonists. Mol. Cell Endocrinol. 2020, 518, 111033. [Google Scholar] [CrossRef]
- Belaya, Z.; Khandaeva, P.; Nonn, L.; Nikitin, A.; Solodovnikov, A.; Sitkin, I.; Grigoriev, A.; Pikunov, M.; Lapshina, A.; Rozhinskaya, L.; et al. Circulating Plasma microRNA to Differentiate Cushing’s Disease From Ectopic ACTH Syndrome. Front. Endocrinol. 2020, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Gu, C.; Yang, Y.; Xue, J.; Sun, Y.; Jian, F.; Chen, D.; Bian, L.; Sun, Q. TSP-1 Is Downregulated and Inversely Correlates with miR-449c Expression in Cushing’s Disease. J. Cell Mol. Med. 2019, 23, 4097–4110. [Google Scholar] [CrossRef]
- Perge, P.; Decmann, Á.; Pezzani, R.; Bancos, I.; Fassina, A.; Luconi, M.; Canu, L.; Tóth, M.; Boscaro, M.; Patócs, A.; et al. Analysis of Circulating Extracellular Vesicle-Associated microRNAs in Cortisol-Producing Adrenocortical Tumors. Endocrine 2018, 59, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Yang, J.; Wang, G.; Wang, C.; Zhang, H. Bioinformatic Analysis of Gene Expression Profiles of Pituitary Gonadotroph Adenomas. Oncol. Lett. 2018, 15, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.T.; Lee, I.N.; Huang, C.; Huang, H.C.; Wu, Y.P.; Chong, Z.Y.; Chen, J.C. ADAM17 Confers Temozolomide Resistance in Human Glioblastoma Cells and miR-145 Regulates Its Expression. Int. J. Mol. Sci. 2023, 24, 7703. [Google Scholar] [CrossRef]
- Whitelaw, B.C. How and When to Use Temozolomide to Treat Aggressive Pituitary Tumours. Endocr. Relat. Cancer 2019, 26, R545–R552. [Google Scholar] [CrossRef] [PubMed]
- Beylerli, O.; Beeraka, N.M.; Gareev, I.; Pavlov, V.; Yang, G.; Liang, Y.; Aliev, G. MiRNAs as Noninvasive Biomarkers and Therapeutic Agents of Pituitary Adenomas. Int. J. Mol. Sci. 2020, 21, 7287. [Google Scholar] [CrossRef]
- Németh, K.; Darvasi, O.; Likó, I.; Szücs, N.; Czirják, S.; Reiniger, L.; Szabó, B.; Krokker, L.; Pállinger, É.; Igaz, P.; et al. Comprehensive Analysis of Circulating miRNAs in the Plasma of Patients With Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2019, 104, 4151–4168. [Google Scholar] [CrossRef]
- Boresowicz, J.; Kober, P.; Rusetska, N.; Maksymowicz, M.; Paziewska, A.; Dąbrowska, M.; Zeber-Lubecka, N.; Kunicki, J.; Bonicki, W.; Ostrowski, J.; et al. The Search of miRNA Related to Invasive Growth of Nonfunctioning Gonadotropic Pituitary Tumors. Int. J. Endocrinol. 2020, 2020, 3730657. [Google Scholar] [CrossRef]
- Duan, J.; Lu, G.; Li, Y.; Zhou, S.; Zhou, D.; Tao, H. miR-137 Functions as a Tumor Suppressor Gene in Pituitary Adenoma by Targeting AKT2. Int. J.Clin. Exp. Pathol. 2019, 12, 1557–1564. [Google Scholar]
- Elston, M.S.; Gill, A.J.; Conaglen, J.V.; Clarkson, A.; Shaw, J.M.; Law, A.J.; Cook, R.J.; Little, N.S.; Clifton-Bligh, R.J.; Robinson, B.G.; et al. Wnt Pathway Inhibitors Are Strongly Down-Regulated in Pituitary Tumors. Endocrinology 2008, 149, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Qian, L.; Jing, G.; Jie, F.; Xiaosong, S.; Chunhui, L.; Yangfang, L.; Guilin, L.; Gao, H.; Yazhuo, Z. Aberrant Expression of the sFRP and WIF1 Genes in Invasive Non-Functioning Pituitary Adenomas. Mol. Cell Endocrinol. 2018, 474, 168–175. [Google Scholar] [CrossRef]
- Du, Q.; Hu, B.; Feng, Y.; Wang, Z.; Wang, X.; Zhu, D.; Zhu, Y.; Jiang, X.; Wang, H. circOMA1-Mediated miR-145-5p Suppresses Tumor Growth of Nonfunctioning Pituitary Adenomas by Targeting TPT1. J. Clin. Endocrinol. Metab. 2019, 104, 2419–2434. [Google Scholar] [CrossRef] [PubMed]
- Gilyazova, I.; Asadullina, D.; Kagirova, E.; Sikka, R.; Mustafin, A.; Ivanova, E.; Bakhtiyarova, K.; Gilyazova, G.; Gupta, S.; Khusnutdinova, E.; et al. MiRNA-146a—A Key Player in Immunity and Diseases. Int. J. Mol. Sci. 2023, 24, 12767. [Google Scholar] [CrossRef]
- Zacharjasz, J.; Sztachera, M.; Smuszkiewicz, M.; Piwecka, M. Micromanaging the Neuroendocrine System—A Review on miR-7 and the Other Physiologically Relevant miRNAs in the Hypothalamic-Pituitary Axis. FEBS Lett. 2024, 598, 1557–1575. [Google Scholar] [CrossRef]
- Niedra, H.; Peculis, R.; Litvina, H.D.; Megnis, K.; Mandrika, I.; Balcere, I.; Romanovs, M.; Steina, L.; Stukens, J.; Breiksa, A.; et al. Genome Wide Analysis of Circulating miRNAs in Growth Hormone Secreting Pituitary Neuroendocrine Tumor Patients’ Plasma. Front. Oncol. 2022, 12, 894317. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Imani, S.; Wu, M.Y.; Wu, R.C. MicroRNA-34 Family in Cancers: Role, Mechanism, and Therapeutic Potential. Cancers 2023, 15, 4723. [Google Scholar] [CrossRef]
- Liang, S.; Chen, L.; Huang, H.; Zhi, D. The Experimental Study of miRNA in Pituitary Adenomas. Turk. Neurosurg. 2013, 23, 721–727. [Google Scholar] [CrossRef]
- Li, X.H.; Wang, E.L.; Zhou, H.M.; Yoshimoto, K.; Qian, Z.R. MicroRNAs in Human Pituitary Adenomas. Int. J. Endocrinol. 2014, 2014, 435171. [Google Scholar] [CrossRef]
- Butz, H.; Likó, I.; Czirják, S.; Igaz, P.; Khan, M.M.; Zivkovic, V.; Bálint, K.; Korbonits, M.; Rácz, K.; Patócs, A. Down-Regulation of Wee1 Kinase by a Specific Subset of microRNA in Human Sporadic Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2010, 95, E181–E191. [Google Scholar] [CrossRef]
- García-Martínez, A.; Fuentes-Fayos, A.C.; Fajardo, C.; Lamas, C.; Cámara, R.; López-Muñoz, B.; Aranda, I.; Luque, R.M.; Picó, A. Differential Expression of MicroRNAs in Silent and Functioning Corticotroph Tumors. J. Clin. Med. 2020, 9, 1838. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.W.; Li, Y.L.; Hou, Y.J.; Xu, Z.D.; Li, Y.Z.; Chang, J.Y. MicroRNA-543 Promotes Cell Invasion and Impedes Apoptosis in Pituitary Adenoma via Activating the Wnt/β-Catenin Pathway by Negative Regulation of Smad7. Biosci. Biotechnol. Biochem. 2019, 83, 1035–1044. [Google Scholar] [CrossRef]
- Gutschner, T.; Diederichs, S. The Hallmarks of Cancer: A Long Non-Coding RNA Point of View. RNA Biol. 2012, 9, 703–719. [Google Scholar] [CrossRef]
- George, T.P.; Subramanian, S.; Supriya, M.H. A Brief Review of Noncoding RNA. Egypt. J. Med. Hum. Genet. 2024, 25, 98. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Qi, Z.; Huang, C.; Zhang, N.; Zhang, W.; Li, Y.; Qiu, M.; Fang, Q.; Hui, G. Genome-Wide Identification of lncRNAs and mRNAs Differentially Expressed in Non-Functioning Pituitary Adenoma and Construction of an lncRNA-mRNA Co-Expression Network. Biol. Open 2019, 8, bio037127. [Google Scholar] [CrossRef]
- Mezzomo, L.C.; Gonzales, P.H.; Pesce, F.G.; Kretzmann Filho, N.; Ferreira, N.P.; Oliveira, M.C.; Kohek, M.B. Expression of Cell Growth Negative Regulators MEG3 and GADD45γ Is Lost in Most Sporadic Human Pituitary Adenomas. Pituitary 2012, 15, 420–427. [Google Scholar] [CrossRef]
- Gejman, R.; Batista, D.L.; Zhong, Y.; Zhou, Y.; Zhang, X.; Swearingen, B.; Stratakis, C.A.; Hedley-Whyte, E.T.; Klibanski, A. Selective Loss of MEG3 Expression and Intergenic Differentially Methylated Region Hypermethylation in the MEG3/DLK1 Locus in Human Clinically Nonfunctioning Pituitary Adenomas. J. Clin. Endocrinol. Metab. 2008, 93, 4119–4125. [Google Scholar] [CrossRef]
- Zhao, J.; Dahle, D.; Zhou, Y.; Zhang, X.; Klibanski, A. Hypermethylation of the Promoter Region Is Associated with the Loss of MEG3 Gene Expression in Human Pituitary Tumors. J. Clin. Endocrinol. Metab. 2005, 90, 2179–2186. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Liu, C.; Yu, S.; Zhang, Y. Expression of the Long Non-Coding RNAs MEG3, HOTAIR, and MALAT-1 in Non-Functioning Pituitary Adenomas and Their Relationship to Tumor Behavior. Pituitary 2015, 18, 42–47. [Google Scholar] [CrossRef]
- Lu, T.; Yu, C.; Ni, H.; Liang, W.; Yan, H.; Jin, W. Expression of the Long Non-Coding RNA H19 and MALAT-1 in Growth Hormone-Secreting Pituitary Adenomas and Its Relationship to Tumor Behavior. Int. J. Dev. Neurosci. 2018, 67, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.R.; Yan, L.; Liu, Y.T.; Cao, L.; Guo, Y.H.; Zhang, Y.; Yao, H.; Cai, L.; Shang, H.B.; Rui, W.W.; et al. Inhibition of mTORC1 by lncRNA H19 via Disrupting 4E-BP1/Raptor Interaction in Pituitary Tumours. Nat. Commun. 2018, 9, 4624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.T.; Tang, H.; Xie, W.Q.; Yao, H.; Gu, W.T.; Zheng, Y.Z.; Shang, H.B.; Wang, Y.; Wei, Y.X.; et al. Exosome-Transmitted lncRNA H19 Inhibits the Growth of Pituitary Adenoma. J. Clin. Endocrinol. Metab. 2019, 104, 6345–6356. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, D.; Mussnich, P.; Sepe, R.; Raia, M.; Del Vecchio, L.; Cappabianca, P.; Pellecchia, S.; Petrosino, S.; Saggio, S.; Solari, D.; et al. RPSAP52 lncRNA Is Overexpressed in Pituitary Tumors and Promotes Cell Proliferation by Acting as miRNA Sponge for HMGA Proteins. J. Mol. Med. 2019, 97, 1019–1032. [Google Scholar] [CrossRef]
- Wang, C.; Tan, C.; Wen, Y.; Zhang, D.; Li, G.; Chang, L.; Su, J.; Wang, X. FOXP1-Induced lncRNA CLRN1-AS1 Acts as a Tumor Suppressor in Pituitary Prolactinoma by Repressing the Autophagy via Inactivating Wnt/β-Catenin Signaling Pathway. Cell Death Dis. 2019, 10, 499. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Abbasi, F.; Nicknam, A.; Hussen, B.M.; Eslami, S.; Akbari Dilmaghani, N.; Taheri, M.; Sharifi, G. Dysregulation of PVT1 and NEAT1 lncRNAs in Pituitary Adenomas. Pathol. Res. Pract. 2023, 248, 154573. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, Y.; Cui, H. Long Noncoding RNA CCAT2 Is Activated by E2F1 and Exerts Oncogenic Properties by Interacting with PTTG1 in Pituitary Adenomas. Am. J. Cancer Res. 2018, 8, 245–255. [Google Scholar]
- Sanchez, A.; Lhuillier, J.; Grosjean, G.; Ayadi, L.; Maenner, S. The Long Non-Coding RNA ANRIL in Cancers. Cancers 2023, 15, 4160. [Google Scholar] [CrossRef]
- Beylerli, O.; Khasanov, D.; Gareev, I.; Valitov, E.; Sokhatskii, A.; Wang, C.; Pavlov, V.; Khasanova, G.; Ahmad, A. Differential Non-Coding RNAs Expression Profiles of Invasive and Non-Invasive Pituitary Adenomas. Noncoding RNA Res. 2021, 6, 115–122. [Google Scholar] [CrossRef]
- Li, J.; Qian, Y.; Zhang, C.; Wang, W.; Qiao, Y.; Song, H.; Li, L.; Guo, J.; Lu, D.; Deng, X. LncRNA LINC00473 Is Involved in the Progression of Invasive Pituitary Adenoma by Upregulating KMT5A via ceRNA-Mediated miR-502-3p Evasion. Cell Death Dis. 2021, 12, 580. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, F.; Fan, H.; Wang, H.; Wang, Q.; Yang, J.; Song, T. Long Non-Coding RNA TUG1/microRNA-187-3p/TESC Axis Modulates Progression of Pituitary Adenoma via Regulating the NF-κB Signaling Pathway. Cell Death Dis. 2021, 12, 524. [Google Scholar] [CrossRef]
- Lu, G.; Duan, J.; Zhou, D. Long-Noncoding RNA IFNG-AS1 Exerts Oncogenic Properties by Interacting with Epithelial Splicing Regulatory Protein 2 (ESRP2) in Pituitary Adenomas. Pathol. Res. Pract. 2018, 214, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, C.; Xie, W.; Wang, Z.; Gao, H.; Cao, L.; Hao, L.; Zhang, Y. Long Non-Coding RNA C5orf66-AS1 is Downregulated in Pituitary Null Cell Adenomas and Is Associated with Their Invasiveness. Oncol. Rep. 2017, 38, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Butz, H. Circulating Noncoding RNAs in Pituitary Neuroendocrine Tumors-Two Sides of the Same Coin. Int. J. Mol. Sci. 2022, 23, 5122. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Chen, L.L. The Biogenesis, Functions, and Challenges of Circular RNAs. Mol. Cell 2018, 71, 428–442. [Google Scholar] [CrossRef]
- Rybak-Wolf, A.; Stottmeister, C.; Glažar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef]
- Du, Q.; Zhang, W.; Feng, Q.; Hao, B.; Cheng, C.; Cheng, Y.; Li, Y.; Fan, X.; Chen, Z. Comprehensive Circular RNA Profiling Reveals That Hsa_circ_0001368 Is Involved in Growth Hormone-Secreting Pituitary Adenoma Development. Brain Res. Bull. 2020, 161, 65–77. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.; Du, Q.; Bian, P.; Chen, Y.; Liu, Z.; Zheng, J.; Sai, K.; Mou, Y.; Chen, Z.; et al. CircVPS13C Promotes Pituitary Adenoma Growth by Decreasing the Stability of IFITM1 mRNA via Interacting with RRBP1. Oncogene 2022, 41, 1550–1562. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Miao, Y.; Shen, Y.; Li, M.; Gong, L.; Wang, H.; He, Y.; Gao, H.; Liu, Q.; et al. A two-circRNA Signature Predicts Tumour Recurrence in Clinical Non-functioning Pituitary Adenoma. Oncol. Rep. 2019, 41, 113–124. [Google Scholar] [CrossRef]
- Wang, J.; Wang, D.; Wan, D.; Ma, Q.; Liu, Q.; Li, J.; Li, Z.; Gao, Y.; Jiang, G.; Ma, L.; et al. Circular RNA In Invasive and Recurrent Clinical Nonfunctioning Pituitary Adenomas: Expression Profiles and Bioinformatic Analysis. World Neurosurg. 2018, 117, e371–e386. [Google Scholar] [CrossRef]
- Hu, T.Y.; Zhu, Q.X.; Duan, Q.Y.; Jin, X.Y.; Wu, R. CircABCB10 Promotes the Proliferation and Migration of Lung Cancer Cells through Down-Regulating microRNA-217 Expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6157–6165. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, N.; Zhang, S.; Zhou, P.; Lv, L.; Richard, S.A.; Ma, W.; Chen, C.; Wang, X.; Huang, S.; et al. Differential Circular RNA Expression Profiles of Invasive and Non-Invasive Non-Functioning Pituitary Adenomas: A Microarray Analysis. Med. Baltim. 2019, 98, e16148. [Google Scholar] [CrossRef]
- Lisiewicz, P.; Szelachowska, M.; Krętowski, A.J.; Siewko, K. The Prospective Roles of Exosomes in Pituitary Tumors. Front. Endocrinol. 2024, 15, 1482756. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, X.; Liu, C.; Zhao, Y. Extracellular Vesicles in Cancers: Mechanisms, Biomarkers, and Therapeutic Strategies. MedComm 2024, 5, e70009. [Google Scholar] [CrossRef]
- Yu, S.; Wang, X.S.; Cao, K.C.; Bao, X.J.; Yu, J. Identification of CDK6 and RHOU in Serum Exosome as Biomarkers for the Invasiveness of Non-Functioning Pituitary Adenoma. Chin. Med. Sci. J. 2019, 34, 168–176. [Google Scholar] [CrossRef]
- Lyu, L.; Li, H.; Chen, C.; Yu, Y.; Wang, L.; Yin, S.; Hu, Y.; Jiang, S.; Ye, F.; Zhou, P. Exosomal miRNA Profiling Is a Potential Screening Route for Non-Functional Pituitary Adenoma. Front. Cell Dev. Biol. 2021, 9, 771354. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhu, D.; Li, W.; Zhang, G.; Zhang, H.; Peng, Q. Exosomal AFAP1-AS1 Promotes the Growth, Metastasis, and Glycolysis of Pituitary Adenoma by Inhibiting HuR Degradation. Mol. Neurobiol. 2025, 62, 2212–2229. [Google Scholar] [CrossRef]
- Wan, H.; Gao, X.; Yang, Z.; Wei, L.; Qu, Y.; Liu, Q. Exosomal CircMFN2 Enhances the Progression of Pituitary Adenoma via the MiR-146a-3p/TRAF6/NF-κB Pathway. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2025, 86, 135–147. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.; Kang, X.; Liu, P.; Qian, L.; Shi, Y.; Osman, R.A.; Yang, Z.; Zhang, G. EMT-Related Markers in Serum Exosomes Are Potential Diagnostic Biomarkers for Invasive Pituitary Adenomas. Neuropsychiatr. Dis. Treat. 2021, 17, 3769–3780. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Bao, X.; Feng, M.; Xing, B.; Lian, W.; Yao, Y.; Wang, R. Diagnosis of Invasive Non-Functional Pituitary Adenomas Using Exosomal Biomarkers. Clin. Chim. Acta 2022, 529, 25–33. [Google Scholar] [CrossRef]
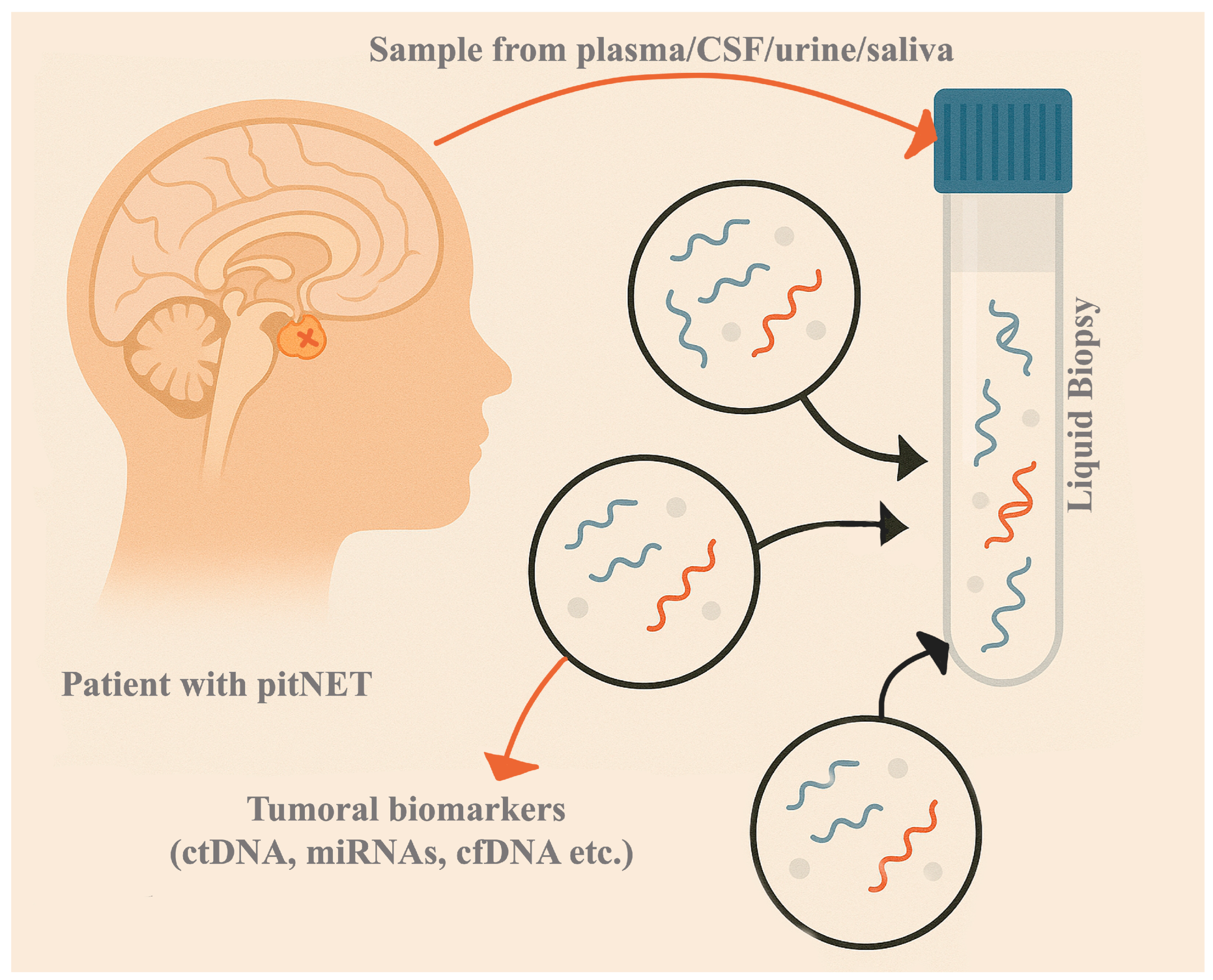
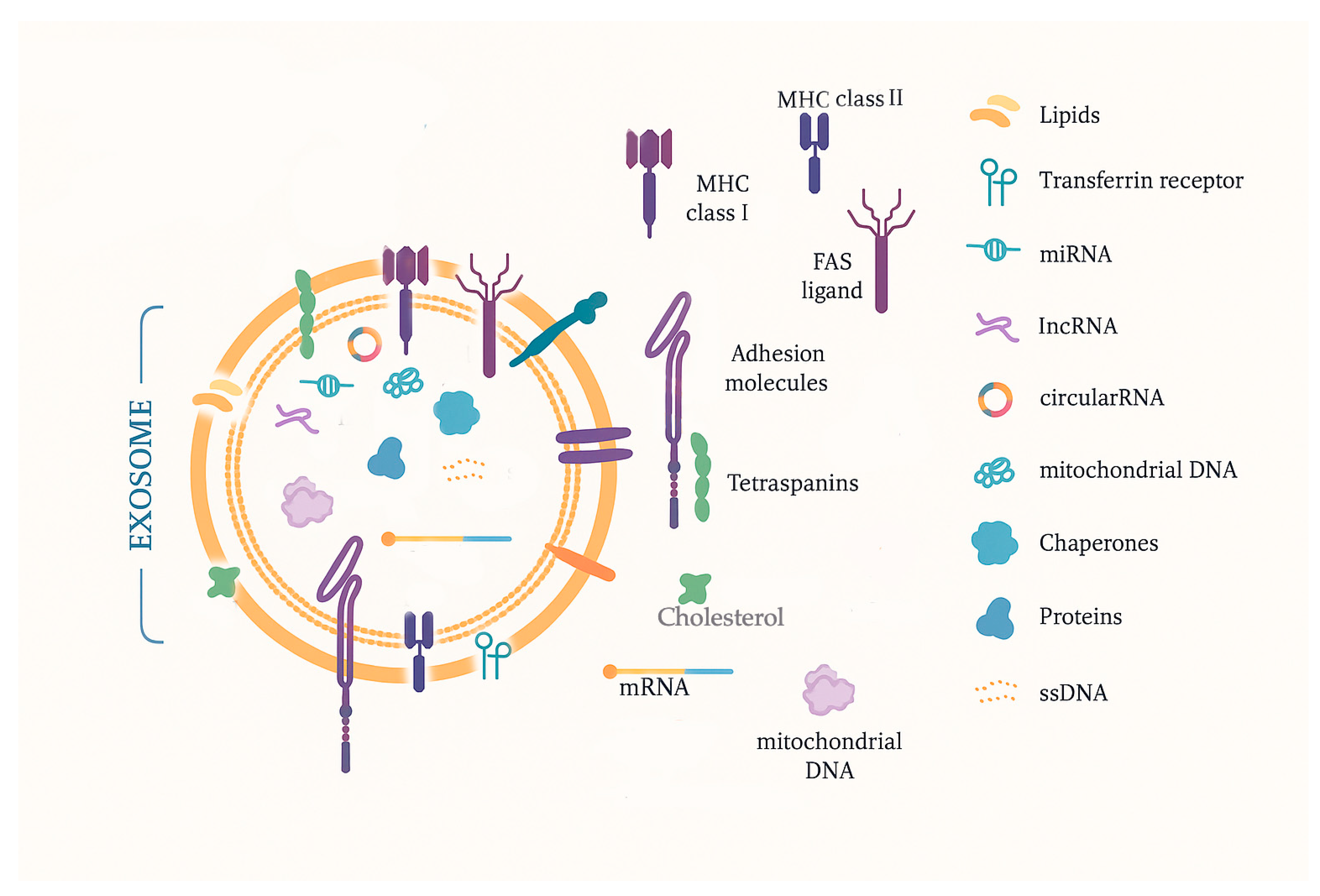
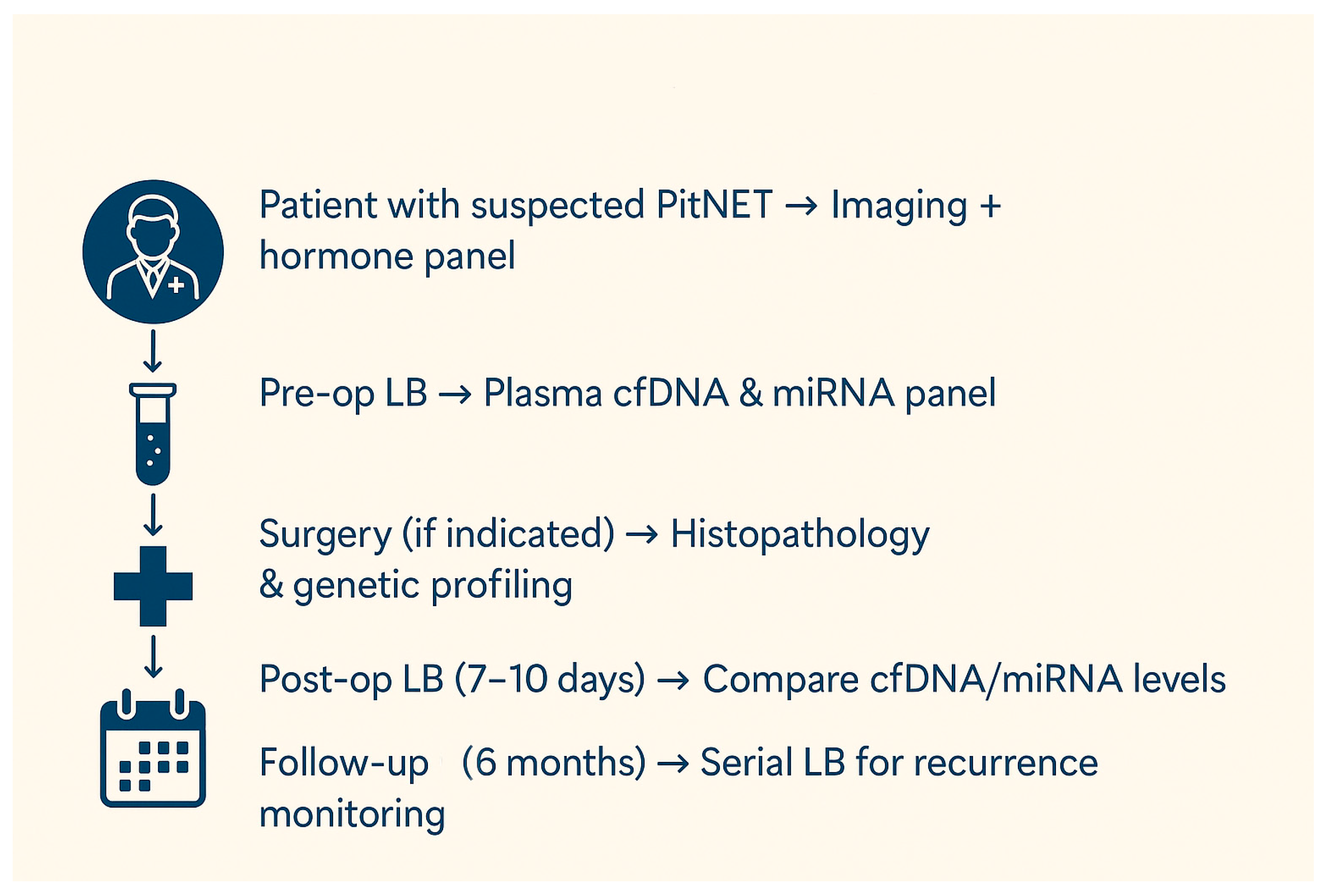
| Biomarker | General Characteristics | Diagnostic Advantages | Diagnostic Limitations | Prognostic Advantages | Prognostic Limitations | Therapeutic Potential | Therapeutic Challenges |
|---|---|---|---|---|---|---|---|
| CTCs | Rare in PitNETs; detached tumor cells in blood; difficult to isolate due to EMT. | Potential early biomarker; detectable even in benign tumors. | Extremely rare; phenotypic changes impair detection. | May indicate metastatic potential if found. | Unclear utility; more studies needed. | Theoretically enables monitoring response to treatment. | No established protocols; research phase. |
| ctDNA | Short half-life; reflects tumor’s genetic/epigenetic profile; allows real-time monitoring. | Allows non-invasive genetic profiling; dynamic tracking. | Low concentration in benign tumors; technical challenges in detection. | Reflects tumor burden and heterogeneity; correlates with progression. | Limited clinical data; interpretation variability. | Changes post-treatment can indicate therapy efficacy. | Not validated in PitNET clinical practice. |
| cfRNA | Includes coding and non-coding RNAs (miRNA, lncRNA, circRNA); high stability in fluids. | Highly stable; specific expression profiles in subtypes. | Complex processing; not yet standard in clinical use. | Subtype-specific expression; potential to indicate aggression. | Role in prognosis still under investigation. | Can reveal resistance mechanisms (e.g., miR-93 in cabergoline resistance). | Many targets not yet clinically actionable. |
| Exosomes | Extracellular vesicles; carry nucleic acids, proteins; reflect tumor status and origin. | Easy to isolate; rich in specific biomarkers (e.g., miR, mRNA). | Requires advanced isolation methods; technical complexity. | Altered profiles (e.g., miR-486-5p) associated with recurrence risk. | Lack of standardization; needs validation. | Therapeutic targets (e.g., exosomal miR, mRNA) identified in resistant tumors. | Experimental stage; more validation required. |
| Genetic Alteration/Syndrome | Clinicopathological Characteristics |
|---|---|
| MEN1 mutation (MEN1 syndrome) | Associated with functional tumors; more invasive, aggressive, drug-resistant. |
| CDKN1B mutation (MEN4 syndrome) | MEN1-like phenotype; loss of cell cycle control, aggressive tumor behavior. |
| PRKAR1A mutation (Carney Complex) | Leads to GH-secreting tumors; linked to cAMP pathway activation and somatotroph tumorigenesis. |
| SDHx/SDHAF2 mutations (3PAs syndrome) | Commonly PRL/GH-secreting or NF-PitNETs; aggressive with poor therapeutic response. |
| DICER1 mutation (DICER1 syndrome) | Leads to rare tumors like pituitary blastoma in infancy; high aggressiveness. |
| GPR101 duplication (X-LAG syndrome) | Early-onset GH/PRL-secreting tumors or hyperplasia; poor response to therapy. |
| AIP mutation (FIPA syndrome) | Aggressive GH-secreting tumors; treatment-resistant; early-onset. |
| GNAS mutation | Somatotroph tumors; increased cAMP and GH; smaller, less invasive, better SSA (somatostatin analogue) response. |
| PTTG1 overexpression | Associated with increased proliferation and aggressiveness in various subtypes. |
| STAT3 overexpression | GH hypersecretion; treatment resistance; aggressive behavior. |
| CDH23 mutation | Somatotroph tumors; impaired adhesion; Wnt pathway involvement. |
| SLC20A1 overexpression | Correlates with tumor size, recurrence, invasiveness in GH tumors. |
| PRDM2 loss | Loss linked to GH tumorigenesis; affects c-Myc regulation. |
| USP8 mutation | Corticotroph tumors; smaller size, higher ACTH; increased recurrence but better surgical outcome. |
| USP48 mutation | Increases ACTH via NF-κB pathway; linked to corticotroph tumor progression. |
| BRAF mutation | Activates POMC transcription; corticotroph tumors; ACTH overproduction. |
| TP53 mutation | Found in aggressive corticotroph tumors; poor prognosis. |
| SF3B1 mutation | Prolactinomas; aberrant splicing affecting estrogen signaling; tumorigenic. |
| HMGA1/HMGA2 overexpression | Overexpression in invasive GH and PRL tumors; chromatin regulation. |
| PIK3CA mutation | Promotes invasiveness; activates PI3K/Akt pathway in various PitNET subtypes. |
| Genetic Alteration/Syndrome | Associated PitNET Subtype | Clinicopathological Characteristics |
|---|---|---|
| GNAS mutation | Somatotroph | Increased cAMP and GH; smaller, less invasive; better response to SSAs. |
| PTTG1 overexpression | Somatotroph | Linked to proliferation; aggressive tumors. |
| STAT3 overexpression | Somatotroph | Leads to GH hypersecretion and therapy resistance. |
| CDH23 mutation | Somatotroph | Impaired cell adhesion; Wnt pathway deregulation. |
| SLC20A1 overexpression | Somatotroph | Correlated with tumor size, invasiveness, and recurrence. |
| PRDM2 loss | Somatotroph | Loss affects c-Myc regulation; involved in tumorigenesis. |
| USP8 mutation | Corticotroph | ACTH excess; better surgical remission but higher recurrence. |
| USP48 mutation | Corticotroph | Promotes ACTH via NF-κB; progressive corticotroph tumors. |
| BRAF mutation | Corticotroph | Activates ACTH transcription; associated with corticotroph tumors. |
| TP53 mutation | Corticotroph | Linked with poor outcomes; aggressive corticotroph tumors. |
| SF3B1 mutation | Prolactinoma | Aberrant splicing; drives estrogen pathway in prolactinomas. |
| MEN1 mutation | Various (familial) | Aggressive, drug-resistant tumors in familial cases. |
| CDKN1B mutation | Various (familial) | Loss of cell cycle control; MEN1-like phenotype. |
| PRKAR1A mutation | GH-secreting | Activates cAMP pathway; GH-producing tumors. |
| SDHx/SDHAF2 mutations | PRL/GH-secreting or NF-PitNET | Aggressive, treatment-resistant; PRL or GH tumors. |
| DICER1 mutation | Pituitary blastoma | Rare aggressive tumor in infants; pituitary blastoma. |
| GPR101 duplication | GH/PRL-secreting or hyperplasia | Early-onset GH/PRL tumors; poor therapeutic response. |
| AIP mutation | GH-secreting | Early-onset, aggressive GH tumors; SSA resistance. |
| PIK3CA mutation | Multiple (ACTH, PRL, NF) | Increased invasiveness via PI3K/Akt; multiple subtypes. |
| HMGA1/HMGA2 overexpression | GH and PRL PitNETs | Chromatin remodeling; overexpression in invasive GH and PRL tumors. |
| Epigenetic Alteration | Associated PitNET Subtype | Clinicopathological Characteristics |
|---|---|---|
| NNAT hypermethylation | Various subtypes | Loss of proliferation inhibition; found in ~70% of PitNETs. |
| CDH13/CDH1 hypermethylation | Various subtypes | Loss of adhesion; associated with tumor aggressiveness. |
| DAPK gene silencing | Various (invasive tumors) | Linked to apoptosis evasion; more aggressive biological behavior. |
| GADD45g loss | Somatotroph/PRL-secreting | Loss of tumor suppressor gene; promotes growth in GH and PRL tumors. |
| LGALS3 methylation | PRL-secreting | Oncogene activity; regulates migration, adhesion, and apoptosis. |
| RASSF1A hypermethylation | All subtypes (especially aggressive) | Correlated with high Ki-67 and aggressiveness. |
| POMC promoter demethylation | Corticotroph | Correlates with ACTH overproduction; USP8-mutant corticotrophs. |
| FGFR2 methylation/MAGE-3 hypomethylation | Corticotroph | FGFR2 silenced (tumor suppressor); MAGE-3 overexpressed (oncogene). |
| TSP-1 downregulation (via miR-449c) | Corticotroph | TSP-1 suppresses proliferation; inhibited by miR-449c in Cushing’s disease. |
| CDKN2A promoter methylation | Gonadotroph, lactotroph, null cell PAs | Inactivation of p16 pathway; promotes proliferation and progression. |
| MEG3 hypermethylation | Non-functioning | Loss of tumor suppressor; linked to progression and poor prognosis. |
| ENC1 methylation | Non-functioning | Lower expression in invasive NFPAs; indicates aggressive behavior. |
| FAM90A1 and ING2 methylation | Non-functioning | Linked to recurrence risk in NF-PitNETs. |
| cfRNAs | Associated PitNET Subtype | Clinicopathological Characteristics |
|---|---|---|
| miR-21 (exosomal) | Somatotroph | Promotes osteoblast proliferation in acromegaly; marker for disease activity. |
| miR-29c-3p | Somatotroph | Lower in uncontrolled acromegaly; potential monitoring marker. |
| miR-423-5p | Somatotroph | Reduces GH secretion; inhibits proliferation and migration in GH-secreting tumors. |
| miR-34a | Various | Tumor suppressor; downregulated in aggressive tumors. |
| miR-93 | Prolactinoma | Mediates cabergoline resistance; regulates autophagy via ATG7. |
| miR-137 | Prolactinoma/Non-functioning | Downregulates Wnt pathway; loss promotes invasiveness. |
| miR-9 | Various | Promotes EMT; linked to aggressive phenotype. |
| miR-145-5p | Non-functioning/ACTH | Downregulation linked to invasiveness; potential TMZ sensitizer. |
| miR-122-5p | Corticotroph | Correlated with ACTH and treatment response in corticotroph tumors. |
| miR-486-5p (exosomal) | Non-functioning | Predicts recurrence in NF-PitNETs; MAPK pathway regulation. |
| miR-320a | Somatotroph | Downregulated in somatotrophs; marker of disease progression. |
| miR-143-3p | Gonadotroph | Reduced post-surgery; correlates with tumor behavior. |
| lncRNA MEG3 | Non-functioning | Tumor suppressor; hypermethylated in NFPAs; loss linked to progression. |
| lncRNA HOTAIR | Non-functioning | Oncogenic role; upregulated in invasive NFPAs. |
| lncRNA H19 | Prolactinoma/Somatotroph | Suppresses proliferation and enhances DA sensitivity; biomarker and target. |
| lncRNA RPSAP52 | GH/PRL-secreting | Sponges miR-15a/16; promotes HMGA2; overexpressed in GH/PRL tumors. |
| lncRNA CLRN1-AS1 | Prolactinoma | Suppresses Wnt pathway; acts as a tumor suppressor. |
| lncRNA THBS1 | Corticotroph | Suppresses TSP-1; involved in Cushing’s disease progression. |
| lncRNA ANRIL | Invasive PitNET | Marker of invasiveness; elevated in invasive PitNETs. |
| lncRNA LINC00473 | Invasive PitNET | Promotes proliferation via cyclin D1/CDK2; invasive tumors. |
| circOMA1 | Non-functioning | Sponges miR-145-5p; promotes invasion in NFPAs. |
| circVPS13C | Non-functioning | Upregulated in high-risk NFPAs; downregulated post-op. |
| hsa_circ_0000066/hsa_circ_0069707 | Non-functioning | Two-circRNA signature predicts recurrence. |
| hsa_circRNA_102597 | Non-functioning | Downregulated in invasive tumors; potential severity marker. |
| Exosome Biomarker/Component | Associated PitNET Subtype | Clinicopathological Significance |
|---|---|---|
| Exosomal mRNA: CDK6 | Non-functioning | Upregulated in invasive NF-PitNETs; cell cycle regulator. |
| Exosomal mRNA: RHOU | Non-functioning | Upregulated in invasive NF-PitNETs; involved in cytoskeletal remodeling. |
| Exosomal mRNA: SPIRE2 | Non-functioning | Linked to invasive behavior; actin nucleation function. |
| Exosomal miR-486-5p | Non-functioning | Most competent predictive biomarker for progression/recurrence; targets MAPK pathways. |
| Exosomal miR-423-5p | Somatotroph | Downregulated in somatotroph tumors; regulates GH and cell proliferation. |
| Exosomal miR-652-3p_R+1 | Non-functioning | Altered in NF-PitNETs; potential diagnostic marker. |
| Exosomal miR-1180-3p | Non-functioning | Altered in NF-PitNETs; prognostic significance unclear. |
| Exosomal miR-151a-5p | Non-functioning | Altered in NF-PitNETs; contributes to the exosomal signature. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tataranu, L.G. Liquid Biopsy in Pituitary Neuroendocrine Tumors—Potential Biomarkers for Diagnosis, Prognosis, and Therapy. Int. J. Mol. Sci. 2025, 26, 4058. https://doi.org/10.3390/ijms26094058
Tataranu LG. Liquid Biopsy in Pituitary Neuroendocrine Tumors—Potential Biomarkers for Diagnosis, Prognosis, and Therapy. International Journal of Molecular Sciences. 2025; 26(9):4058. https://doi.org/10.3390/ijms26094058
Chicago/Turabian StyleTataranu, Ligia Gabriela. 2025. "Liquid Biopsy in Pituitary Neuroendocrine Tumors—Potential Biomarkers for Diagnosis, Prognosis, and Therapy" International Journal of Molecular Sciences 26, no. 9: 4058. https://doi.org/10.3390/ijms26094058
APA StyleTataranu, L. G. (2025). Liquid Biopsy in Pituitary Neuroendocrine Tumors—Potential Biomarkers for Diagnosis, Prognosis, and Therapy. International Journal of Molecular Sciences, 26(9), 4058. https://doi.org/10.3390/ijms26094058







