Iris germanica L. Rhizome-Derived Exosomes Ameliorated Dihydrotestosterone-Damaged Human Follicle Dermal Papilla Cells Through the Activation of Wnt/β-Catenin Pathway
Abstract
:1. Introduction
2. Results
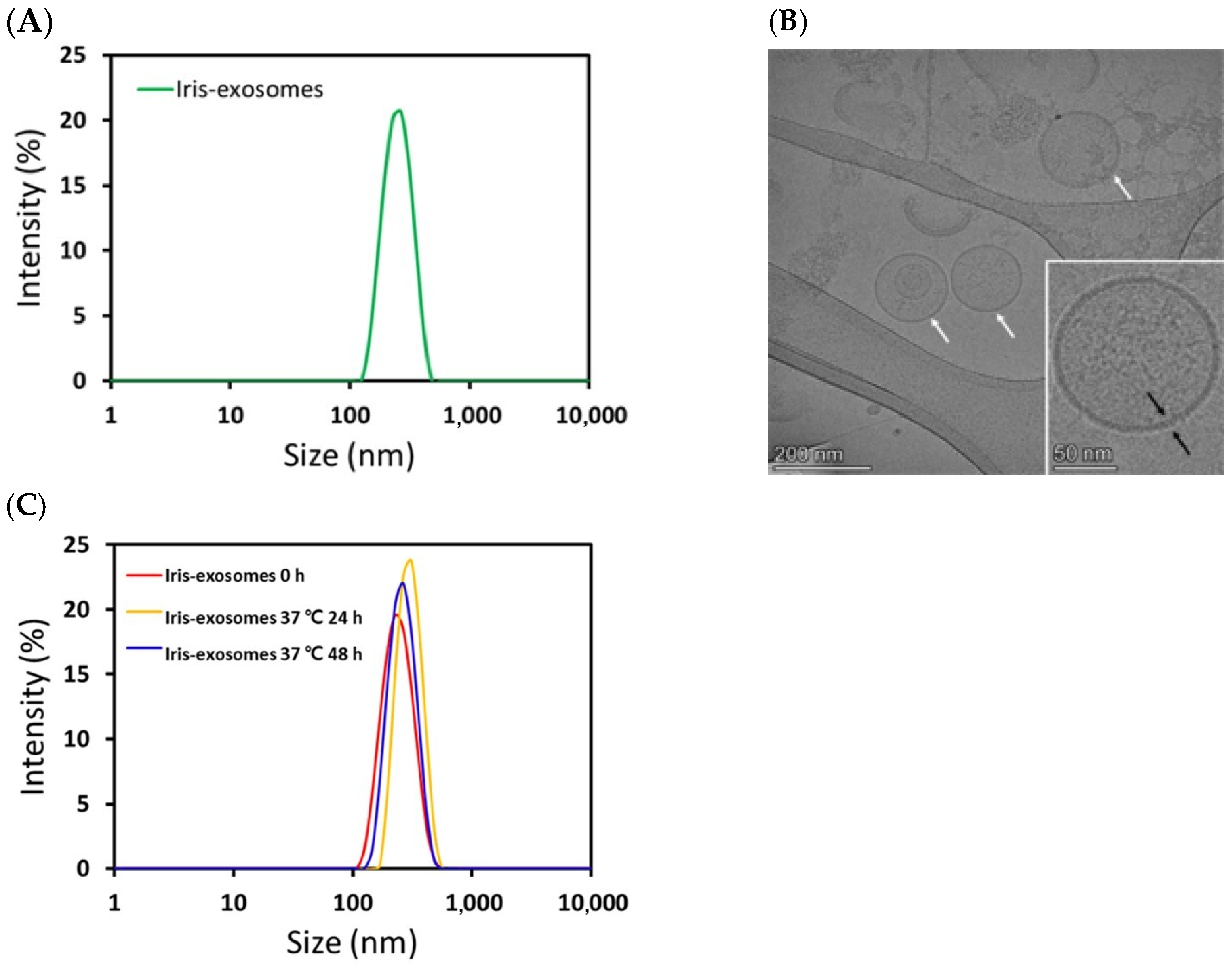
2.1. Characterization of Iris Exosomes Derived from Iris germanica L. Rhizome
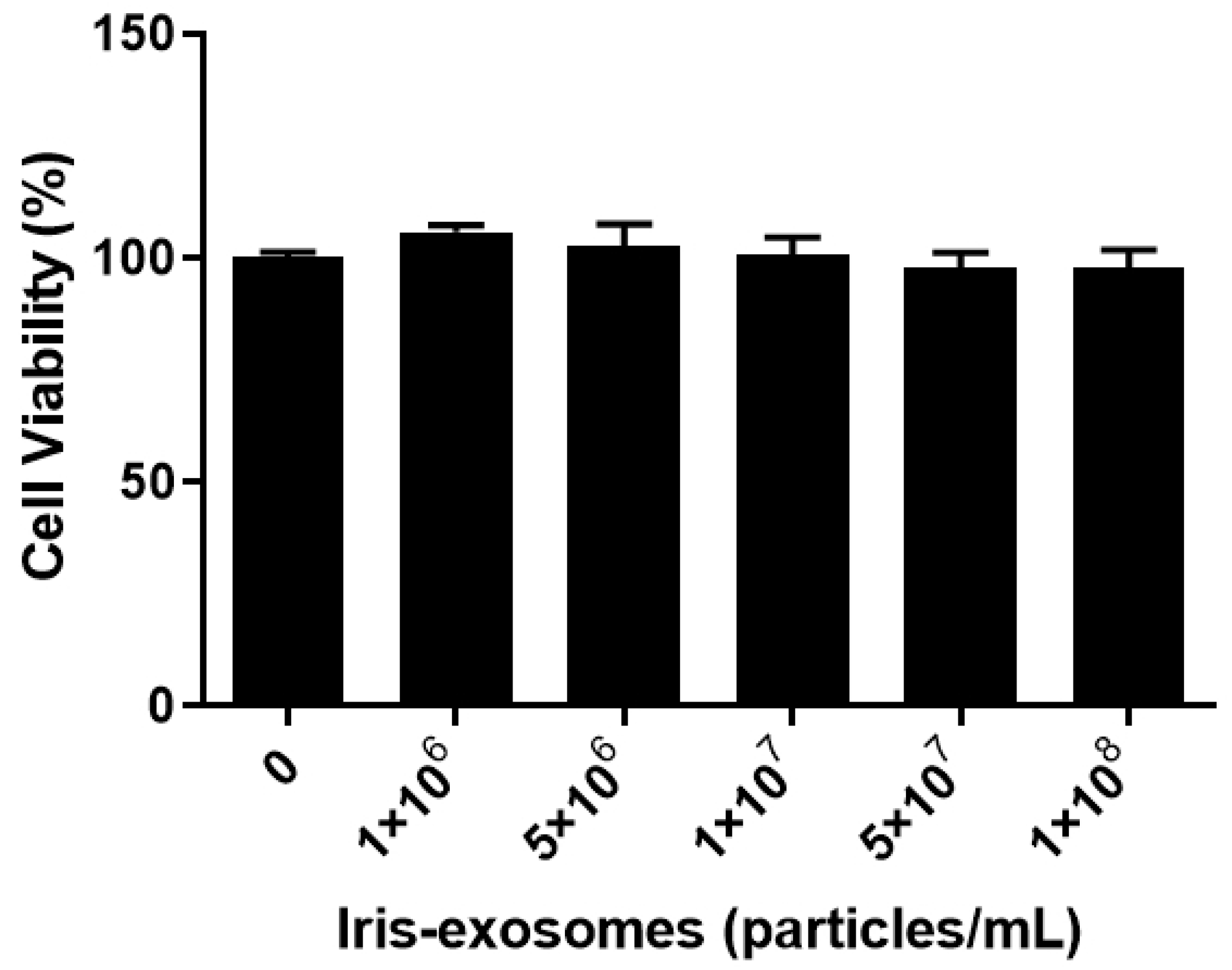
2.2. Effect of Iris-Exosome Treatment on the Cell Viability of HFDPCs
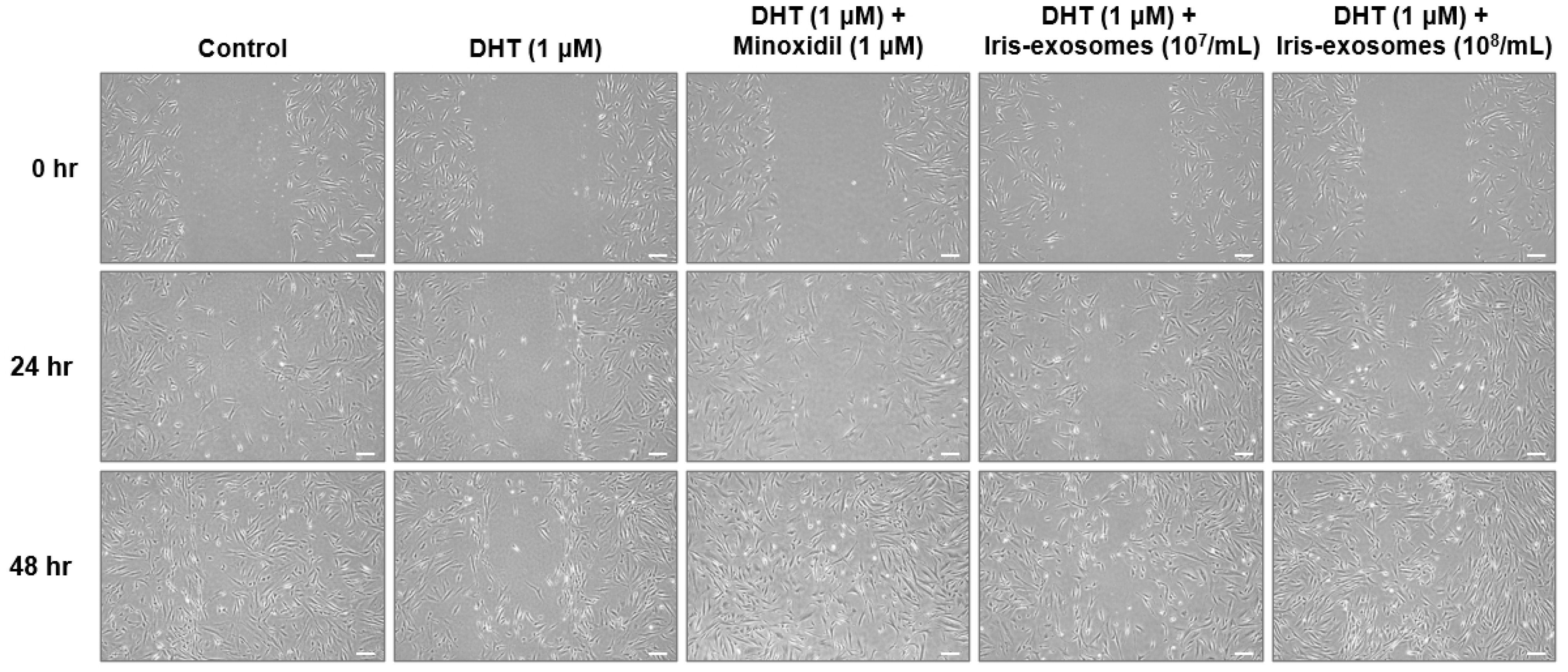
2.3. Iris-Exosomes Increase the Migration of DHT-Damaged HFDPCs
2.4. Iris-Exosomes Enhance Alkaline Phosphatase Expression in DHT-Damaged HFDPCs
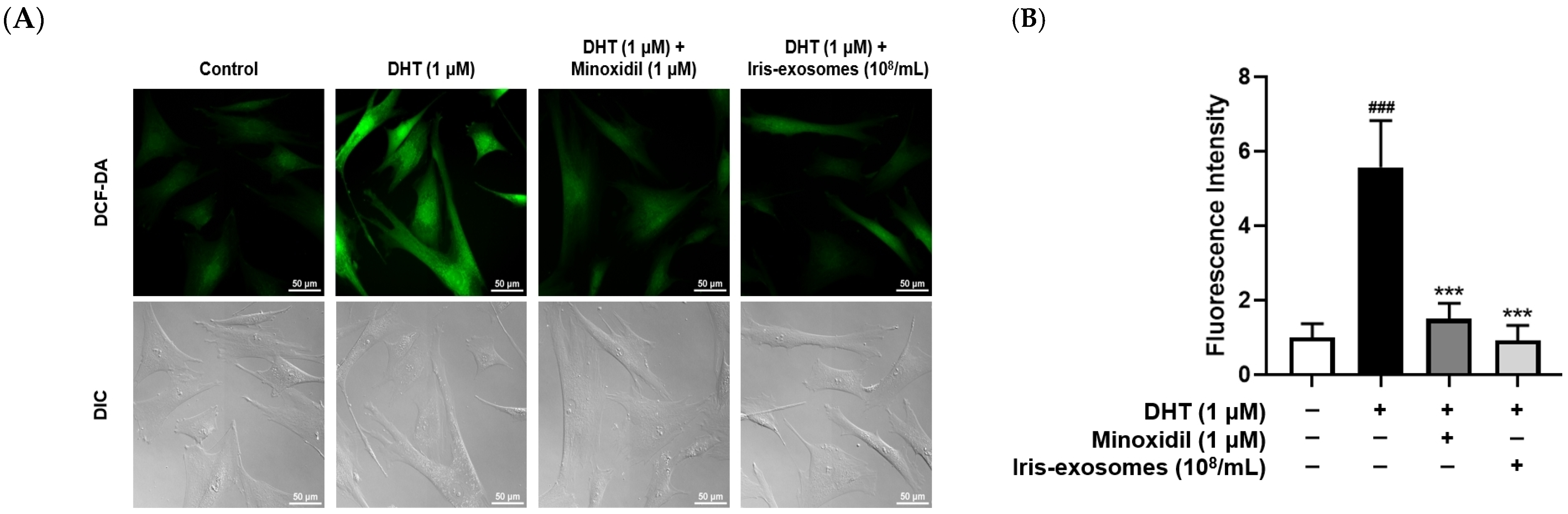
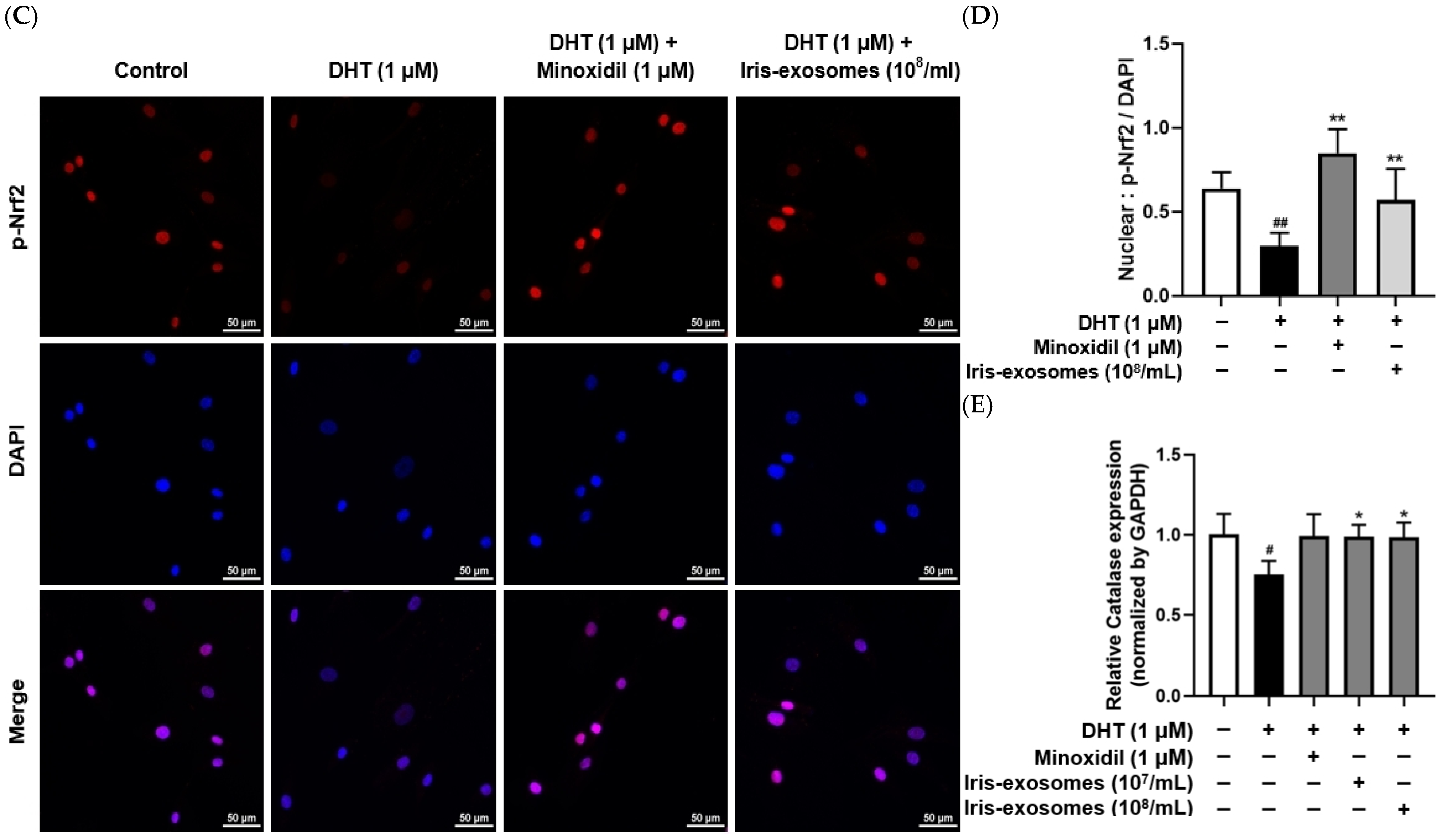
2.5. Iris-Exosomes Reduce ROS Levels in DHT-Damaged HFDPCs
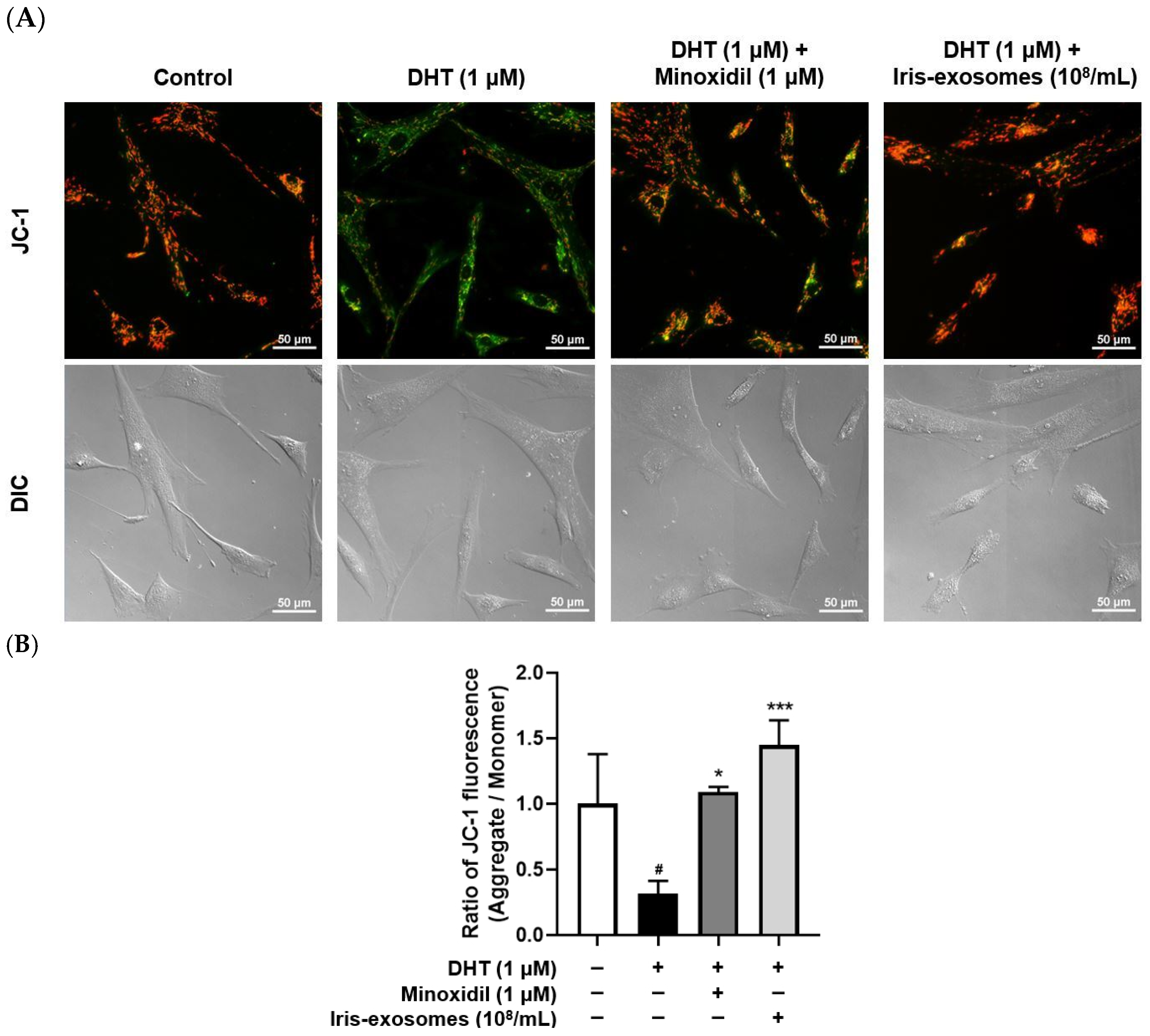
2.6. Iris-Exosomes Restore the Mitochondrial Membrane Potential in DHT-Damaged HFDPCs
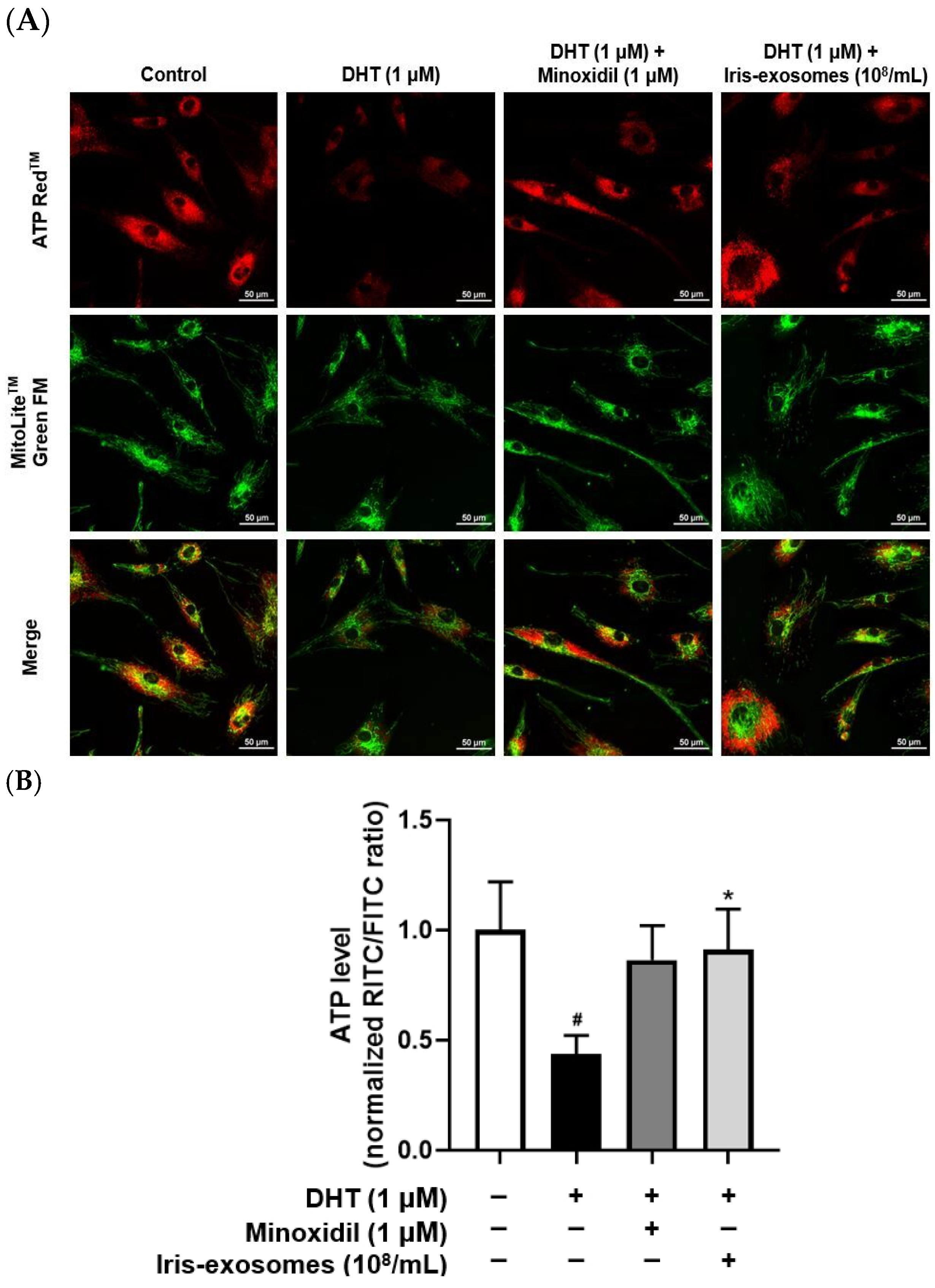
2.7. Iris-Exosomes Enhance ATP Levels in DHT-Damaged HFDPCs
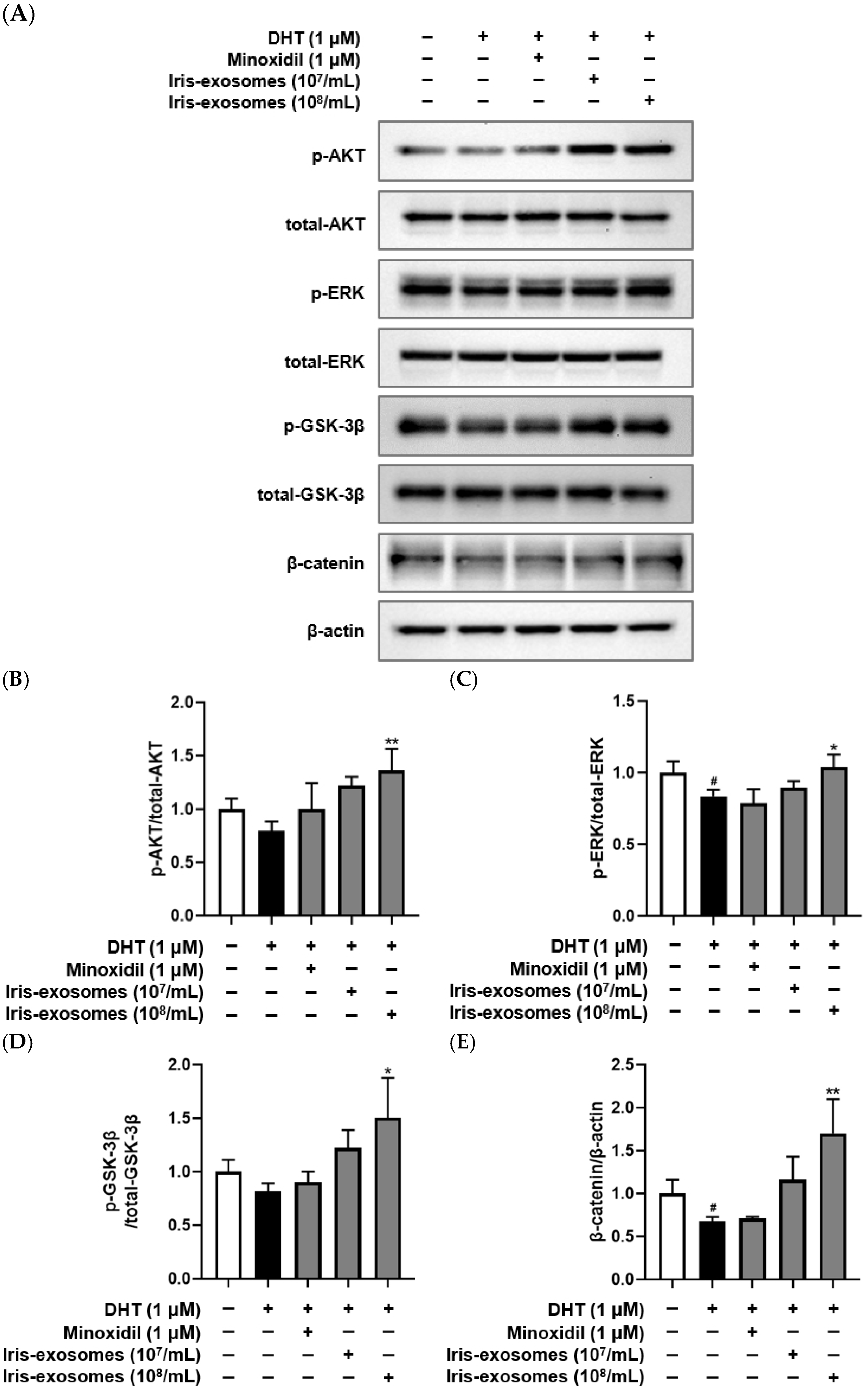
2.8. Iris-Exosomes Upregulate the Phosphorylation Levels of AKT, ERK, and GSK-3β and the Expression of β-Catenin in DHT-Damaged HFDPCs
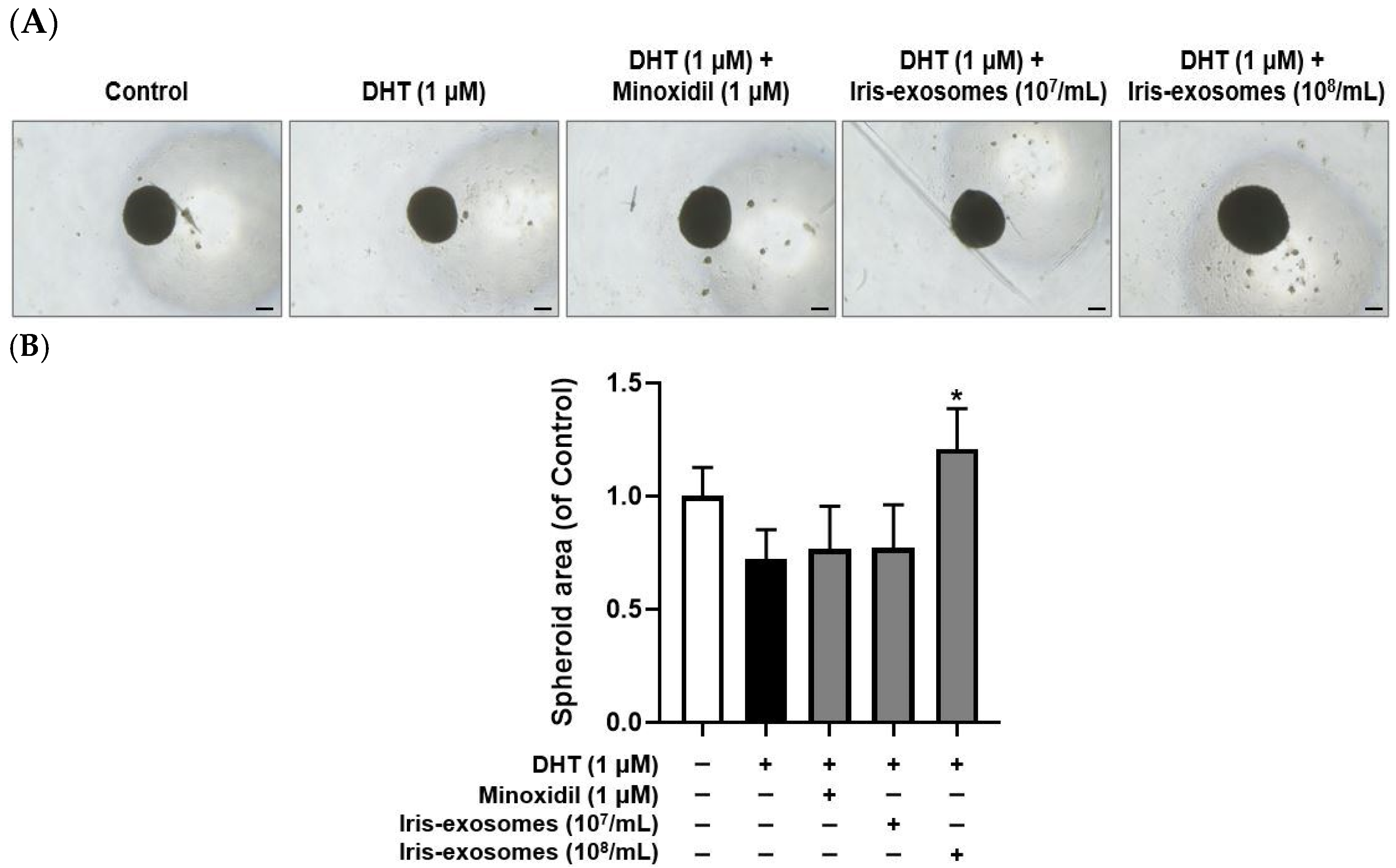
2.9. Iris-Exosomes Increase the 3D Spheroid Size in DHT-Damaged HFDPCs
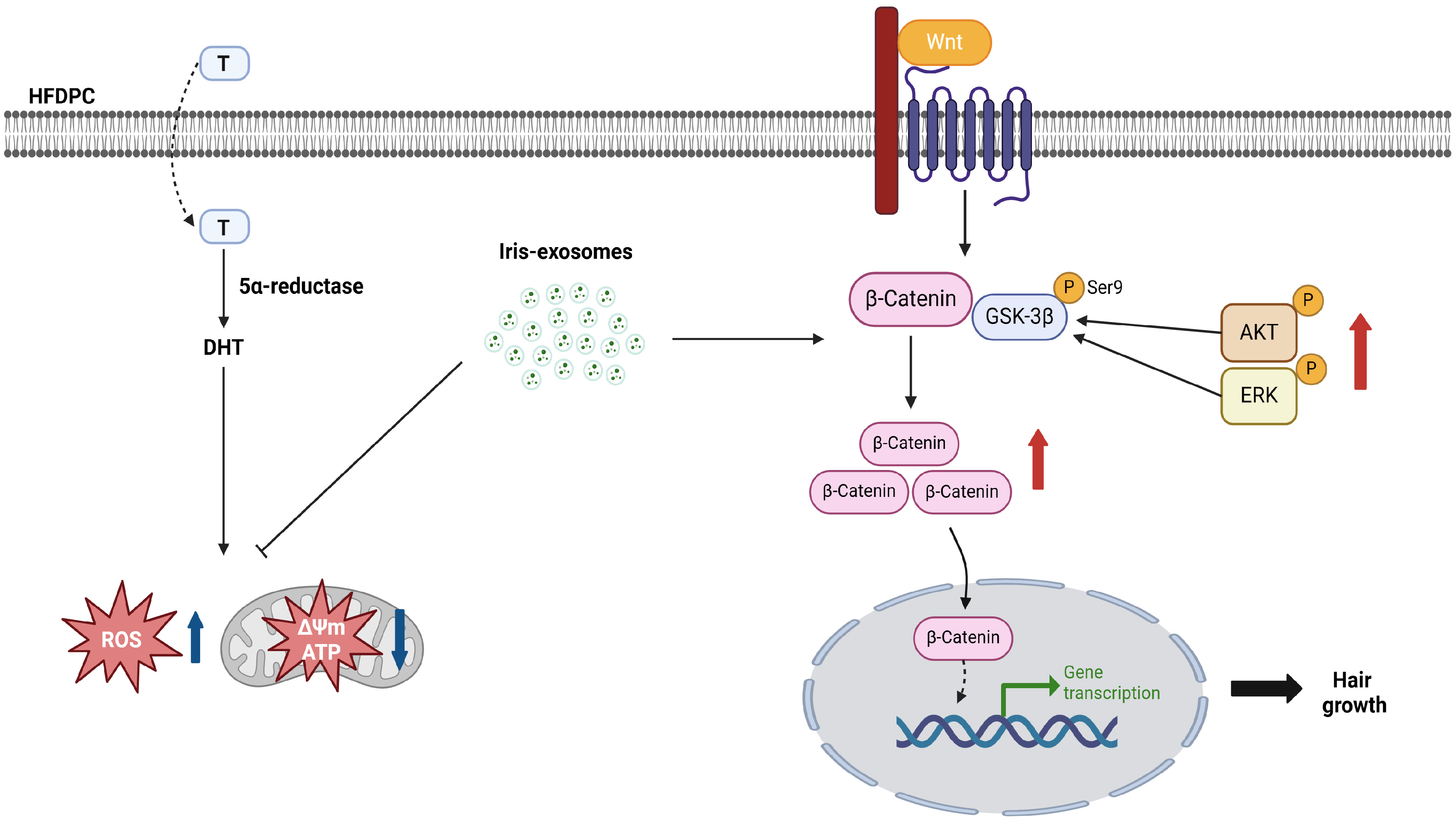
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of Iris-Exosomes
4.2. Dynamic Light Scattering
4.3. Cryo-TEM Analysis
4.4. Nanoparticle Tracking Analysis
4.5. Protein Quantification of Iris-Exosomes
4.6. Cell Culture
4.7. Cell Viability Assay
4.8. Wound Healing Assay
4.9. Alkaline Phosphatase Staining Assay
4.10. DCF-DA ROS Assay
4.11. Quantitative Real-Time Polymerase Chain Reaction
4.12. Immunofluorescence Analysis
4.13. Measurement of Mitochondrial Membrane Potential
4.14. ATP Assay
4.15. Western Blot Analysis
4.16. 3D Spheroid Formation of HFDPCs
4.17. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.B.; Collie, S.P.; Parent, C.A. The ins-and-outs of exosome biogenesis, secretion, and internalization. Trends Cell Biol. 2024, 34, 90. [Google Scholar] [CrossRef]
- Février, B.; Raposo, G. Exosomes: Endosomal-derived vesicles shipping extracellular messages. Curr. Opin. Cell Biol. 2004, 16, 415. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef]
- Lee, J.H.; Won, Y.J.; Kim, H.; Choi, M.; Lee, E.; Ryoou, B.; Lee, S.; Cho, B.S. Adipose Tissue-Derived Mesenchymal Stem Cell-Derived Exosomes Promote Wound Healing and Tissue Regeneration. Int. J. Mol. Sci. 2023, 24, 10434. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Zolbanin, N.M.; Jafari, R. Mesenchymal stem cell derived-exosomes: A modern approach in translational medicine. J. Transl. Med. 2020, 18, 449. [Google Scholar] [CrossRef]
- Kalluri, R.; Lebleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2017, 156, 16. [Google Scholar] [CrossRef]
- Rajendran, R.L.; Gangadaran, P.; Bak, S.S.; Oh, J.M.; Kalimuthu, S.; Lee, H.W.; Baek, S.H.; Zhu, L.; Sung, Y.K.; Jeong, S.Y.; et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 2017, 7, 15560. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Seo, C.H.; Gangadaran, P.; Ahn, B.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp. Dermatol. 2019, 28, 854. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Hong, Y.D.; Kim, D.; Park, S.J.; Kim, J.S.; Kim, H.; Yoon, E.J.; Cho, J. Confirmation of plant-derived exosomes as bioactive substances for skin application through comparative analysis of keratinocyte transcriptome. Appl. Biol. Chem. 2022, 65, 8. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 69. [Google Scholar] [CrossRef]
- Thakur, A.; Shah, D.; Rai, D.; Parra, D.C.; Pathikonda, S.; Kurilova, S.; Cili, A. Therapeutic Values of Exosomes in Cosmetics, Skin Care, Tissue Regeneration, and Dermatological Diseases. Cosmetics 2023, 10, 65. [Google Scholar] [CrossRef]
- Pratt, C.H.; King, L.E.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic alopecia: A review. Endocrine 2017, 57, 9. [Google Scholar] [CrossRef]
- Alessandrini, A.; Bruni, F.; Piraccini, B.M.; Starace, M. Common causes of hair loss—Clinical manifestations, trichoscopy and therapy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 629–640. [Google Scholar] [CrossRef]
- Griggs, J.; Trüeb, R.M.; Gavazzoni Dias, M.F.R.; Hordinsky, M.; Tosti, A. Fibrosing alopecia in a pattern distribution. J. Am. Acad. Dermatol. 2020, 85, 1557. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, M.; He, Y.; Liu, F.; Chen, L.; Xiong, X. Cellular Senescence: Ageing and Androgenetic Alopecia. Dermatology 2024, 239, 533. [Google Scholar] [CrossRef]
- Hobo, Y.; Nishikawa, J.; Taniguchi Asai, N.; Yoneyama, K.; Watanabe, Y.; Miyashiro, Y.; Fujikata, A. Evaluation of the therapeutic effects of AGA drugs by measuring finasteride, dutasteride, and dihydrotestosterone in hair. Clin. Chim. Acta 2023, 547, 117456. [Google Scholar] [CrossRef]
- Owecka, B.; Tomaszewska, A.; Dobrzeniecki, K.; Owecki, M. The Hormonal Background of Hair Loss in Non-Scarring Alopecias. Biomedicines 2024, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Huang, J.; Li, K.; Chen, Y.; He, Y.; Sun, Y.; Guo, Y.; Du, L.; Qu, Q.; Miao, Y.; et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. 2021, 137, 111247. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, H.; Erel-Akbaba, G.; Başpınar, Y.; Şentürk, Ş. Design of Liposome Formulations for CRISPR/Cas9 Enzyme Immobilization: Evaluation of 5-Alpha-Reductase Enzyme Knockout for Androgenic Disorders. ACS Omega 2023, 8, 46101. [Google Scholar] [CrossRef]
- Gubelin Harcha, W.; Barboza Martínez, J.; Tsai, T.; Katsuoka, K.; Kawashima, M.; Tsuboi, R.; Barnes, A.; Ferron-Brady, G.; Chetty, D. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J. Am. Acad. Dermatol. 2014, 70, 489. [Google Scholar] [CrossRef]
- Fertig, R.M.; Gamret, A.C.; Darwin, E.; Gaudi, S. Sexual side effects of 5-α-reductase inhibitors finasteride and dutasteride: A comprehensive review. Dermatol. Online J. 2017, 23, 3. [Google Scholar] [CrossRef]
- Rossi, A.; Cantisani, C.; Melis, L.; Iorio, A.; Scali, E.; Calvieri, S. Minoxidil use in dermatology, side effects and recent patents. Recent. Pat. Inflamm. Allergy Drug Discov. 2012, 6, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Borrelli, M.R.; Tapking, C.; Popp, D.; Puladi, B.; Ooms, M.; Chelliah, M.P.; Rein, S.; Pförringer, D.; Thor, D.; et al. Molecular Mechanisms of Hair Growth and Regeneration: Current Understanding and Novel Paradigms. Dermatology 2024, 236, 271. [Google Scholar] [CrossRef]
- Woo, W.; Zhen, H.H.; Oro, A.E. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin–Shh regulatory loop. Genes. Dev. 2012, 26, 1235. [Google Scholar] [CrossRef]
- Kageyama, T.; Seo, J.; Yan, L.; Fukuda, J. Effects of oxytocin on the hair growth ability of dermal papilla cells. Sci. Rep. 2023, 13, 15587. [Google Scholar] [CrossRef]
- Kwack, M.H.; Yang, J.M.; Won, G.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Establishment and characterization of five immortalized human scalp dermal papilla cell lines. Biochem. Biophys. Res. Commun. 2018, 496, 346. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, J.; Kang, D.; Zhou, Y.; Du, L.; Qu, Q.; Wang, J.; Wen, L.; Fu, D.; Hu, Z.; et al. Microenvironmental reprogramming of human dermal papilla cells for hair follicle tissue engineering. Acta Biomater. 2022, 165, 31. [Google Scholar] [CrossRef] [PubMed]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of Hair Follicle Dermal Papilla cells asin vitroscreening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429. [Google Scholar] [CrossRef]
- Qiu, W.; Lei, M.; Zhou, L.; Bai, X.; Lai, X.; Yu, Y.; Yang, T.; Lian, X. Hair follicle stem cell proliferation, Akt and Wnt signaling activation in TPA-induced hair regeneration. Histochem. Cell Biol. 2017, 147, 749. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Guo, H.; Qiu, W.; Lai, X.; Yang, T.; Widelitz, R.B.; Chuong, C.; Lian, X.; Yang, L. Modulating hair follicle size with Wnt10b/DKK1 during hair regeneration. Exp. Dermatol. 2014, 23, 407. [Google Scholar] [CrossRef]
- Chen, X.; Liu, B.; Li, Y.; Han, L.; Tang, X.; Deng, W.; Lai, W.; Wan, M. Dihydrotestosterone Regulates Hair Growth Through the Wnt/β-Catenin Pathway in C57BL/6 Mice and In Vitro Organ Culture. Front. Pharmacol. 2020, 10, 1528. [Google Scholar] [CrossRef]
- Leirós, G.J.; Ceruti, J.M.; Castellanos, M.L.; Kusinsky, A.G.; Balañá, M.E. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol. Cell. Endocrinol. 2016, 439, 26. [Google Scholar] [CrossRef]
- Lin, C.; Yuan, Y.; Chen, X.; Li, H.; Cai, B.; Liu, Y.; Zhang, H.; Li, Y.; Huang, K. Expression of Wnt/β-catenin signaling, stem-cell markers and proliferating cell markers in rat whisker hair follicles. J. Mol. Hist. 2015, 46, 233. [Google Scholar] [CrossRef]
- Ryu, Y.C.; Lee, D.; Shim, J.; Park, J.; Kim, Y.; Choi, S.; Bak, S.S.; Sung, Y.K.; Lee, S.; Choi, K. KY19382, a novel activator of Wnt/beta-catenin signalling, promotes hair regrowth and hair follicle neogenesis. Br. J. Pharmacol. 2021, 178, 2533–2546. [Google Scholar] [CrossRef]
- Bin Rubaian, N.; Alzamami, H.; Amir, B.A. An Overview of Commonly Used Natural Alternatives for the Treatment of Androgenetic Alopecia, with Special Emphasis on Rosemary Oil. Clin. Cosmet. Investig. Dermatol. 2024, 17, 2495. [Google Scholar] [CrossRef]
- Dhariwala, M.Y.; Ravikumar, P. An overview of herbal alternatives in androgenetic alopecia. J. Cosmet. Dermatol. 2019, 18, 966. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Boo, M.Y.; Boo, Y.C. Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets. Molecules 2024, 29, 2288. [Google Scholar] [CrossRef]
- Rahman, A.; Nasim, S.; Baig, I.; Jalil, S.; Orhan, I.; Sener, B.; Choudhary, M.I. Anti-inflammatory isoflavonoids from the rhizomes of Iris germanica. J. Ethnopharmacol. 2003, 86, 177. [Google Scholar] [CrossRef]
- Sayyed, K.; Hdayed, I.; Tabcheh, M.; Abdel-Razzak, Z.; El-Bitar, H. Antioxidant properties of the Lebanese plant Iris x germanica L. crude extracts and antagonism of chlorpromazine toxicity on Saccharomyces cerevisiae. Drug Chem. Toxicol. 2020, 45, 1168. [Google Scholar] [CrossRef] [PubMed]
- Yousefsani, B.S.; Boozari, M.; Shirani, K.; Jamshidi, A.; Dadmehr, M. A review on phytochemical and therapeutic potential of Iris germanica. J. Pharm. Pharmacol. 2021, 73, 611. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.Ž.; Gašić, U.M.; Pešić, M.B.; Stanojević, S.P.; Barać, M.B.; Mačukanović-jocić, M.P.; Avramov, S.N.; Tešić, Ž.L. Phytochemical Analysis and Total Antioxidant Capacity of Rhizome, Above-Ground Vegetative Parts and Flower of Three Iris Species. Chem. Biodivers. 2019, 16, e1800565. [Google Scholar] [CrossRef]
- Ullah, F.; Ayaz, M.; Sadiq, A.; Hussain, A.; Ahmad, S.; Imran, M.; Zeb, A. Phenolic, flavonoid contents, anticholinesterase and antioxidant evaluation of Iris germanica var; florentina. Nat. Prod. Res. 2015, 30, 1440. [Google Scholar] [CrossRef]
- Philpott, M.P.; Kealey, T. Effects of EGF on the morphology and patterns of DNA synthesis in isolated human hair follicles. J. Investig. Dermatol. 1994, 102, 186–191. [Google Scholar] [CrossRef]
- Elliott, K.; Stephenson, T.J.; Messenger, A.G. Differences in hair follicle dermal papilla volume are due to extracellular matrix volume and cell number: Implications for the control of hair follicle size and androgen responses. J. Investig. Dermatol. 1999, 113, 873–877. [Google Scholar] [CrossRef]
- Yang, C.; Cotsarelis, G. Review of hair follicle dermal cells. J. Dermatol. Sci. 2011, 57, 2. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.; Min, D.S.; Kim, H.; Choi, K. Valproic Acid Induces Hair Regeneration in Murine Model and Activates Alkaline Phosphatase Activity in Human Dermal Papilla Cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Jang, Y.J.; Won, G.H.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Overexpression of alkaline phosphatase improves the hair-inductive capacity of cultured human dermal papilla spheres. J. Dermatol. Sci. 2019, 95, 126. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Oh, C.T.; Choi, E.J.; Park, H.M.; Han, H.J.; Ji, H.J.; Kim, B.J. Human placental extract exerts hair growth-promoting effects through the GSK-3β signaling pathway in human dermal papilla cells. Int. J. Mol. Med. 2015, 36, 1088. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Kim, T.; Kyung, J.; Kim, D.; Park, D.; Choi, E.; Lee, S.; Yang, W.; Kang, M.; Kim, Y. Effectiveness of the combinational treatment of Laminaria japonica and Cistanche tubulosa extracts in hair growth. Lab. Anim. Res. 2015, 31, 24–32. [Google Scholar] [CrossRef]
- Tobin, D.J. Introduction to skin aging. J. Tissue Viability 2017, 26, 37. [Google Scholar] [CrossRef]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 799. [Google Scholar] [CrossRef]
- Poljšak, B.; Dahmane, R. Free Radicals and Extrinsic Skin Aging. Dermatol. Res. Pract. 2012, 2012, 135206. [Google Scholar] [CrossRef]
- Zheng, M.; Jang, Y.; Choi, N.; Kim, D.Y.; Han, T.W.; Yeo, J.H.; Lee, J.; Sung, J.-H. Hypoxia improves hair inductivity of dermal papilla cells via nuclear NADPH oxidase 4-mediated reactive oxygen species generation’. Br. J. Dermatol. 2019, 181, 523. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Zhu, H.; Itoh, K.; Yamamoto, M.; Zweier, J.L.; Li, Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: Protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005, 579, 3029. [Google Scholar] [CrossRef]
- Suomalainen, A.; Battersby, B.J. Mitochondrial diseases: The contribution of organelle stress responses to pathology. Nat. Rev. Mol. Cell Biol. 2017, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Geyfman, M.; Plikus, M.V.; Treffeisen, E.; Andersen, B.; Paus, R. Resting no more: Re-defining telogen, the maintenance stage of the hair growth cycle. Biol. Rev. 2016, 90, 1179. [Google Scholar] [CrossRef]
- Bodemer, C.; Rötig, A.; Rustin, P.; Cormier, V.; Niaudet, P.; Saudubray, J.; Rabier, D.; Munnich, A.; De Prost, Y. Hair and Skin Disorders as Signs of Mitochondrial Disease. Pediatrics 1999, 103, 428. [Google Scholar] [CrossRef] [PubMed]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It is all about energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef]
- Li, F.; Liu, H.; Wu, X.; Song, Z.; Tang, H.; Gong, M.; Liu, L.; Li, F. Tetrathiomolybdate Decreases the Expression of Alkaline Phosphatase in Dermal Papilla Cells by Increasing Mitochondrial ROS Production. Int. J. Mol. Sci. 2023, 24, 3123. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chae, C.W.; Choi, G.E.; Shin, H.C.; Lim, J.R.; Chang, H.S.; Park, J.; Cho, J.H.; Park, M.R.; Lee, H.J.; et al. Cyanidin 3-O-arabinoside suppresses DHT-induced dermal papilla cell senescence by modulating p38-dependent ER-mitochondria contacts. J. Biomed. Sci. 2022, 29, 17. [Google Scholar] [CrossRef]
- Lee, S.; Ohn, J.; Kang, B.M.; Hwang, S.T.; Kwon, O. Activation of mitochondrial aldehyde dehydrogenase 2 promotes hair growth in human hair follicles. J. Adv. Res. 2023, 64, 237. [Google Scholar] [CrossRef] [PubMed]
- Veltri, A.; Lang, C.; Lien, W. Concise Review: Wnt Signaling Pathways in Skin Development and Epidermal Stem Cells. Stem Cells 2017, 36, 22. [Google Scholar] [CrossRef]
- Shin, D.W. The Molecular Mechanism of Natural Products Activating Wnt/β-Catenin Signaling Pathway for Improving Hair Loss. Life 2022, 12, 1856. [Google Scholar] [CrossRef]
- Leirós, G.J.; Attorresi, A.I.; Balañá, M.E. Hair follicle stem cell differentiation is inhibited through cross-talk between Wnt/β-catenin and androgen signalling in dermal papilla cells from patients with androgenetic alopecia. Br. J. Dermatol. 2011, 166, 1035. [Google Scholar] [CrossRef]
- Doumpas, N.; Lampart, F.; Robinson, M.D.; Lentini, A.; Nestor, C.E.; Cantù, C.; Basler, K. TCF/LEFdependent and independent transcriptional regulation of Wnt/β-catenin target genes. EMBO J. 2018, 38, e98873. [Google Scholar] [CrossRef]
- Shah, K.; Kazi, J.U. Phosphorylation-Dependent Regulation of WNT/Beta-Catenin Signaling. Front. Oncol. 2022, 12, 858782. [Google Scholar] [CrossRef] [PubMed]
- Taghiabadi, E.; Nilforoushzadeh, M.A.; Aghdami, N. Maintaining Hair Inductivity in Human Dermal Papilla Cells: A Review of Effective Methods. Skin Pharmacol. Physiol. 2020, 33, 280. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Miao, Y.; Wang, J.; Fan, Z.; Du, L.; Su, Y.; Liu, B.; Hu, Z.; Xing, M. Surface Tension Guided Hanging-Drop: Producing Controllable 3D Spheroid of High-Passaged Human Dermal Papilla Cells and Forming Inductive Microtissues for Hair-Follicle Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 5906. [Google Scholar] [CrossRef]
- Topouzi, H.; Logan, N.J.; Williams, G.; Higgins, C.A. Methods for the isolation and 3D culture of dermal papilla cells from human hair follicles. Exp. Dermatol. 2017, 26, 491. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Kobayashi, T.; Sasaki, T.; Shimizu, A.; Amagai, M. Restoration of the intrinsic properties of human dermal papilla in vitro. J. Cell Sci. 2012, 125, 4114–4125. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, X.; Liao, X. Dermal Papilla Cells: From Basic Research to Translational Applications. Biology 2024, 13, 842. [Google Scholar] [CrossRef]
- Lu, K.; Han, Q.; Ma, Z.; Yan, Q.; Pei, Y.; Shi, P.; Zhang, J.; Rong, K.; Ma, K.; Li, P.; et al. Injectable platelet rich fibrin facilitates hair follicle regeneration by promoting human dermal papilla cell proliferation, migration, and trichogenic inductivity. Exp. Cell Res. 2021, 409, 112888. [Google Scholar] [CrossRef]
- McElwee, K.J.; Kissling, S.; Wenzel, E.; Huth, A.; Hoffmann, R. Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J. Investig. Dermatol. 2003, 121, 1267–1275. [Google Scholar] [CrossRef]
- Iida, M.; Ihara, S.; Matsuzaki, T. Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev. Growth Differ. 2007, 49, 185. [Google Scholar] [CrossRef]
- Rajendran, R.L.; Gangadaran, P.; Kwack, M.H.; Oh, J.M.; Hong, C.M.; Sung, Y.K.; Lee, J.; Ahn, B. Human fibroblast-derived extracellular vesicles promote hair growth in cultured human hair follicles. FEBS Lett. 2021, 595, 942. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.; Oliva, A.K.; Ke, M.S.; Ferdousi, F.; Isoda, H. 3D Spheroid Human Dermal Papilla Cell as an Effective Model for the Screening of Hair Growth Promoting Compounds: Examples of Minoxidil and 3,4,5-Tri-O-caffeoylquinic acid (TCQA). Cells 2022, 11, 2093. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lim, K.M.; Cha, H.J.; An, I.; Lee, J.P.; Lee, K.S.; Lee, G.T.; Lee, K.K.; Jung, H.J.; Ahn, K.J.; et al. Arctiin blocks hydrogen peroxide-induced senescence and cell death though microRNA expression changes in human dermal papilla cells. Biol. Res. 2014, 47, 50. [Google Scholar] [CrossRef] [PubMed]
- Goren, A.; Naccarato, T.; Situm, M.; Kovacevic, M.; Lotti, T.; McCoy, J. Mechanism of action of minoxidil in the treatment of androgenetic alopecia is likely mediated by mitochondrial adenosine triphosphate synthase-induced stem cell differentiation. J. Biol. Regul. Homeost. Agents 2017, 31, 1049–1053. [Google Scholar]
- Wang, Y.; Hekimi, S. Mitochondrial dysfunction and longevity in animals: Untangling the knot. Science 2015, 350, 1204–1207. [Google Scholar] [CrossRef]
- Chew, E.G.Y.; Lim, T.C.; Leong, M.F.; Liu, X.; Sia, Y.Y.; Leong, S.T.; Yan-jiang, B.C.; Stoecklin, C.; Borhan, R.; Heilmann-heimbach, S.; et al. Observations that suggest a contribution of altered dermal papilla mitochondrial function to androgenetic alopecia. Exp. Dermatol. 2022, 31, 906. [Google Scholar] [CrossRef]
- Hadjihannas, M.V.; Bernkopf, D.B.; Brückner, M.; Behrens, J. Cell cycle control of Wnt/β-catenin signalling by conductin/axin2 through CDC20. EMBO Rep. 2012, 13, 347. [Google Scholar] [CrossRef]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef]
- Dong, J.; Xu, X.; Zhang, Q.; Yuan, Z.; Tan, B. The PI3K/AKT pathway promotes fracture healing through its crosstalk with Wnt/beta-catenin. Exp.Cell Res. 2020, 394, 112137. [Google Scholar] [CrossRef]
- Shimizu, Y.; Ntege, E.H.; Sunami, H.; Inoue, Y. Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature. Regen. Ther. 2022, 21, 527. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.; Yoon, E.; Lee, H.; Ji, Y.; Kim, Y.; Park, S.; Kim, J.; Bae, S. Protective Effect of Iris germanica L. Rhizome-Derived Exosome against Oxidative-Stress-Induced Cellular Senescence in Human Epidermal Keratinocytes. Appl. Sci. 2023, 13, 11681. [Google Scholar] [CrossRef]
- Kang, J.Y.; Yoon, B.K.; Baek, H.; Ko, Y.; Bhang, S.H.; Jackman, J.A.; Kim, J.W. Facile and scalable fabrication of exosome-mimicking nanovesicles through PEGylated lipid detergent-aided cell extrusion. Nanoscale 2022, 14, 16581–16589. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Woo, J.; Kim, J.; Choi, M.; Shin, H.J.; Kim, Y.; Kim, J.; Shin, D.W. Iris germanica L. Rhizome-Derived Exosomes Ameliorated Dihydrotestosterone-Damaged Human Follicle Dermal Papilla Cells Through the Activation of Wnt/β-Catenin Pathway. Int. J. Mol. Sci. 2025, 26, 4070. https://doi.org/10.3390/ijms26094070
Kim M, Woo J, Kim J, Choi M, Shin HJ, Kim Y, Kim J, Shin DW. Iris germanica L. Rhizome-Derived Exosomes Ameliorated Dihydrotestosterone-Damaged Human Follicle Dermal Papilla Cells Through the Activation of Wnt/β-Catenin Pathway. International Journal of Molecular Sciences. 2025; 26(9):4070. https://doi.org/10.3390/ijms26094070
Chicago/Turabian StyleKim, Mujun, Jung Woo, Jinsick Kim, Minah Choi, Hee Jung Shin, Youngseok Kim, Junoh Kim, and Dong Wook Shin. 2025. "Iris germanica L. Rhizome-Derived Exosomes Ameliorated Dihydrotestosterone-Damaged Human Follicle Dermal Papilla Cells Through the Activation of Wnt/β-Catenin Pathway" International Journal of Molecular Sciences 26, no. 9: 4070. https://doi.org/10.3390/ijms26094070
APA StyleKim, M., Woo, J., Kim, J., Choi, M., Shin, H. J., Kim, Y., Kim, J., & Shin, D. W. (2025). Iris germanica L. Rhizome-Derived Exosomes Ameliorated Dihydrotestosterone-Damaged Human Follicle Dermal Papilla Cells Through the Activation of Wnt/β-Catenin Pathway. International Journal of Molecular Sciences, 26(9), 4070. https://doi.org/10.3390/ijms26094070






