Epicatechin Decreases UCP2 Gene Expression in MDA-MB-231 Breast Cancer Cells by the Presence of a Regulatory Element in the Promoter
Abstract
:1. Introduction
2. Results
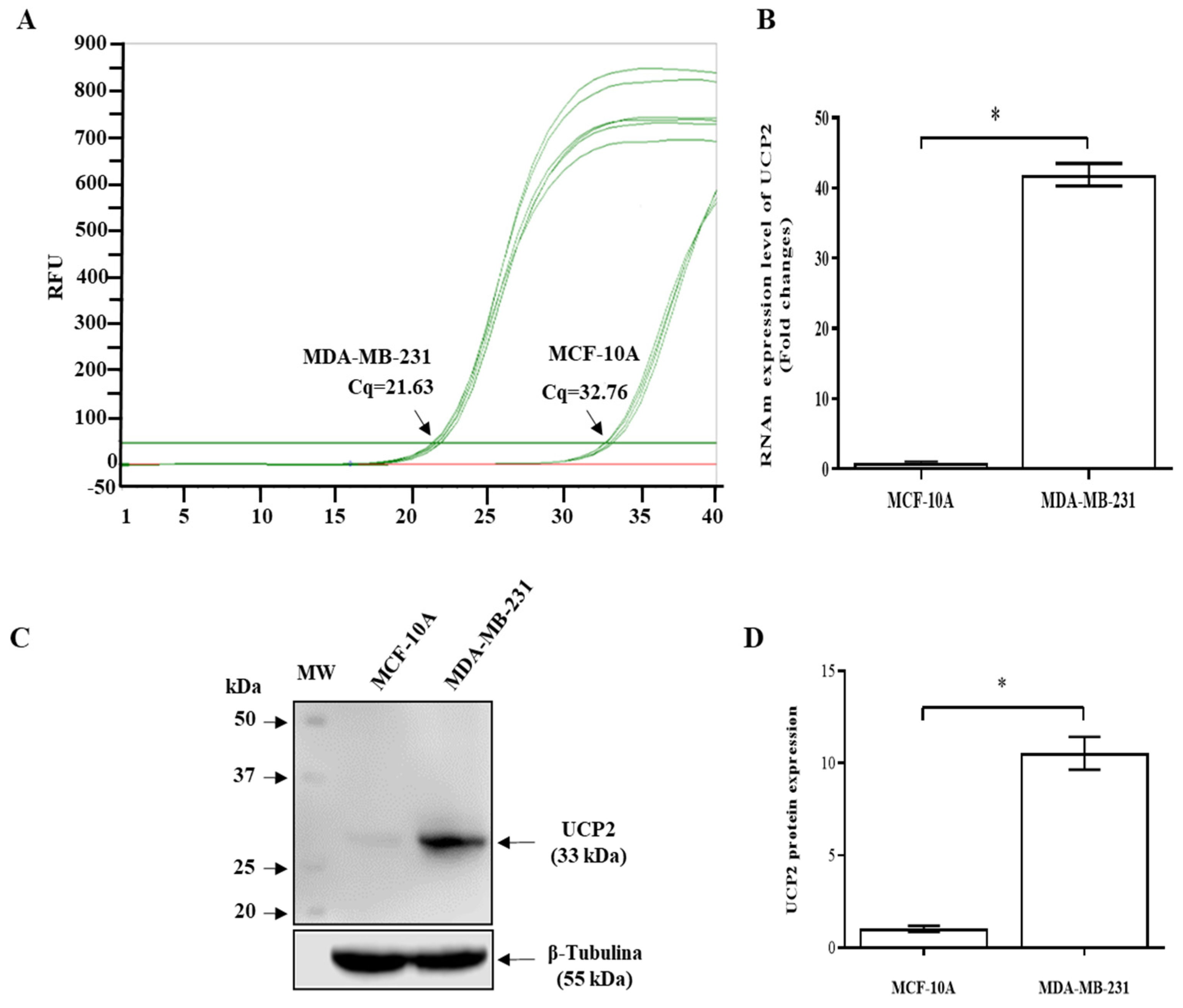
2.1. Differential UCP2 Expression Among MDA-MB-231 Breast Cancer Cells and MCF10A Cells (Breast Noncancerous Cells)
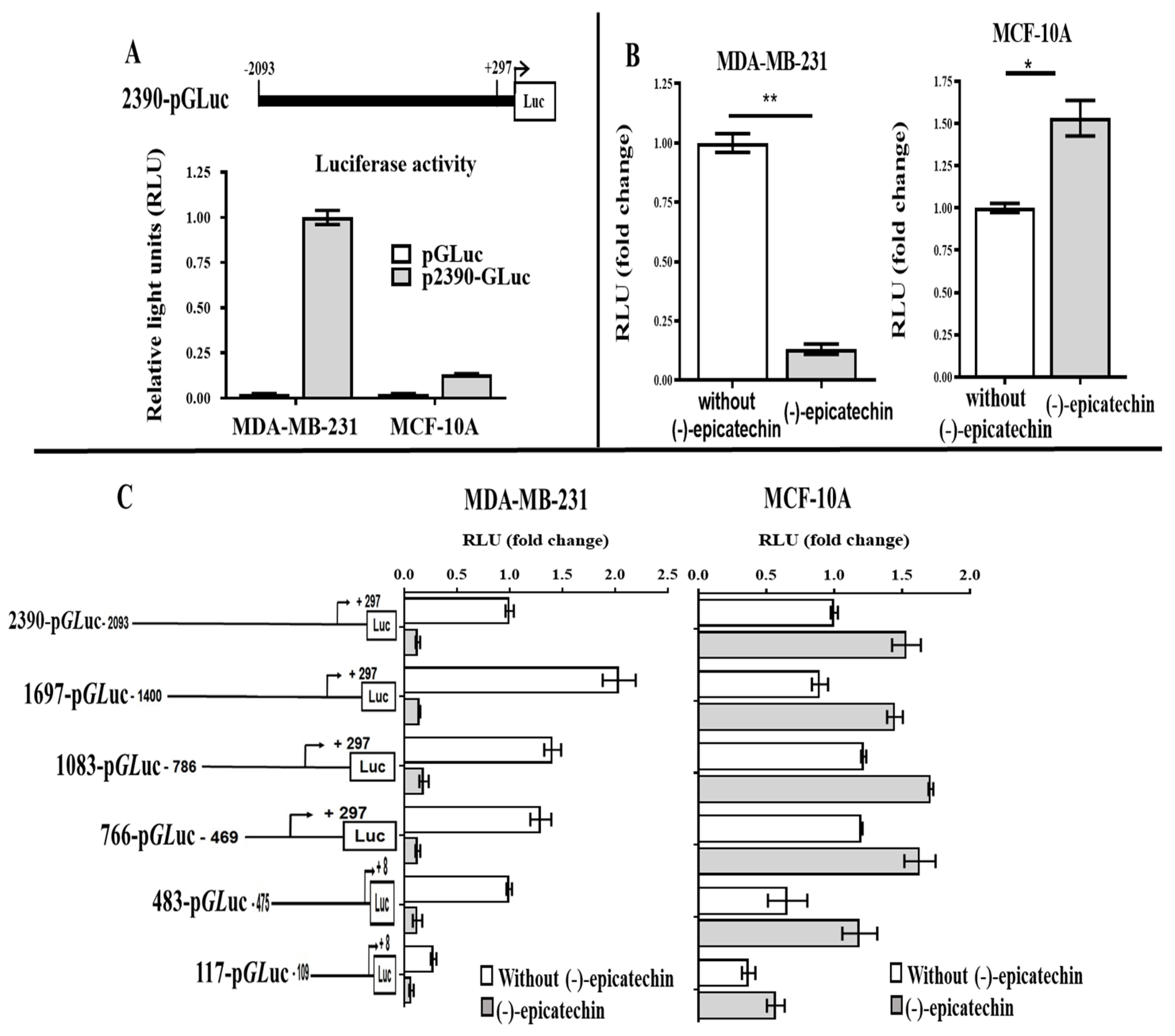
2.2. Treatment with (-)-Epicatechin Decreased UCP2 Expression in MDA-MB-231 Cells but Not in MCF10A Cells
2.3. Transcriptional Regulation of the Human UCP2 Gene by (-)-Epicatechin
2.4. The 5′ Region of 117 Bp Is a Minimal Promoter Region That Contains the Elements That Respond to (-)-Epicatechin and the Basal Promoter Activity of the UCP2 Gene
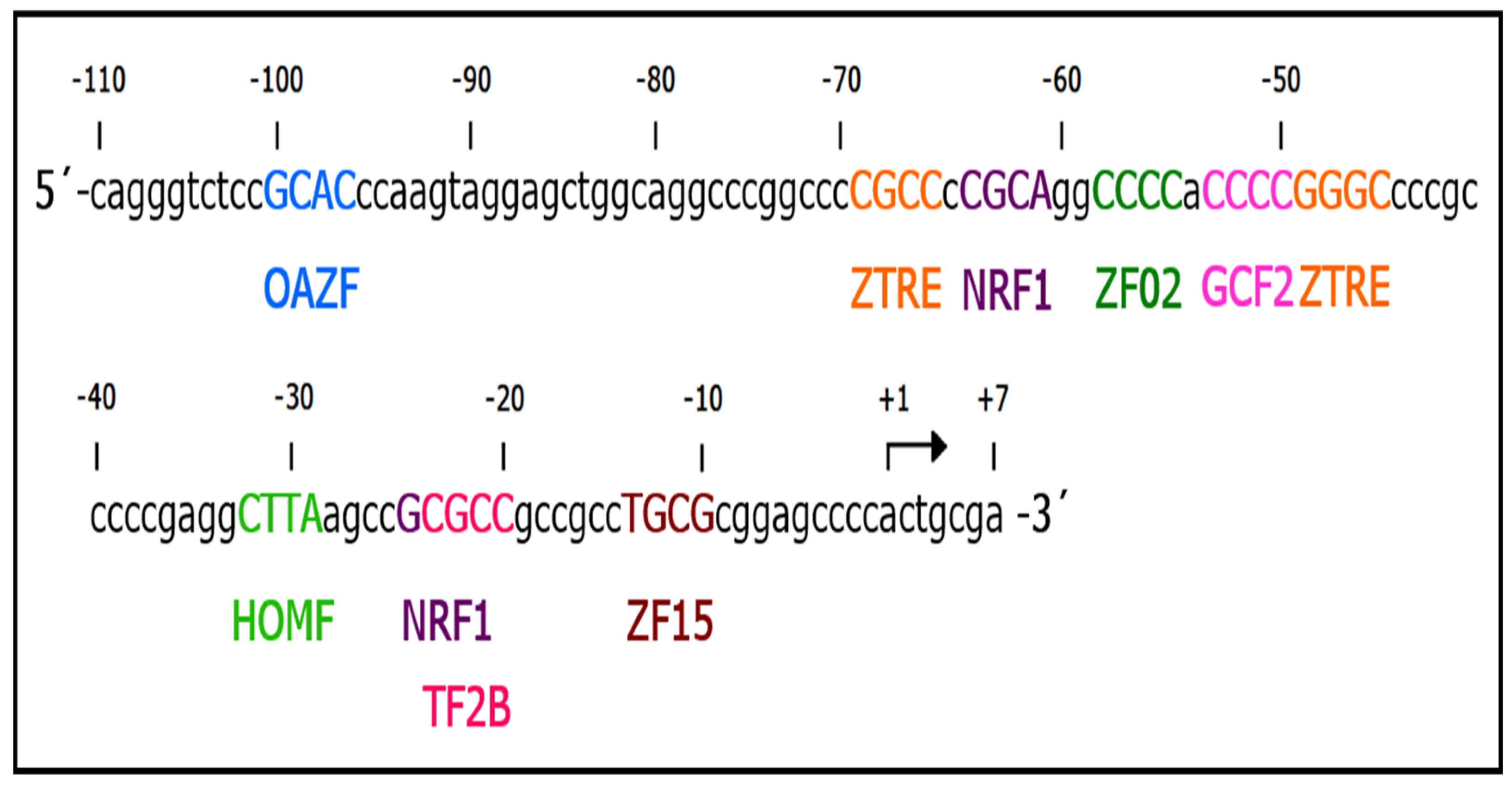
2.5. One Element in the 5′ Region Is Required for the Regulatory Effect of (-)-Epicatechin on UCP2 Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture and (-)-Epicatechin Treatment
4.2. RNA Isolation and qRT–PCR
4.3. Purification of UCP2 Protein and Western Blotting
4.4. Cloning, Subcloning and Analysis of the Basal Promoter Activity of the Human UCP2 Gene
4.5. Generation of UCP2 Promoter Deletion-Luciferase Fusion
4.6. Transfection, (-)-Epicatechin Treatment and Promoter Activity
4.7. Electrophoretic Mobility Shift Assays (EMSAs)
4.8. Bioinformatic Analysis of the Promoter Region (−82/+8) of the UCP2 Gene
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Echtay, K.S.; Bienengraeber, M.; Mayinger, P.; Heimpel, S.; Winkler, E.; Druhmann, D.; Frischmuth, K.; Kamp, F.; Huang, S.-G. Uncoupling Proteins: Martin Klingenberg’s Contributions for 40 Years. Arch. Biochem. Biophys. 2018, 657, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. Forty years of Mitchell’s proton circuit: From little grey books to little grey cells. Biochim. Biophys. Acta. 2008, 1777, 550–556. [Google Scholar] [CrossRef]
- Nicholls, D.G. Mitochondrial proton leaks and uncoupling proteins. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148428. [Google Scholar] [CrossRef]
- Horimoto, M.; Fulop, P.; Derdak, Z.; Wands, J.R.; Baffy, G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology 2004, 39, 386–392. [Google Scholar] [CrossRef]
- Mailloux, R.J.; Harper, M.E. Mitochondrial proticity and ROS signaling: Lessons from the uncoupling proteins. Trends Endocrinol. Metab. 2012, 23, 451–458. [Google Scholar] [CrossRef]
- Mao, W.; Yu, X.X.; Zhong, A.; Li, W.; Brush, J.; Sherwood, S.W.; Adams, S.H.; Pan, G. UCP4, a novel brain-specific mitochondrial protein that reduces membrane potential in mammalian cells. FEBS Lett. 1999, 443, 326–330. [Google Scholar] [CrossRef]
- Ramsden, D.B.; Ho, P.W.; Ho, J.W.; Liu, H.F.; So, D.H.; Tse, H.M.; Chan, K.H.; Ho, S.L. Human neuronal uncoupling proteins 4 and 5(UCP4 and UCP5): Structural properties, regulation, and physiological role in protection against oxidative stress and mitochondrial dysfunction. Brain Behav. 2012, 2, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.X.; Mao, W.; Zhong, A.; Schow, P.; Brush, J.; Sherwood, S.W.; Adams, S.H.; Pan, G. Characterization of novel UCP5/BMCP1 isoforms and differential regulation of UCP4 and UCP5 expression through dietary or temperature manipulation. FASEB J. 2000, 14, 1611–1618. [Google Scholar] [CrossRef]
- Ayyasamy, V.; Owens, K.M.; Desouki, M.M.; Liang, P.; Bakin, A.; Thangaraj, K.; Buchsbaum, D.J.; LoBuglio, A.F.; Singh, K.K. Cellular Model of Warburg Effect Identifies Tumor Promoting Function of UCP2 in Breast Cancer and Its Suppression by Genipin. PLoS ONE 2011, 6, e24792. [Google Scholar] [CrossRef]
- Yu, X.; Shi, M.; Wu, Q.; Wei, W.; Sun, S.; Zhu, S. Identification of UCP1 and UCP2 as Potential Prognostic Markers in Breast Cancer: A Study Based on Immunohistochemical Analysis and Bioinformatics. Front. Cell Dev. Biol. 2022, 10, 891731. [Google Scholar] [CrossRef]
- Park, H.K.; Choi, Y.D.; Shim, H.J.; Choi, Y.; Chung, I.J.; Yun, S.J. Comparative Whole-Genome Sequencing Analysis of In-Situ and Invasive Acral Lentiginous Melanoma: Markedly Increased Copy Number Gains of GAB2, PAK1, UCP2, and CCND1 Are Associated with Melanoma Invasion. Am. J. Surg. Pathol. 2024, 48, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Shi, L.; Lin, W.; Lu, B.; Zhao, Y. UCP2 Promotes Proliferation and Chemoresistance through Regulating the NF-κB/β-Catenin Axis and Mitochondrial ROS in Gallbladder Cancer. Biochem. Pharmacol. 2020, 172, 113745. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Liu, J.; Xu, K.; Dong, J. Uncoupling Protein 2 Mediates Resistance to Gemcitabine-Induced Apoptosis in Hepatocellular Carcinoma Cell Lines. Biosci. Rep. 2015, 35, e00231. [Google Scholar] [CrossRef]
- Esteves, P.; Pecqueur, C.; Alves-Guerra, M.-C. UCP2 Induces Metabolic Reprogramming to Inhibit Proliferation of Cancer Cells. Mol. Cell. Oncol. 2015, 2, e975024. [Google Scholar] [CrossRef]
- Cheng, W.-C.; Tsui, Y.-C.; Ragusa, S.; Koelzer, V.H.; Mina, M.; Franco, F.; Läubli, H.; Tschumi, B.; Speiser, D.; Romero, P.; et al. Uncoupling Protein 2 Reprograms the Tumor Microenvironment to Support the Anti-Tumor Immune Cycle. Nat. Immunol. 2019, 20, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, A.; Meng, Z.; Luciani, G.; Chen, L.-C.; Bennington, J.L.; Dairkee, S.H. Negative Regulation of UCP2 by TGFβ Signaling Characterizes Low and Intermediate-Grade Primary Breast Cancer. Cell Death Dis. 2010, 1, e53. [Google Scholar] [CrossRef]
- Hua, J.; Zhang, Z.; Zhang, L.; Sun, Y.; Yuan, Y. UCP-2 Inhibitor Enhanced the Efficacy of Trastuzumab against HER2 Positive Breast Cancer Cells. Cancer Chemother. Cancer Chemother. Pharmacol. 2021, 88, 633–642. [Google Scholar] [CrossRef]
- Cassard-Doulcier, A.M.; Gelly, C.; Fox, N.; Schrementi, J.; Raimbault, S.; Klaus, S.; Forest, C.; Bouillaud, F.; Ricquier, D. Tissue-Specific and Beta-Adrenergic Regulation of the Mitochondrial Uncoupling Protein Gene: Control by Cis-Acting Elements in the 5’-Flanking Region. Mol. Endocrinol. 1993, 7, 497–506. [Google Scholar]
- Boyer, B.B.; Kozak, L.P. The Mitochondrial Uncoupling Protein Gene in Brown Fat: Correlation between DNase I Hypersensitivity and Expression in Transgenic Mice. Mol. Cell. Biol. 1991, 11, 4147–4156. [Google Scholar]
- Yoshitomi, H.; Yamazaki, K.; Tanaka, I. Mechanism of Ubiquitous Expression of Mouse Uncoupling Protein 2 mRNA: Control by Cis-Acting DNA Element in 5’-Flanking Region. Biochem. J. 1999, 340, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Park, M.Y.; Kim, Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Kim, G.S. Function and Application of Flavonoids in the Breast Cancer. Int. J. Mol. Sci. 2022, 23, 7732. [Google Scholar] [CrossRef]
- Hatono, M.; Ikeda, H.; Suzuki, Y.; Kajiwara, Y.; Kawada, K.; Tsukioki, T.; Kochi, M.; Suzawa, K.; Iwamoto, T.; Yamamoto, H.; et al. Effect of Isoflavones on Breast Cancer Cell Development and Their Impact on Breast Cancer Treatments. Breast Cancer Res. Treat. 2021, 185, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Hussain, A.; Sharma, R. Molecular insight into apoptosis mediated by flavones in cancer (Review). World Acad. Sci. J. 2020, 2, 6. [Google Scholar] [CrossRef]
- Pereyra-Vergara, F.; Olivares-Corichi, I.M.; Perez-Ruiz, A.G.; Luna-Arias, J.P.; García-Sánchez, J.R. Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells Is Mediated by Reactive Oxygen Species. Molecules 2020, 25, 1020. [Google Scholar] [CrossRef]
- Osorio-Cruz, Y.; Olivares-Corichi, I.M.; Correa-Basurto, J.; González-Garrido, J.A.; Pereyra-Vergara, F.; Rivera, G.; García-Sánchez, J.R. The Autoxidized Mixture of (-)-Epicatechin Contains Procyanidins and Shows Antiproliferative and Apoptotic Activity in Breast Cancer Cells. Pharmaceuticals 2024, 17, 258. [Google Scholar] [CrossRef]
- Kothinti, R.; Tabatabai, N.M.; Petering, D.H. Electrophoretic mobility shift assay of zinc finger proteins: Competition for Zn(2+) bound to Sp1 in protocols including EDTA. J. Inorg. Biochem. 2011, 105, 569–576. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-Antioxidant Activity Relationships of Luteolin and Catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Leyva-Soto, A.; Alejandra Chavez-Santoscoy, R.; Porras, O.; Hidalgo-Ledesma, M.; Serrano-Medina, A.; Alejandra Ramírez-Rodríguez, A.; Alejandra Castillo-Martinez, N. Epicatechin and Quercetin Exhibit in Vitro Antioxidant Effect, Improve Biochemical Parameters Related to Metabolic Syndrome, and Decrease Cellular Genotoxicity in Humans. Food Res. Int. 2021, 142, 110101. [Google Scholar] [CrossRef]
- German, I.J.S.; Pomini, K.T.; Andreo, J.C.; Shindo, J.V.T.C.; Castro, M.V.M.D.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Fornari Laurindo, L.; Bueno, P.C.D.S.; et al. New Trends to Treat Muscular Atrophy: A Systematic Review of Epicatechin. Nutrients 2024, 16, 326. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.; Haddad, Z.; Deisling, S.; Ellinger, S. Effect of an (–)-Epicatechin Intake on Cardiometabolic Parameters—A Systematic Review of Randomized Controlled Trials. Nutrients 2022, 14, 4500. [Google Scholar] [CrossRef]
- Cheng, J.; Nanayakkara, G.; Shao, Y.; Cueto, R.; Wang, L.; Yang, W.Y.; Tian, Y.; Wang, H.; Yang, X. Mitochondrial Proton Leak Plays a Critical Role in Pathogenesis of Cardiovascular Diseases. Adv. Exp. Med. Biol. 2017, 982, 359–370. [Google Scholar] [PubMed]
- Vallejo, F.A.; Vanni, S.; Graham, R.M. UCP2 as a Potential Biomarker for Adjunctive Metabolic Therapies in Tumor Management. Front. Oncol. 2021, 11, 640720. [Google Scholar] [CrossRef]
- Hirschenson, J.; Melgar-Bermudez, E.; Mailloux, R.J. The Uncoupling Proteins: A Systematic Review on the Mechanism Used in the Prevention of Oxidative Stress. Antioxidants 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Raho, S.; Capobianco, L.; Malivindi, R.; Vozza, A.; Piazzolla, C.; De Leonardis, F.; Gorgoglione, R.; Scarcia, P.; Pezzuto, F.; Agrimi, G.; et al. KRAS-Regulated Glutamine Metabolism Requires UCP2-Mediated Aspartate Transport to Support Pancreatic Cancer Growth. Nat. Metab. 2020, 2, 1373–1381. [Google Scholar] [CrossRef]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. UCP2 Transports C4 Metabolites out of Mitochondria, Regulating Glucose and Glutamine Oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef]
- Gorgoglione, R.; Impedovo, V.; Riley, C.L.; Fratantonio, D.; Tiziani, S.; Palmieri, L.; Dolce, V.; Fiermonte, G. Glutamine-Derived Aspartate Biosynthesis in Cancer Cells: Role of Mitochondrial Transporters and New Therapeutic Perspectives. Cancers 2022, 14, 245. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Arasu, A.; Velusamy, T. Targeting Altered Calcium Homeostasis and Uncoupling Protein-2 Promotes Sensitivity in Drug-resistant Breast Cancer Cells. J. Biochem. Mol. Toxicol. 2024, 38, e23575. [Google Scholar] [CrossRef]
- Tu, N.; Chen, H.; Winnikes, U.; Reinert, I.; Marmann, G.; Pirke, K.M.; Lentes, K.-U. Molecular Cloning and Functional Characterization of the Promoter Region of the Human Uncoupling Protein-2 Gene. Biochem. Biophys. Res. Commun. 1999, 265, 326–334. [Google Scholar] [CrossRef]
- Qin, J.; Fu, M.; Wang, J.; Huang, F.; Liu, H.; Huangfu, M.; Yu, D.; Liu, H.; Li, X.; Guan, X.; et al. PTEN/AKT/mTOR signaling mediates anticancer effects of epigallocatechin-3-gallate in ovarian cancer. Oncol. Rep. 2020, 43, 1885–1896. [Google Scholar] [CrossRef]
- Ahmadi, N.; Mohamed, S.; Sulaiman Rahman, H.; Rosli, R. Epicatechin and scopoletin-rich Morinda citrifolia leaf ameliorated leukemia via anti-inflammatory, anti-angiogenesis, and apoptosis pathways in vitro and in vivo. J. Food Biochem. 2019, 43, e12868. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.W.; Xu, S.P. Galangin inhibits the cell progression and induces cell apoptosis through activating PTEN and Caspase-3 pathways in retinoblastoma. Biomed. Pharmacother. 2018, 97, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Y.; Wang, Y.; Chan, A.M. Multifaceted Regulation of PTEN Subcellular Distributions and Biological Functions. Cancers 2019, 11, 1247. [Google Scholar] [CrossRef]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nature reviews. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar]
- Žáčková, M.; Škobisová, E.; Urbánková, E.; Ježek, P. Activating ω-6 Polyunsaturated Fatty Acids and Inhibitory Purine Nucleotides Are High Affinity Ligands for Novel Mitochondrial Uncoupling Proteins UCP2 and UCP3. J. Biol. Chem. 2003, 278, 20761–20769. [Google Scholar] [CrossRef]
- Kreiter, J.; Rupprecht, A.; Zimmermann, L.; Moschinger, M.; Rokitskaya, T.I.; Antonenko, Y.N.; Gille, L.; Fedorova, M.; Pohl, E.E. Molecular Mechanisms Responsible for Pharmacological Effects of Genipin on Mitochondrial Proteins. Biophys. J. 2019, 117, 1845–1857. [Google Scholar] [CrossRef]
- Liu, C.H.; Huang, Z.H.; Dong, X.Y.; Zhang, X.Q.; Li, Y.H.; Zhao, G.; Sun, B.S.; Shen, Y.N. Inhibition of Uncoupling Protein 2 Enhances the Radiosensitivity of Cervical Cancer Cells by Promoting the Production of Reactive Oxygen Species. Oxid. Med. Cell. Longev. 2020, 2020, 5135893. [Google Scholar] [CrossRef]
- Derdak, Z.; Garcia, T.A.; Baffy, G. Detection of Uncoupling Protein-2 (UCP2) As a Mitochondrial Modulator of Apoptosis. In Apoptosis; Erhardt, P., Toth, A., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2009; Volume 559, pp. 205–217. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Hara, T.; Ishido, Y.; Yoshihara, A.; Oda, K.; Makino, M.; Ishii, N.; Hiroi, N.; Suzuki, K. Rapid Preparation of High-Purity Nuclear Proteins from a Small Number of Cultured Cells for Use in Electrophoretic Mobility Shift Assays. BMC Immunol. 2014, 15, 586. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.-W.; Alqadah, A.; Chuang, C.-F. An Optimized Protocol for Electrophoretic Mobility Shift Assay Using Infrared Fluorescent Dye-Labeled Oligonucleotides. J. Vis. Exp. 2016, 117, 54863. [Google Scholar]
- Cartharius, K.; Frech, K.; Grote, K.; Klocke, B.; Haltmeier, M.; Klingenhoff, A.; Frisch, M.; Bayerlein, M.; Werner, T. MatInspector and beyond: Promoter Analysis Based on Transcription Factor Binding Sites. Bioinformatics 2005, 21, 2933–2942. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereyra-Vergara, F.; Olivares-Corichi, I.M.; Luna-Arias, J.P.; Méndez-Luna, D.; García-Sánchez, J.R. Epicatechin Decreases UCP2 Gene Expression in MDA-MB-231 Breast Cancer Cells by the Presence of a Regulatory Element in the Promoter. Int. J. Mol. Sci. 2025, 26, 4102. https://doi.org/10.3390/ijms26094102
Pereyra-Vergara F, Olivares-Corichi IM, Luna-Arias JP, Méndez-Luna D, García-Sánchez JR. Epicatechin Decreases UCP2 Gene Expression in MDA-MB-231 Breast Cancer Cells by the Presence of a Regulatory Element in the Promoter. International Journal of Molecular Sciences. 2025; 26(9):4102. https://doi.org/10.3390/ijms26094102
Chicago/Turabian StylePereyra-Vergara, Fernando, Ivonne María Olivares-Corichi, Juan Pedro Luna-Arias, David Méndez-Luna, and José Rubén García-Sánchez. 2025. "Epicatechin Decreases UCP2 Gene Expression in MDA-MB-231 Breast Cancer Cells by the Presence of a Regulatory Element in the Promoter" International Journal of Molecular Sciences 26, no. 9: 4102. https://doi.org/10.3390/ijms26094102
APA StylePereyra-Vergara, F., Olivares-Corichi, I. M., Luna-Arias, J. P., Méndez-Luna, D., & García-Sánchez, J. R. (2025). Epicatechin Decreases UCP2 Gene Expression in MDA-MB-231 Breast Cancer Cells by the Presence of a Regulatory Element in the Promoter. International Journal of Molecular Sciences, 26(9), 4102. https://doi.org/10.3390/ijms26094102





