Thidiazuron Enhances Strawberry Shoot Multiplication by Regulating Hormone Signal Transduction Pathways
Abstract
:1. Introduction
2. Results
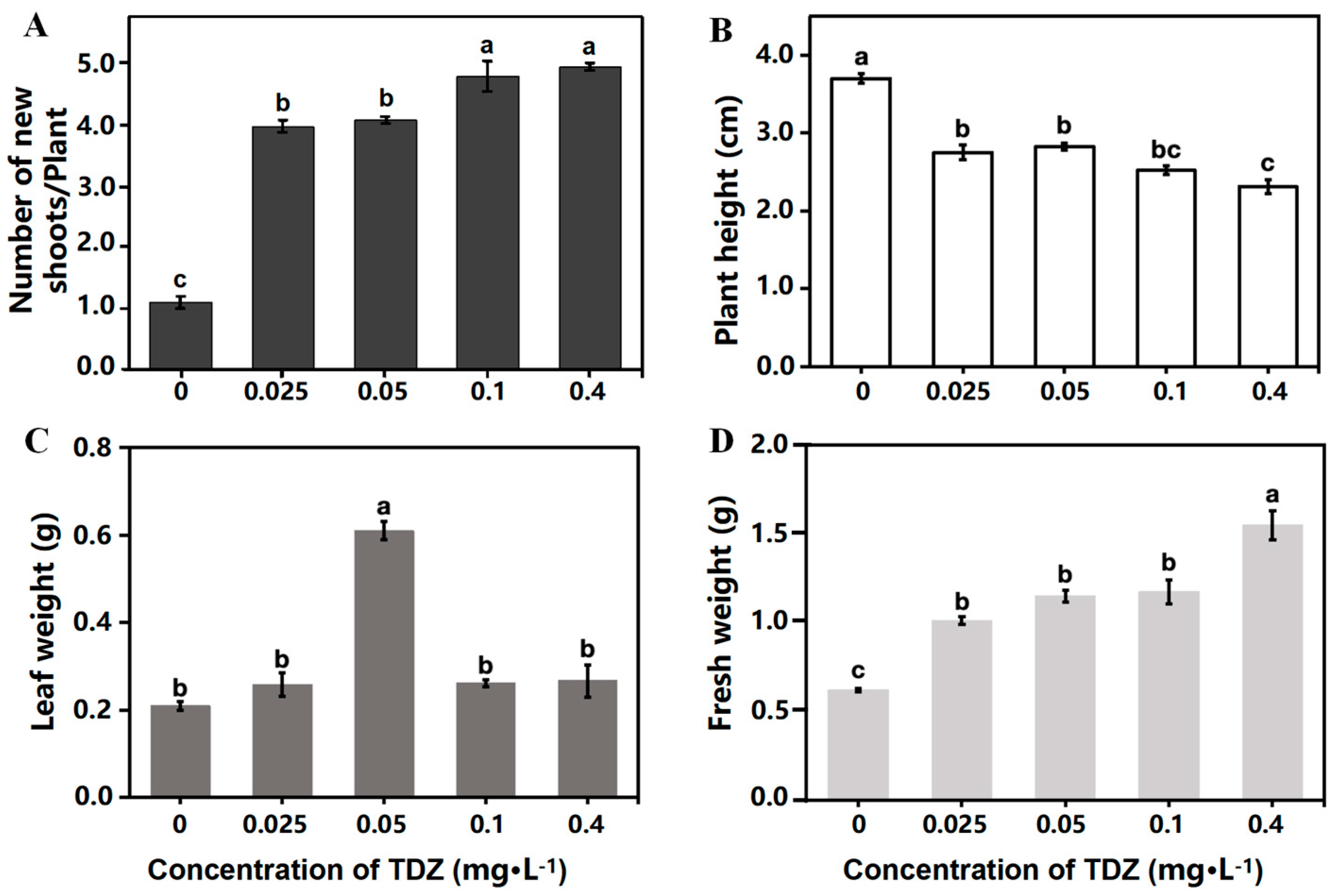
2.1. Plant Growth and Multiplication
2.2. Contents of Chlorophyll and Carotenoid
2.3. Contents of Soluble Sugar and Protein
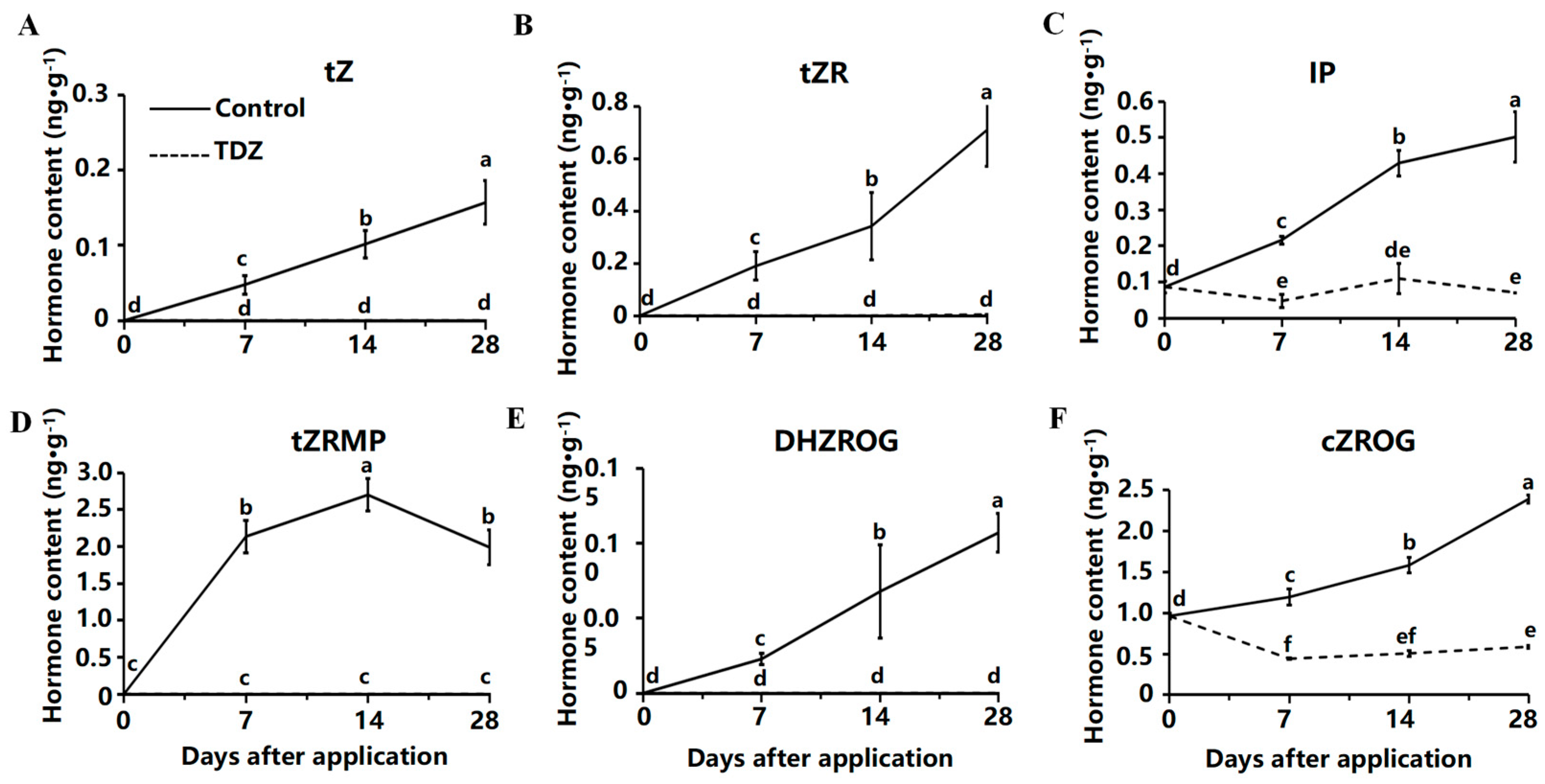
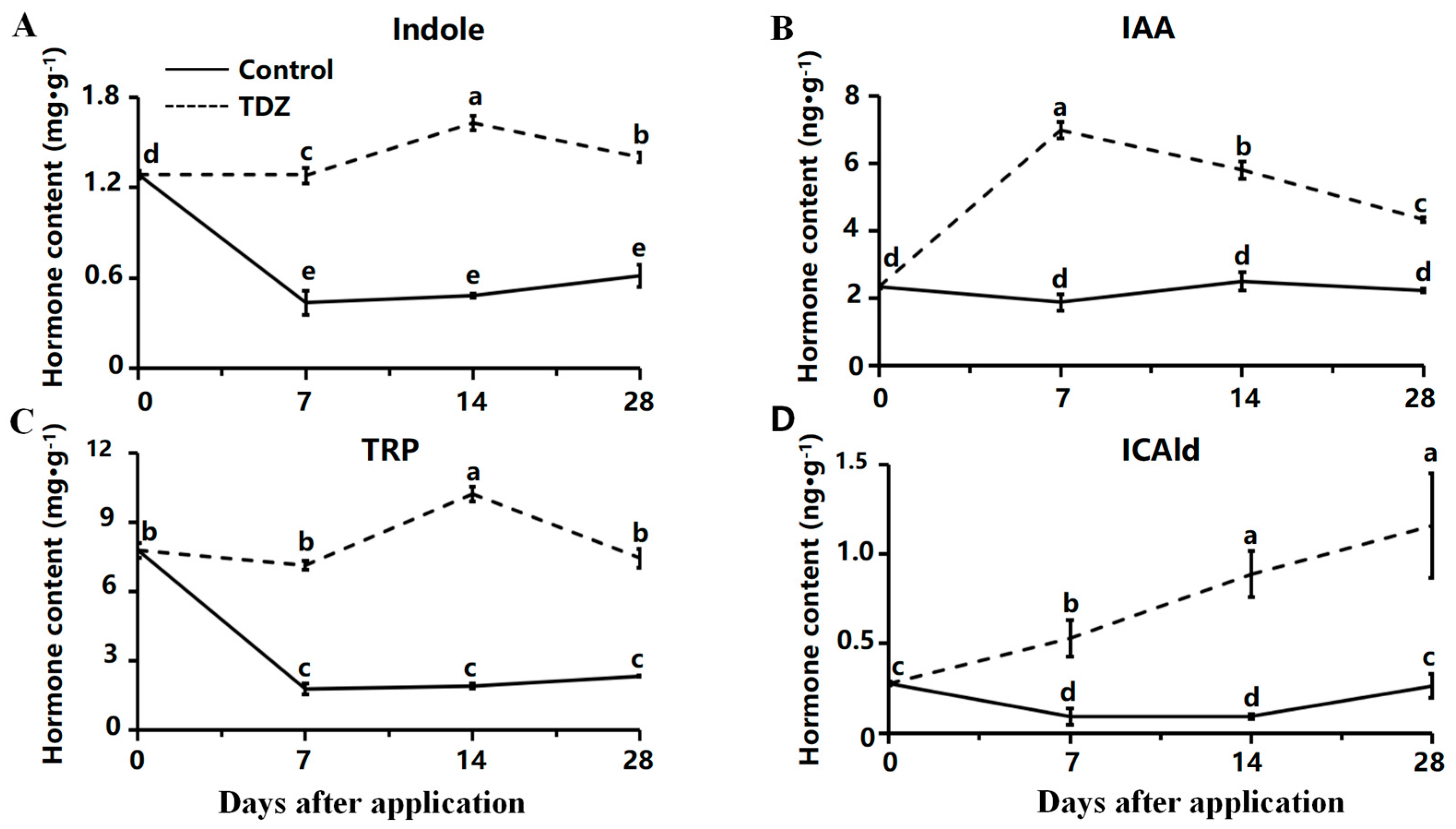
2.4. Contents of Cytokinin and Auxin Contents
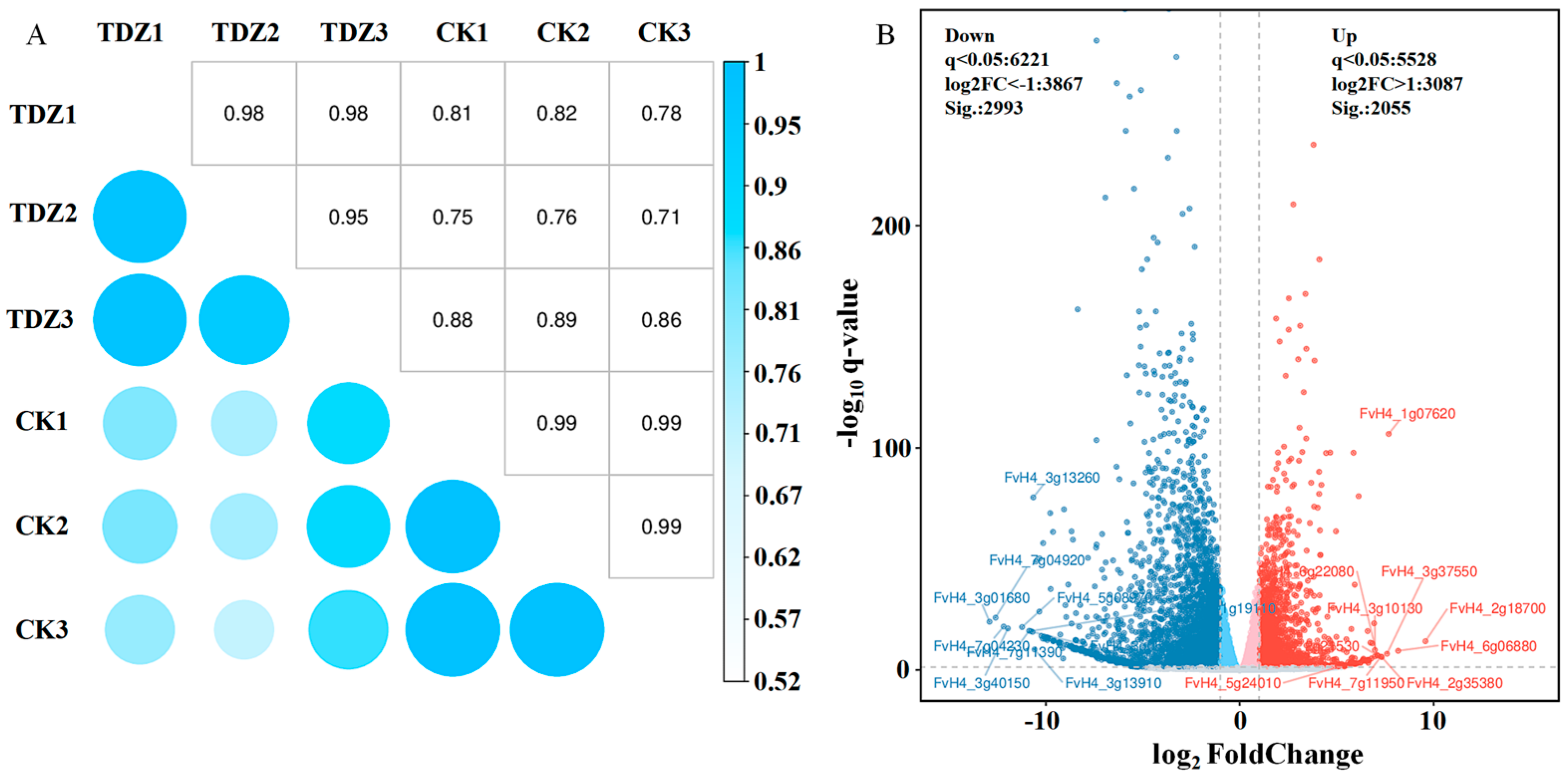
2.5. Transcriptome Analysis
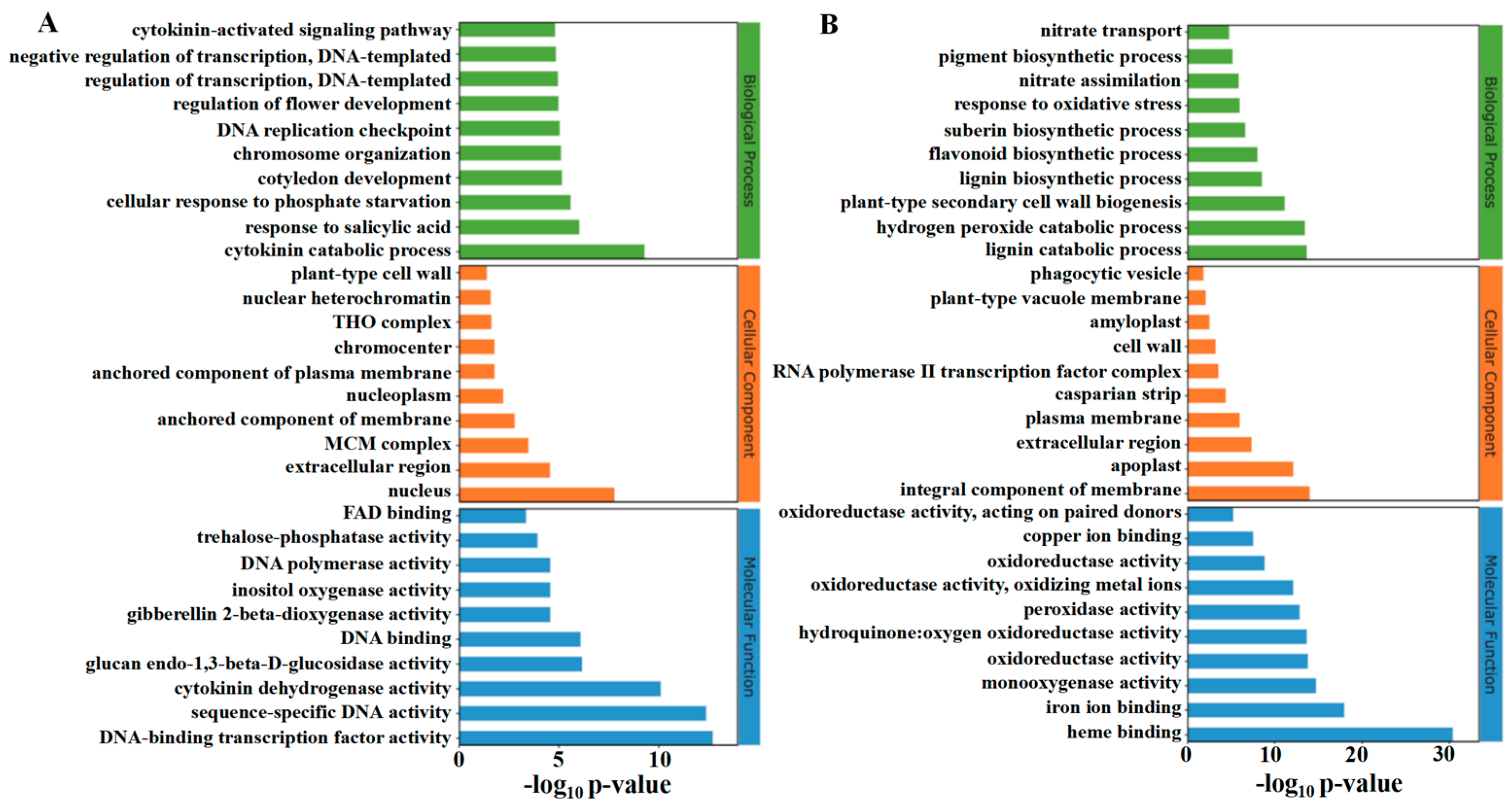
2.5.1. GO Analysis
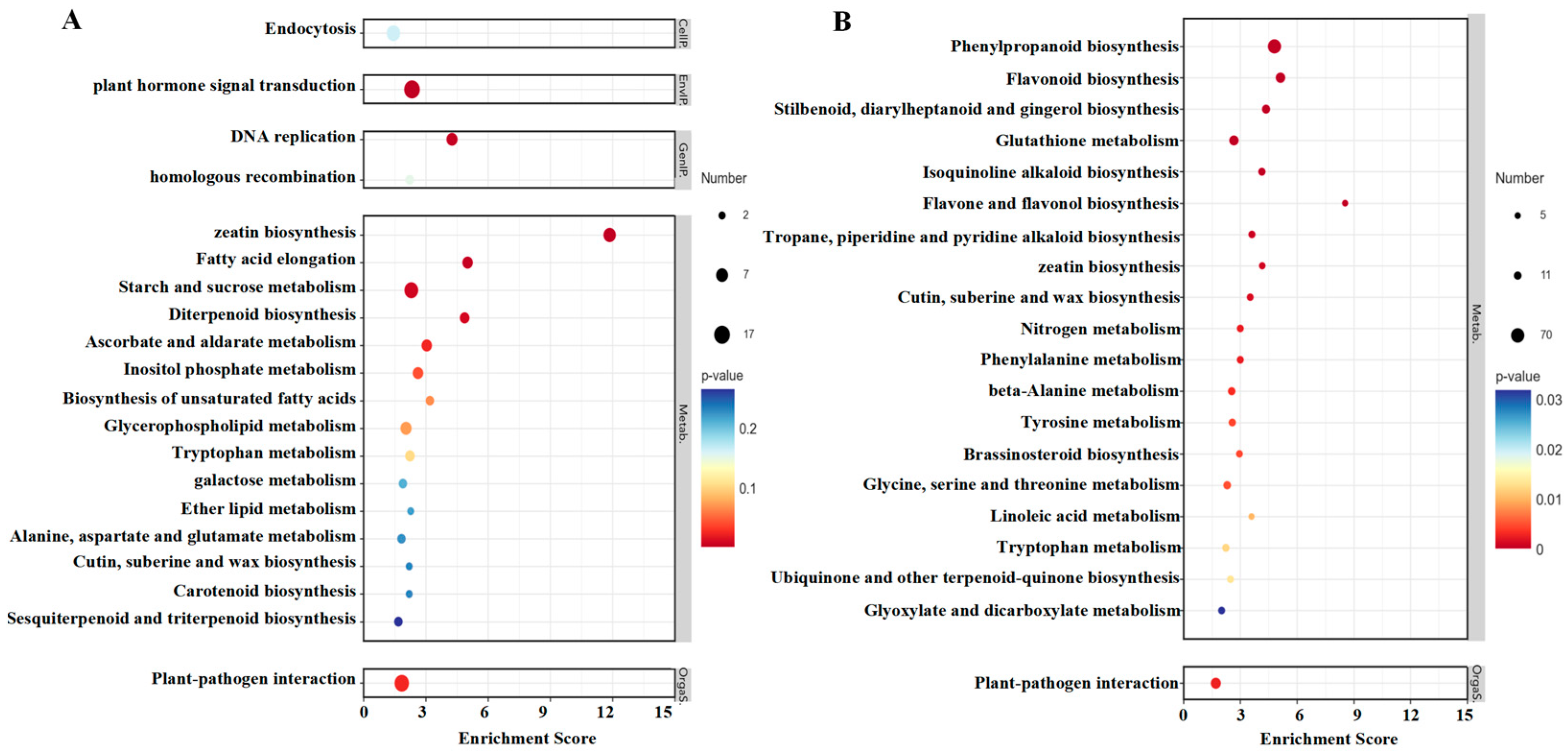
2.5.2. KEGG Analysis
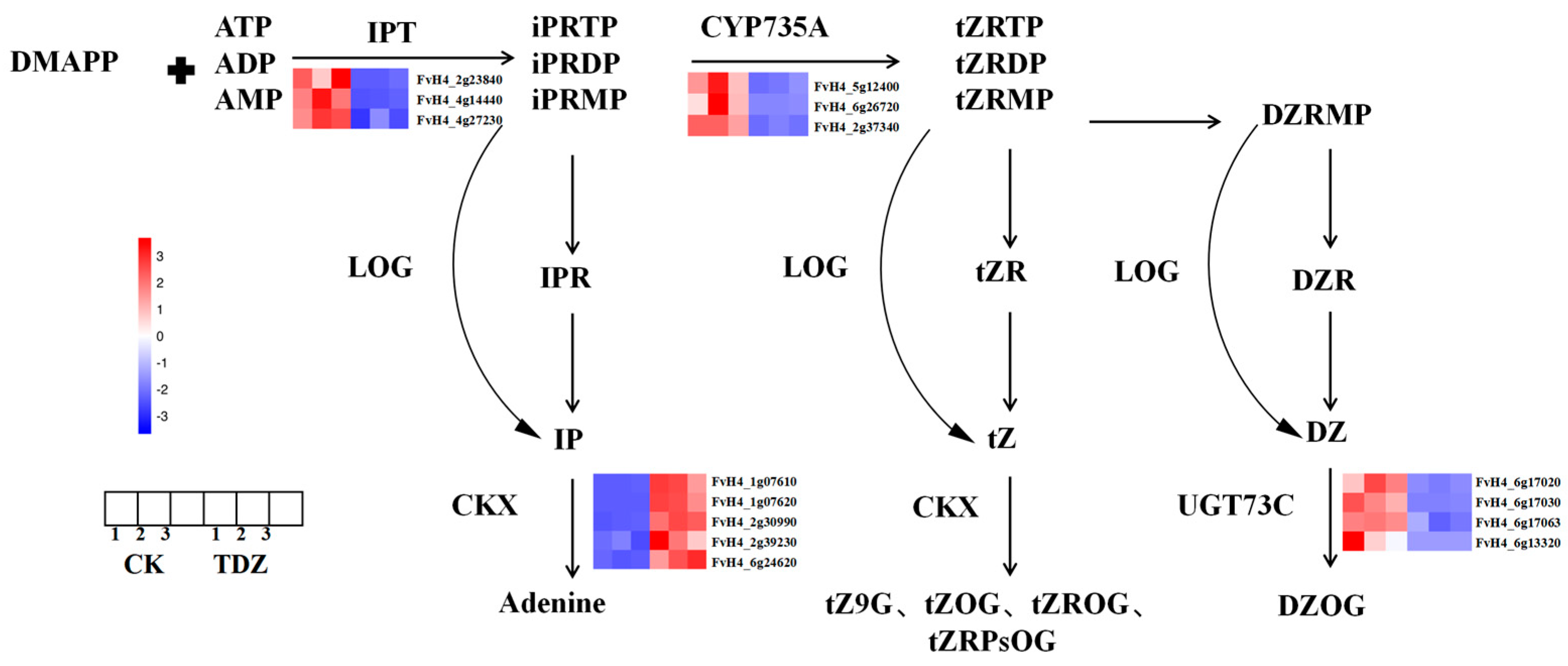
2.5.3. Zeatin Synthesis
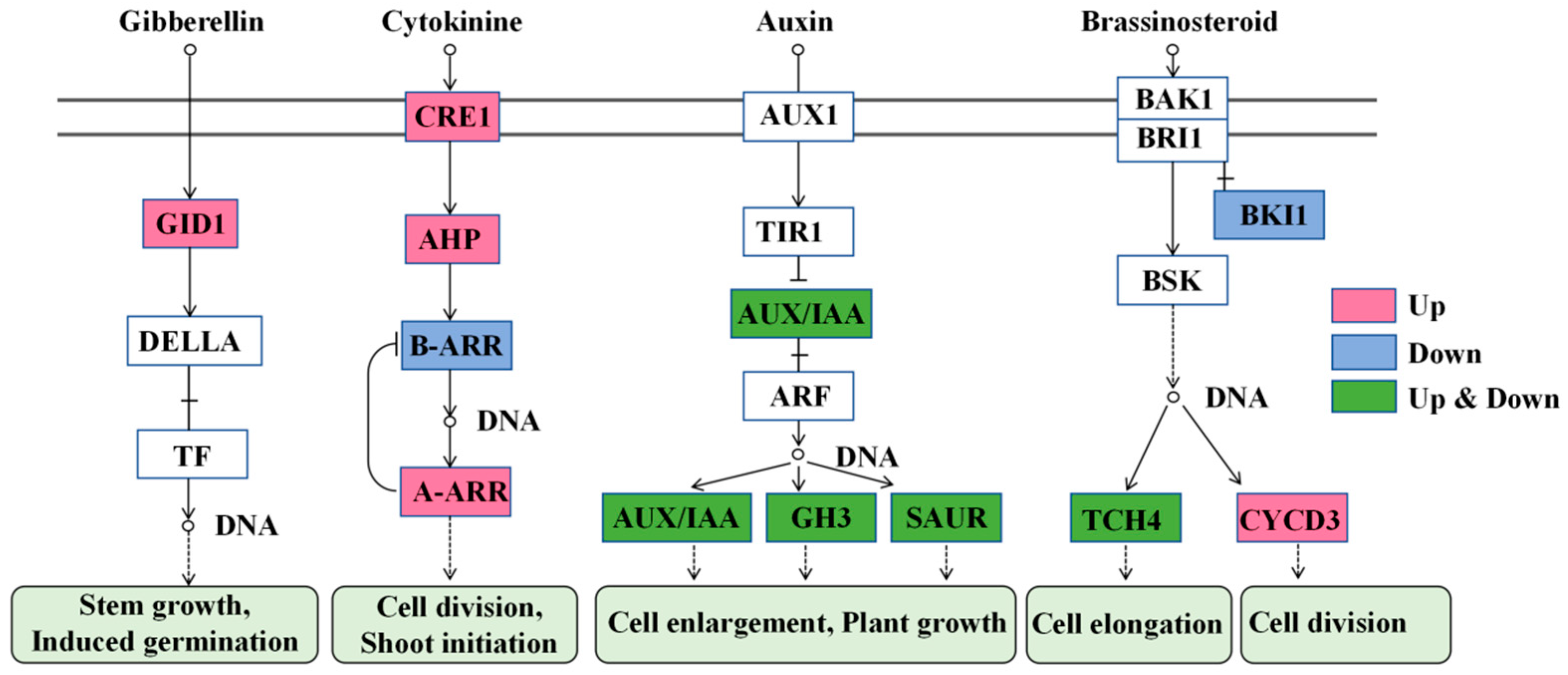
2.5.4. Plant Hormone Signal Transduction
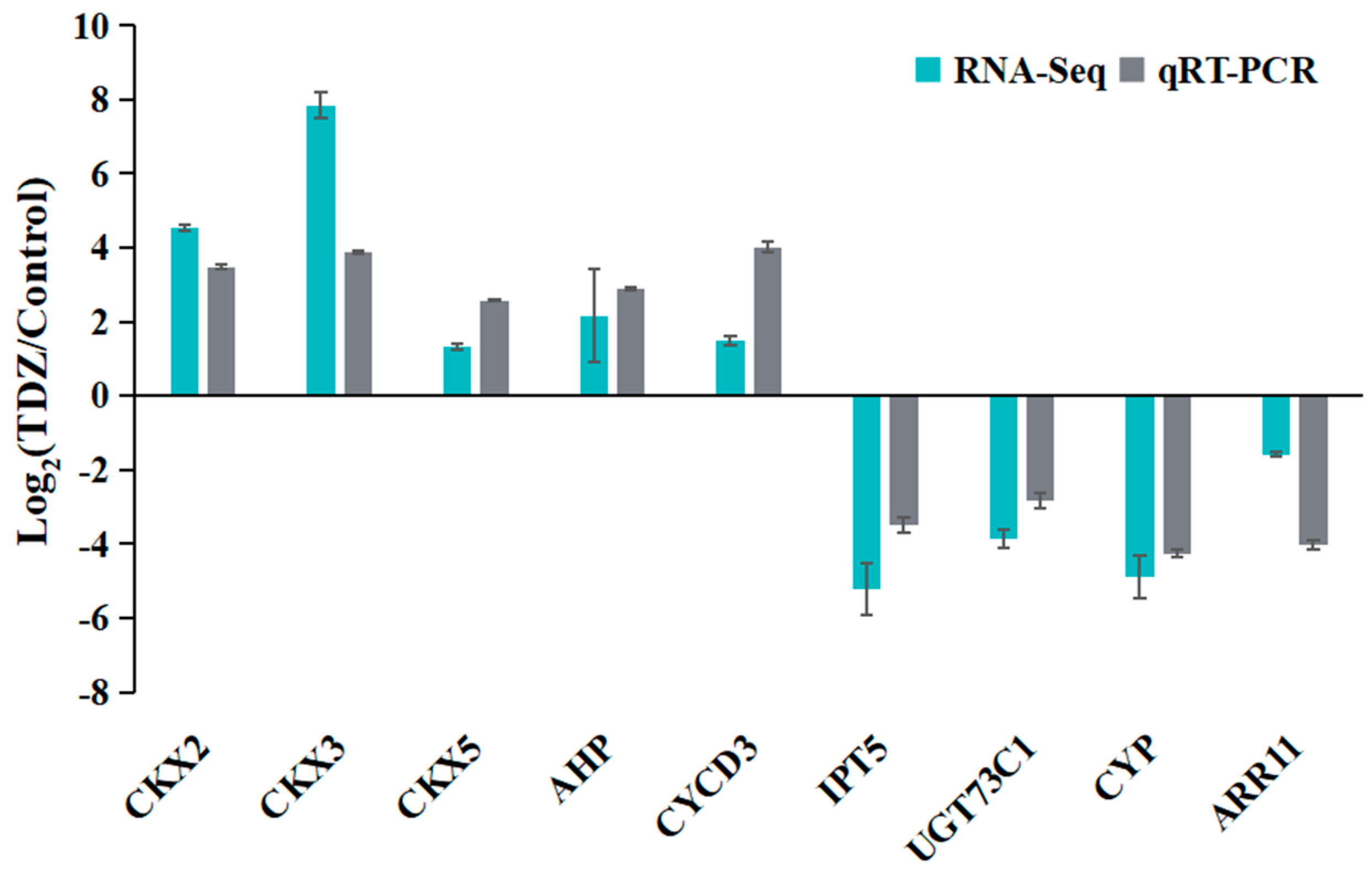
2.6. Expression Profile Validation
3. Discussion
4. Materials and Methods
4.1. Materials Preparation
4.2. TDZ Supplement and Plant Growth Measurement
4.3. Determination of Chlorophyll Contents
4.4. Determination of Soluble Sugar and Protein Contents
4.5. Quantification of Endogenous Cytokinins and Auxins
4.6. RNA Extraction and cDNA Library Construction
4.7. RNA-Seq and DEGs Enrichment Analysis
4.8. Validation of Gene Expression Profiles by qRT-PCR
4.9. Data Collection and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Khayri, J.M.; Islam, R. Genetic Improvement of Strawberry (Fragaria × ananassa Duchesne). In Advances in Plant Breeding Strategies: Fruits; Springer: Berlin/Heidelberg, Germany, 2018; Volume 3, pp. 217–275. [Google Scholar]
- Chung, H.-H.; Ouyang, H.-Y. Use of thidiazuron for high-frequency callus induction and organogenesis of wild strawberry (Fragaria vesca). Plants 2020, 10, 67. [Google Scholar] [CrossRef]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef] [PubMed]
- George, E.F.; Hall, M.A.; Klerk, G.J.D. Plant Propagation by Tissue Culture; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Shelepova, O.V.; Baranova, E.N.; Tkacheva, E.V.; Evdokimenkova, Y.B.; Ivanovskii, A.A.; Konovalova, L.N.; Gulevich, A.A. Aromatic Plants Metabolic Engineering: A Review. Agronomy 2022, 12, 3131. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Vázquez-Flota, F. An introduction to plant cell culture: Back to the future. In Plant Cell Culture Protocols; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–8. [Google Scholar]
- Nehra, N.S.; Kartha, K.K.; Stushnoff, C.; Giles, K.L. Effect of in vitro propagation methods on field performance of two strawberry cultivars. Euphytica 1994, 76, 107–115. [Google Scholar] [CrossRef]
- Qiu, Y.; Guan, S.C.; Wen, C.; Li, P.; Gao, Z.; Chen, X. Auxin and cytokinin coordinate the dormancy and outgrowth of axillary bud in strawberry runner. BMC Plant Biol. 2019, 19, 528. [Google Scholar] [CrossRef]
- El-Sayed, S.; El-Sawy, A.M.; Taha, S.S.; Gomah, M.S. Effect of Benzylaminopurine concentration and number of subcultures on behavior of some strawberry cultivars in vitro. Egypt. J. Plant Breed. 2017, 21, 1–12. [Google Scholar] [CrossRef]
- Müller, D.; Waldie, T.; Miyawaki, K.; To, J.P.; Melnyk, C.W.; Kieber, J.J.; Kakimoto, T.; Leyser, O. Cytokinin is required for escape but not release from auxin mediated apical dominance. Plant J. 2015, 82, 874–886. [Google Scholar] [CrossRef]
- Zeng, X.-F.; Zhao, D.-G. Expression of IPT in Asakura-sanshoo (Zanthoxylum piperitum (L.) DC. f. inerme Makino) alters tree architecture, delays leaf senescence, and changes leaf essential oil composition. Plant Mol. Biol. Report. 2016, 34, 649–658. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Vaňková, R.; Malbeck, J.; Li, A.; Li, Y.; McAvoy, R. Enhancement of flowering and branching phenotype in chrysanthemum by expression of ipt under the control of a 0.821 kb fragment of the LEACO1 gene promoter. Plant Cell Rep. 2009, 28, 1351–1362. [Google Scholar] [CrossRef]
- Guo, Y.; Gan, S. AtMYB2 regulates whole plant senescence by inhibiting cytokinin-mediated branching at late stages of development in Arabidopsis. Plant Physiol. 2011, 156, 1612–1619. [Google Scholar] [CrossRef]
- Hutchison, C.E.; Li, J.; Argueso, C.; Gonzalez, M.; Lee, E.; Lewis, M.W.; Maxwell, B.B.; Perdue, T.D.; Schaller, G.E.; Alonso, J.M.; et al. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 2006, 18, 3073–3087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-M.; Li, J.X.; Zhang, T.Q.; Xu, Z.G.; Ma, M.L.; Zhang, P.; Wang, J.W. The structure of B-ARR reveals the molecular basis of transcriptional activation by cytokinin. Proc. Natl. Acad. Sci. USA 2024, 121, e2319335121. [Google Scholar] [CrossRef]
- Taniguchi, M.; Sasaki, N.; Tsuge, T.; Aoyama, T.; Oka, A. ARR1 directly activates cytokinin response genes that encode proteins with diverse regulatory functions. Plant Cell Physiol. 2007, 48, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liu, Z.; Qiao, M.; Li, J.; Li, S.; Xiang, F. ARR12 promotes de novo shoot regeneration in Arabidopsis thaliana via activation of WUSCHEL expression. J. Integr. Plant Biol. 2017, 59, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Kamiuchi, Y.; Yamamoto, K.; Furutani, M.; Tasaka, M.; Aida, M. The CUC1 and CUC2 genes promote carpel margin meristem formation during Arabidopsis gynoecium development. Front. Plant Sci. 2014, 5, 165. [Google Scholar] [CrossRef]
- Burian, A.; De Reuille, P.B.; Kuhlemeier, C. Patterns of stem cell divisions contribute to plant longevity. Curr. Biol. 2016, 26, 1385–1394. [Google Scholar] [CrossRef]
- Waldie, T.; Leyser, O. Cytokinin targets auxin transport to promote shoot branching. Plant Physiol. 2018, 177, 803–818. [Google Scholar] [CrossRef]
- Nisler, J. TDZ: Mode of action, use and potential in agriculture. In Thidiazuron: From Urea Derivative to Plant Growth Regulator; Springer: Berlin/Heidelberg, Germany, 2018; pp. 37–59. [Google Scholar]
- Dewir, Y.H.; Nurmansyah Naidoo, Y.; Teixeira da Silva, J.A. Thidiazuron-induced abnormalities in plant tissue cultures. Plant Cell Rep. 2018, 37, 1451–1470. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Xiao, J.; Guo, G.; Jeong, B.R. Foliar thidiazuron promotes the growth of axillary buds in Strawberry. Agronomy 2021, 11, 594. [Google Scholar] [CrossRef]
- Peddaboina, V.; Thamidala, C.; Karampuri, S. In vitro shoot multiplication and plant regeneration in four Capsicum species using thidiazuron. Sci. Hortic. 2006, 107, 117–122. [Google Scholar] [CrossRef]
- Faisal, M.; Ahmad, N.; Anis, M. Shoot multiplication in Rauvolfia tetraphylla L. using thidiazuron. Plant Cell Tissue Organ Cult. 2005, 80, 187–190. [Google Scholar] [CrossRef]
- Parveen, S.; Shahzad, A. TDZ-induced high frequency shoot regeneration in Cassia sophera Linn. via cotyledonary node explants. Physiol. Mol. Biol. Plants 2010, 16, 201–206. [Google Scholar] [CrossRef]
- Tsutomu, I.; Higuchi, M.; Hashimoto, Y.; Seki, M.; Kobayashi, M.; Kato, T.; Tabata, S.; Shinozaki, K.; Kakimoto, T. Identification of CRE1 as a Cytokinin Receptor from Arabidopsis. Nature 2001, 409, 1060–1063. [Google Scholar]
- Ueguchi, C.; Sato, S.; Kato, T.; Tabata, S. The AHK4 gene involved in the cytokinin-signaling pathway as a direct receptor molecule in Arabidopsis thaliana. Plant Cell Physiol. 2001, 42, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Li, G.F.; Chen, X.L.; Xing, L.B.; Ma, J.J.; Zhang, D.; Ge, H.J.; Han, M.Y.; Sha, G.L.; An, N. Role of cytokinin, strigolactone, and auxin export on outgrowth of axillary buds in apple. Front. Plant Sci. 2019, 10, 616. [Google Scholar] [CrossRef]
- Cui, X.; Ge, C.; Wang, R.; Wang, H.; Chen, W.; Fu, Z.; Jiang, X.; Li, J.; Wang, Y. The BUD2 mutation affects plant architecture through altering cytokinin and auxin responses in Arabidopsis. Cell Res. 2010, 20, 576–586. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Beveridge, C.A. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 2009, 149, 1929–1944. [Google Scholar] [CrossRef]
- Jones, B.; Gunnerås, S.A.; Petersson, S.V.; Tarkowski, P.; Graham, N.; May, S.; Dolezal, K.; Sandberg, G.; Ljung, K. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction. Plant Cell 2010, 22, 2956–2969. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.-J.; Zhang, J.-Z. Aux/IAA gene family in plants: Molecular structure, regulation, and function. Int. J. Mol. Sci. 2018, 19, 259. [Google Scholar] [CrossRef]
- Park, J.-E.; Park, J.Y.; Kim, Y.S.; Staswick, P.E.; Jeon, J.; Yun, J.; Kim, S.Y.; Kim, J.M.; Lee, Y.H.; Park, C.M. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J. Biol. Chem. 2007, 282, 10036–10046. [Google Scholar] [CrossRef]
- Bao, D.; Chang, S.; Li, X.; Qi, Y. Advances in the study of auxin early response genes: Aux/IAA, GH3, and SAUR. Crop J. 2024, 12, 964–978. [Google Scholar] [CrossRef]
- Stortenbeker, N.; Bemer, M. The SAUR gene family: The plant’s toolbox for adaptation of growth and development. J. Exp. Bot. 2019, 70, 17–27. [Google Scholar] [CrossRef]
- Hardtke, C.S.; Dorcey, E.; Osmont, K.S.; Sibout, R. Phytohormone collaboration: Zooming in on auxin–brassinosteroid interactions. Trends Cell Biol. 2007, 17, 485–492. [Google Scholar] [CrossRef]
- Ross, J.; O’neill, D.P.; Wolbang, C.M.; Symons, G.M.; Reid, J. Auxin-Gibberellin Interactions and Their Role in Plant Growth; University of Tasmania: Hobart, Australia, 2002. [Google Scholar]
- O’Neill, D.P.; Ross, J.J. Auxin regulation of the gibberellin pathway in pea. Plant Physiol. 2002, 130, 1974–1982. [Google Scholar] [CrossRef]
- Chung, Y.; Maharjan, P.M.; Lee, O.; Fujioka, S.; Jang, S.Y.; Kim, B.K.; Tsujimoto, M.; Kim, H.B.; Cho, S.; Park, T.S.; et al. Auxin stimulates DWARF4 expression and brassinosteroid biosynthesis in Arabidopsis. Plant J. 2011, 66, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhao, Y.; Chen, Y.; Xu, J.; Jiang, C.; Wang, X.; Zhuo, R.; Lu, M.Z.; Zhang, J. Lignin biosynthesis and accumulation in response to abiotic stresses in woody plants. For. Res. 2022, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Beisson, F.; Li, Y.; Ohlrogge, J.B. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci. 2008, 13, 236–246. [Google Scholar] [CrossRef]
- Kerbler, S.M.L.; Armijos-Jaramillo, V.; Lunn, J.E.; Vicente, R. The trehalose 6-phosphate phosphatase family in plants. Physiol. Plant. 2023, 175, e14096. [Google Scholar] [CrossRef]
- Lara-Núñez, A.; García-Ayala, B.B.; Garza-Aguilar, S.M.; Flores-Sánchez, J.; Sánchez-Camargo, V.A.; Bravo-Alberto, C.E.; Vázquez-Santana, S.; Vázquez-Ramos, J.M. Glucose and sucrose differentially modify cell proliferation in maize during germination. Plant Physiol. Biochem. 2017, 113, 20–31. [Google Scholar] [CrossRef]
- Mishra, B.S.; Sharma, M.; Laxmi, A. Role of sugar and auxin crosstalk in plant growth and development. Physiol. Plant. 2022, 174, e13546. [Google Scholar] [CrossRef]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant–environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhou, H.; Liu, X.; Wang, N.; Xu, Q.; Yan, G. Correlation analysis of the transcriptome and metabolome reveals the role of the flavonoid biosynthesis pathway in regulating axillary buds in upland cotton (Gossypium hirsutum L.). Planta 2021, 254, 7. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cao, S.; Wang, X.; Liu, Y.; Sun, Z.; Zhang, Y.; Li, M.; Wang, Y.; He, W.; Zhang, Y.; et al. Foliar application of sodium selenite affects the growth, antioxidant system, and fruit quality of strawberry. Front. Plant Sci. 2024, 15, 1449157. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; She, M.; Zhao, M.; Yu, H.; Xiao, W.; Zhang, Y.; Li, M.; Chen, Q.; Zhang, Y.; Wang, Y.; et al. Genome-wide analysis and functional validation reveal the role of late embryogenesis abundant genes in strawberry (Fragaria × ananassa) fruit ripening. BMC Genom. 2024, 25, 228. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, H.W.; Wang, X.R.; Xie, X.L.; Yue, X.Y.; Tang, H.R. An alternative cetyltrimethylammonium bromide-based protocol for RNA isolation from blackberry (Rubus, L.). Genet. Mol. Res. GMR 2012, 11, 1773–1782. [Google Scholar] [CrossRef]
- Wang, L.; Lin, Y.; Hou, G.; Yang, M.; Peng, Y.; Jiang, Y.; He, C.; She, M.; Chen, Q.; Li, M.; et al. A histone deacetylase, FaSRT1-2, plays multiple roles in regulating fruit ripening, plant growth and stresses resistance of cultivated strawberry. Plant Cell Environ. 2024, 47, 2258–2273. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Li, Y.; Pang, Y.; Hu, J.; Kang, X.; Qian, C. Thidiazuron Enhances Strawberry Shoot Multiplication by Regulating Hormone Signal Transduction Pathways. Int. J. Mol. Sci. 2025, 26, 4060. https://doi.org/10.3390/ijms26094060
Wang F, Li Y, Pang Y, Hu J, Kang X, Qian C. Thidiazuron Enhances Strawberry Shoot Multiplication by Regulating Hormone Signal Transduction Pathways. International Journal of Molecular Sciences. 2025; 26(9):4060. https://doi.org/10.3390/ijms26094060
Chicago/Turabian StyleWang, Fang, Yali Li, Yadan Pang, Jiangtao Hu, Xinna Kang, and Chun Qian. 2025. "Thidiazuron Enhances Strawberry Shoot Multiplication by Regulating Hormone Signal Transduction Pathways" International Journal of Molecular Sciences 26, no. 9: 4060. https://doi.org/10.3390/ijms26094060
APA StyleWang, F., Li, Y., Pang, Y., Hu, J., Kang, X., & Qian, C. (2025). Thidiazuron Enhances Strawberry Shoot Multiplication by Regulating Hormone Signal Transduction Pathways. International Journal of Molecular Sciences, 26(9), 4060. https://doi.org/10.3390/ijms26094060





