The Role of Insulin in the Proliferation and Differentiation of Bovine Muscle Satellite (Stem) Cells for Cultured Meat Production
Abstract
:1. Introduction
2. Results
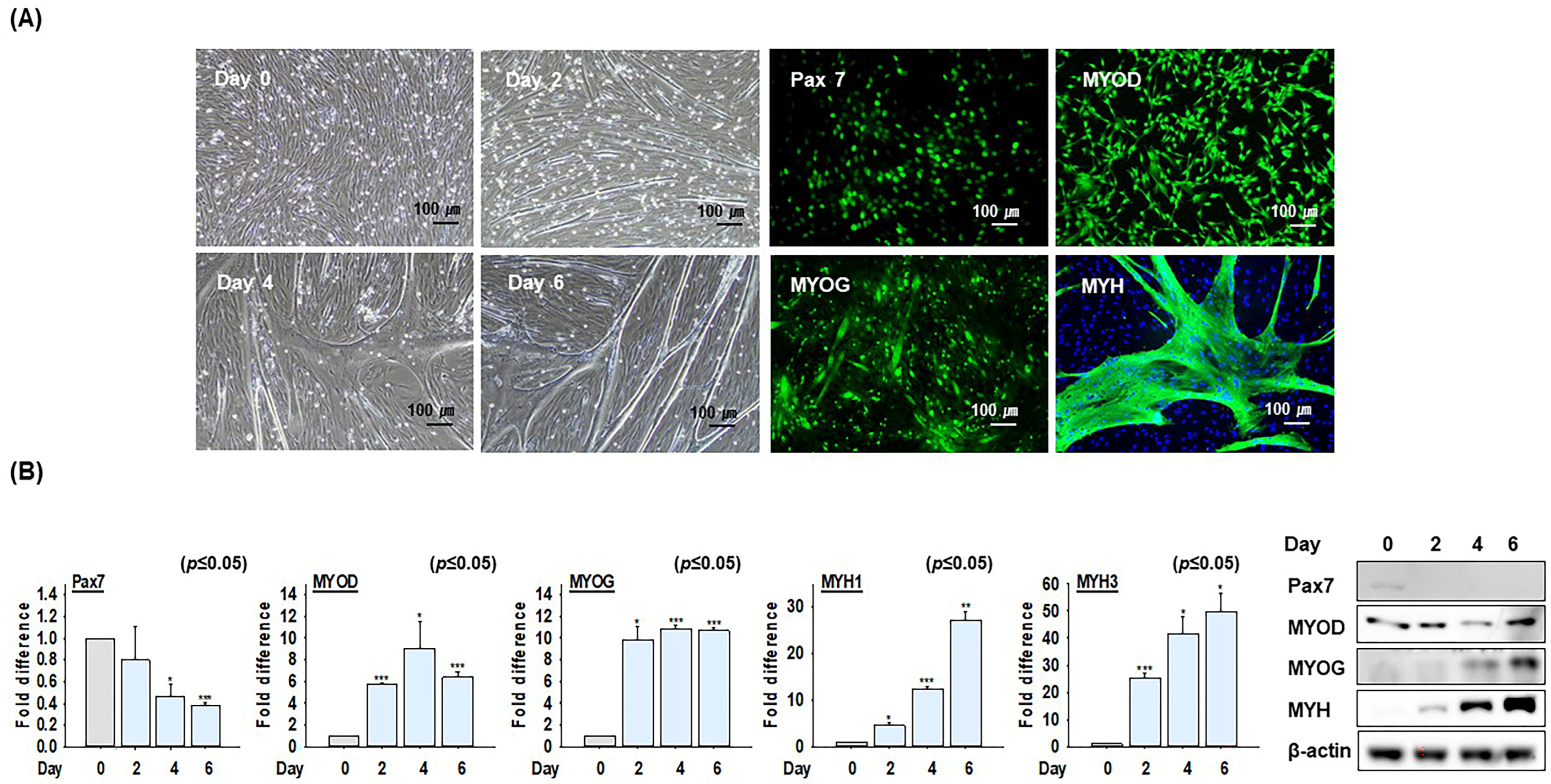
2.1. The Differentiation Ability of Bovine MSCs
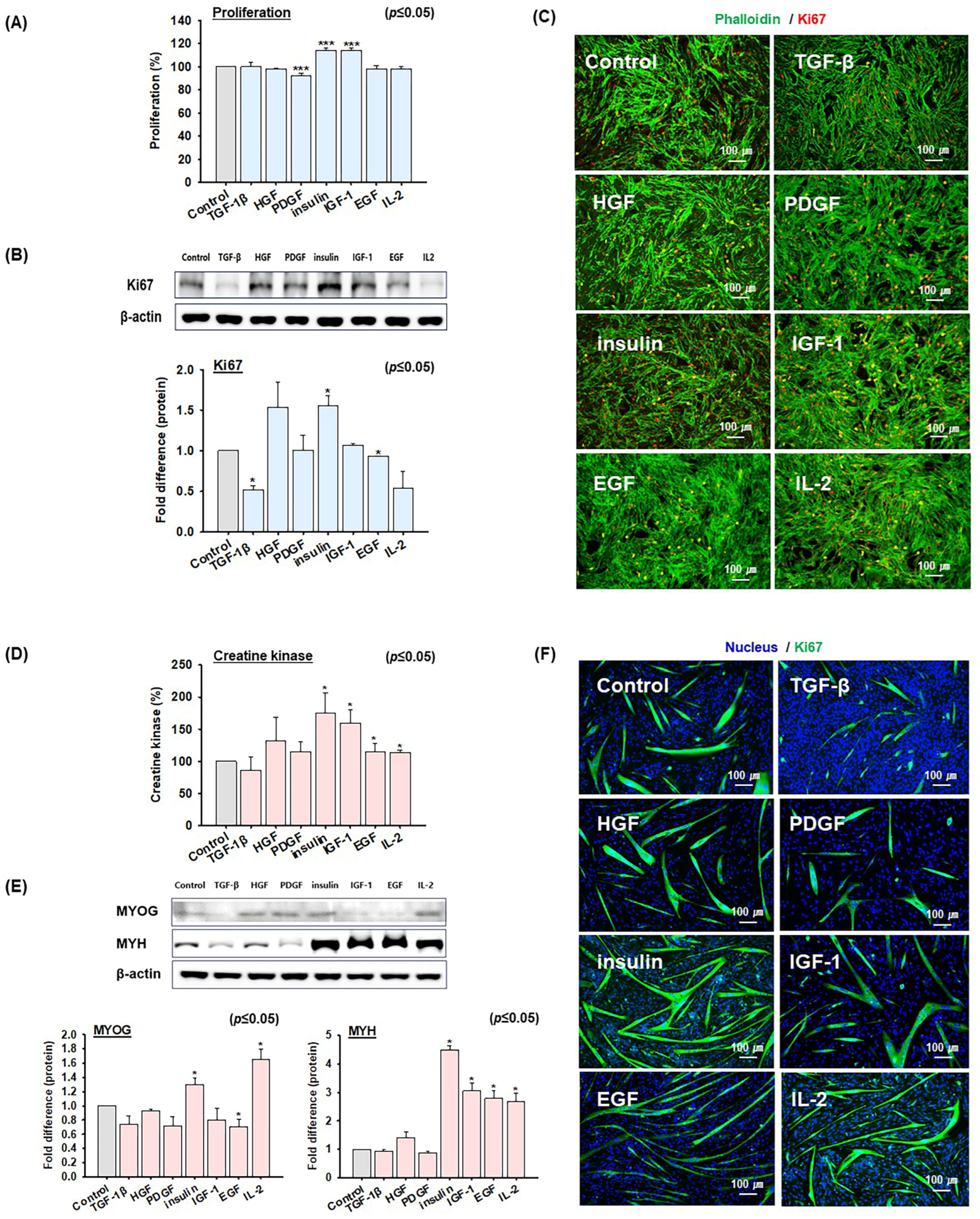
2.2. Screening of Growth Factors and Cytokine That Promote MSC Proliferation and Differentiation
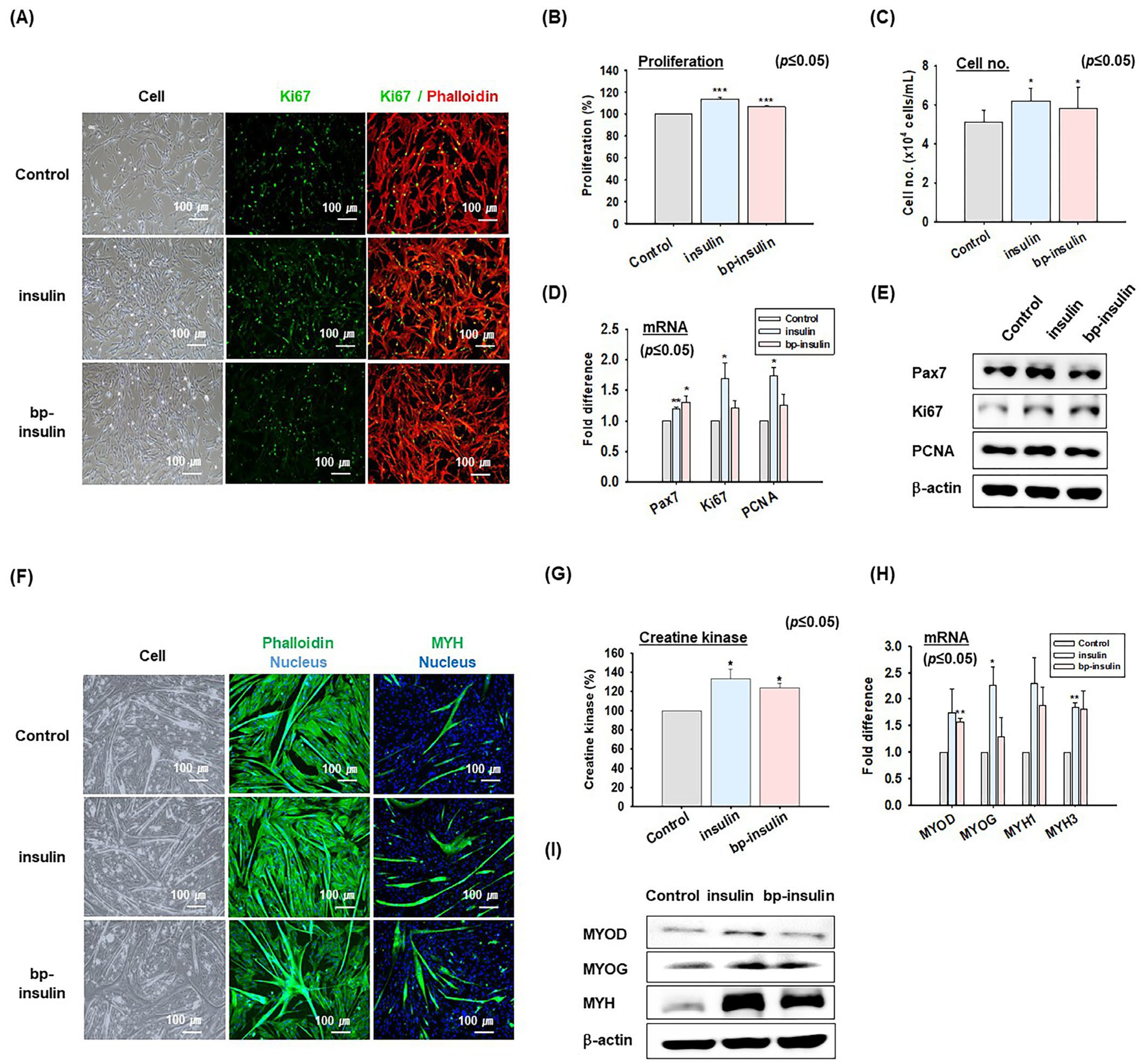
2.3. Effects of Insulin on MSC Proliferation and Differentiation
2.4. Effect of Insulin on MSC Proliferation and Differentiation at Different FBS Concentrations
3. Discussion
4. Materials and Methods
4.1. Bovine MSC Isolation
4.2. Bovine MSC Proliferation and Differentiation
4.3. Growth Factor or Cytokine Treatment
4.4. Counting MSCs
4.5. MTS Analysis
4.6. Creatine Kinase Activity
4.7. cDNA Synthesis and Real-Time RT-PCR
4.8. Western Blot
4.9. Phalloidin Staining
4.10. Immunocytochemistry
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, M.; Park, S.; Choi, B.; Choi, W.; Lee, H.; Lee, J.M.; Lee, S.T.; Yoo, K.H.; Han, D.; Bang, G.; et al. Cultured meat with enriched organoleptic properties by regulating cell differentiation. Nat. Commun. 2024, 15, 77. [Google Scholar] [CrossRef]
- Post, M.J.; Levenberg, S.; Kaplan, D.L.; Genovese, N.; Fu, J.; Bryant, C.J.; Negowetti, N.; Verzijden, K.; Moutsatsou, P. Scientific, sustainability and regulatory challenges of cultured meat. Nat. Food 2020, 1, 403–415. [Google Scholar] [CrossRef]
- Lee, E.J.; Jan, A.T.; Baig, M.H.; Ashraf, J.M.; Nahm, S.S.; Kim, Y.W.; Park, S.Y.; Choi, I. Fibromodulin: A master regulator of myostatin controlling progression of satellite cells through a myogenic program. FASEB J. 2016, 30, 2708–2719. [Google Scholar] [CrossRef]
- Shaikh, S.; Lee, E.; Ahmad, K.; Ahmad, S.S.; Chun, H.; Lim, J.; Lee, Y.; Choi, I. Cell Types Used for Cultured Meat Production and the Importance of Myokines. Foods 2021, 10, 2318. [Google Scholar] [CrossRef]
- Treich, N. Cultured Meat: Promises and Challenges. Environ. Resour. Econ. 2021, 79, 33–61. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef]
- Sousa-Victor, P.; Garcia-Prat, L.; Munoz-Canoves, P. Control of satellite cell function in muscle regeneration and its disruption in ageing. Nat. Rev. Mol. Cell Biol. 2022, 23, 204–226. [Google Scholar] [CrossRef]
- Jan, A.T.; Lee, E.J.; Ahmad, S.; Choi, I. Meeting the meat: Delineating the molecular machinery of muscle development. J. Anim. Sci. Technol. 2016, 58, 18. [Google Scholar] [CrossRef]
- Shirakawa, T.; Toyono, T.; Inoue, A.; Matsubara, T.; Kawamoto, T.; Kokabu, S. Factors regulating or regulated by myogenic regulatory factors in skeletal muscle stem cells. Cells 2022, 11, 1493. [Google Scholar] [CrossRef]
- Yin, H.; Price, F.; Rudnicki, M.A. Satellite cells and the muscle stem cell niche. Physiol. Rev. 2013, 93, 23–67. [Google Scholar] [CrossRef]
- Kim, T.; Ahmad, K.; Shaikh, S.; Jan, A.T.; Seo, M.G.; Lee, E.J.; Choi, I. Dermatopontin in Skeletal Muscle Extracellular Matrix Regulates Myogenesis. Cells 2019, 8, 332. [Google Scholar] [CrossRef]
- Lim, J.H.; Ahmad, K.; Chun, H.J.; Hwang, Y.C.; Qadri, A.F.; Ali, S.; Ahmad, S.S.; Shaikh, S.; Choi, J.; Kim, J.; et al. IgLON4 Regulates Myogenesis via Promoting Cell Adhesion and Maintaining Myotube Orientation. Cells 2022, 11, 3265. [Google Scholar] [CrossRef]
- Lim, J.H.; Beg, M.M.A.; Ahmad, K.; Shaikh, S.; Ahmad, S.S.; Chun, H.J.; Choi, D.; Lee, W.J.; Jin, J.O.; Kim, J.; et al. IgLON5 Regulates the Adhesion and Differentiation of Myoblasts. Cells 2021, 10, 417. [Google Scholar] [CrossRef]
- Ahmad, S.S.; Lim, J.H.; Ahmad, K.; Chun, H.J.; Hur, S.J.; Lee, E.J.; Choi, I. Targeting myostatin using quercetin as a media supplement to improve myogenesis for cultured meat production: An in silico and in vitro study. Curr. Res. Food Sci. 2024, 8, 100678. [Google Scholar] [CrossRef]
- Boyer, J.G.; Huo, J.; Han, S.; Havens, J.R.; Prasad, V.; Lin, B.L.; Kass, D.A.; Song, T.; Sadayappan, S.; Khairallah, R.J.; et al. Depletion of skeletal muscle satellite cells attenuates pathology in muscular dystrophy. Nat. Commun. 2022, 13, 2940. [Google Scholar] [CrossRef]
- Benedetti, A.; Cera, G.; De Meo, D.; Villani, C.; Bouche, M.; Lozanoska-Ochser, B. A novel approach for the isolation and long-term expansion of pure satellite cells based on ice-cold treatment. Skelet. Muscle 2021, 11, 7. [Google Scholar] [CrossRef]
- Tzimorotas, D.; Solberg, N.T.; Andreassen, R.C.; Moutsatsou, P.; Bodiou, V.; Pedersen, M.E.; Ronning, S.B. Expansion of bovine skeletal muscle stem cells from spinner flasks to benchtop stirred-tank bioreactors for up to 38 days. Front. Nutr. 2023, 10, 1192365. [Google Scholar] [CrossRef]
- Lee, D.Y.; Yun, S.H.; Lee, S.Y.; Lee, J.; Jr Mariano, E.; Joo, S.T.; Choi, I.; Choi, J.S.; Kim, G.D.; Lee, J.; et al. Analysis of commercial fetal bovine serum (FBS) and its substitutes in the development of cultured meat. Food Res. Int. 2023, 174, 113617. [Google Scholar] [CrossRef]
- Humbird, D. Scale-up economics for cultured meat. Biotechnol. Bioeng. 2021, 118, 3239–3250. [Google Scholar] [CrossRef]
- Lee, D.M.; Bajracharya, P.; Lee, E.J.; Kim, J.E.; Lee, H.J.; Chun, T.; Kim, J.; Cho, K.H.; Chang, J.; Hong, S.; et al. Effects of gender-specific adult bovine serum on myogenic satellite cell proliferation, differentiation and lipid accumulation. In Vitro Cell Dev. Biol. Anim. 2011, 47, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Gstraunthaler, G.; Lindl, T.; van der Valk, J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology 2013, 65, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Subbiahanadar Chelladurai, K.; Selvan Christyraj, J.D.; Rajagopalan, K.; Yesudhason, B.V.; Venkatachalam, S.; Mohan, M.; Chellathurai Vasantha, N.; Selvan Christyraj, J.R.S. Alternative to FBS in animal cell culture—An overview and future perspective. Heliyon 2021, 7, e07686. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Chun, H.J.; Ahmad, K.; Shaikh, S.; Lim, J.H.; Ali, S.; Han, S.S.; Hur, S.J.; Sohn, J.H.; Lee, E.J.; et al. The roles of growth factors and hormones in the regulation of muscle satellite cells for cultured meat production. J. Anim. Sci. Technol. 2023, 65, 16–31. [Google Scholar] [CrossRef]
- Allen, R.E.; Boxhorn, L.K. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J. Cell Physiol. 1987, 133, 567–572. [Google Scholar] [CrossRef]
- Rathbone, C.R.; Yamanouchi, K.; Chen, X.K.; Nevoret-Bell, C.J.; Rhoads, R.P.; Allen, R.E. Effects of transforming growth factor-beta (TGF-beta1) on satellite cell activation and survival during oxidative stress. J. Muscle Res. Cell Motil. 2011, 32, 99–109. [Google Scholar] [CrossRef]
- Sheehan, S.M.; Tatsumi, R.; Temm-Grove, C.J.; Allen, R.E. HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve 2000, 23, 239–245. [Google Scholar] [CrossRef]
- Hamaguchi, H.; Dohi, K.; Sakai, T.; Taoka, M.; Isobe, T.; Matsui, T.S.; Deguchi, S.; Furuichi, Y.; Fujii, N.L.; Manabe, Y. PDGF-B secreted from skeletal muscle enhances myoblast proliferation and myotube maturation via activation of the PDGFR signaling cascade. Biochem. Biophys. Res. Commun. 2023, 639, 169–175. [Google Scholar] [CrossRef]
- Pinol-Jurado, P.; Gallardo, E.; de Luna, N.; Suarez-Calvet, X.; Sanchez-Riera, C.; Fernandez-Simon, E.; Gomis, C.; Illa, I.; Diaz-Manera, J. Platelet-Derived Growth Factor BB Influences Muscle Regeneration in Duchenne Muscle Dystrophy. Am. J. Pathol. 2017, 187, 1814–1827. [Google Scholar] [CrossRef]
- Cassar-Malek, I.; Langlois, N.; Picard, B.; Geay, Y. Regulation of bovine satellite cell proliferation and differentiation by insulin and triiodothyronine. Domest. Anim. Endocrinol. 1999, 17, 373–388. [Google Scholar] [CrossRef]
- Godoy-Parejo, C.; Deng, C.; Liu, W.; Chen, G. Insulin Stimulates PI3K/AKT and Cell Adhesion to Promote the Survival of Individualized Human Embryonic Stem Cells. Stem Cells 2019, 37, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Sylow, L.; Tokarz, V.L.; Richter, E.A.; Klip, A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021, 33, 758–780. [Google Scholar] [CrossRef] [PubMed]
- Aboalola, D.; Han, V.K.M. Different Effects of Insulin-Like Growth Factor-1 and Insulin-Like Growth Factor-2 on Myogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells Int. 2017, 2017, 8286248. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zhang, Y.; Jiang, H. Signaling pathways mediating the effects of insulin-like growth factor-I in bovine muscle satellite cells. Mol. Cell Endocrinol. 2013, 372, 23–29. [Google Scholar] [CrossRef]
- Wroblewski, O.M.; Vega-Soto, E.E.; Nguyen, M.H.; Cederna, P.S.; Larkin, L.M. Impact of Human Epidermal Growth Factor on Tissue-Engineered Skeletal Muscle Structure and Function. Tissue Eng. Part. A 2021, 27, 1151–1159. [Google Scholar] [CrossRef]
- Rosa Neto, J.C.; Lira, F.S.; Zanchi, N.E.; Oyama, L.M.; Pimentel, G.D.; Santos, R.V.; Seelaender, M.; Oller do Nascimento, C.M. Acute exhaustive exercise regulates IL-2, IL-4 and MyoD in skeletal muscle but not adipose tissue in rats. Lipids Health Dis. 2011, 10, 97. [Google Scholar] [CrossRef]
- Clunn, G.F.; Refson, J.S.; Lymn, J.S.; Hughes, A.D. Platelet-derived growth factor beta-receptors can both promote and inhibit chemotaxis in human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2622–2629. [Google Scholar] [CrossRef]
- Machida, S.; Spangenburg, E.E.; Booth, F.W. Forkhead transcription factor FoxO1 transduces insulin-like growth factor’s signal to p27Kip1 in primary skeletal muscle satellite cells. J. Cell Physiol. 2003, 196, 523–531. [Google Scholar] [CrossRef]
- Miller, K.J.; Thaloor, D.; Matteson, S.; Pavlath, G.K. Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am. J. Physiol. Cell Physiol. 2000, 278, C174–C181. [Google Scholar] [CrossRef]
- Ottolenghi, A.; Bolel, P.; Sarkar, R.; Greenshpan, Y.; Iraqi, M.; Ghosh, S.; Bhattacharya, B.; Taylor, Z.V.; Kundu, K.; Radinsky, O.; et al. Life-extended glycosylated IL-2 promotes Treg induction and suppression of autoimmunity. Sci. Rep. 2021, 11, 7676. [Google Scholar] [CrossRef]
- Pferdehirt, L.; Guo, P.; Lu, A.; Huard, M.; Guilak, F.; Huard, J. In vitro analysis of genome-engineered muscle-derived stem cells for autoregulated anti-inflammatory and antifibrotic activity. J. Orthop. Res. 2022, 40, 2937–2946. [Google Scholar] [CrossRef] [PubMed]
- Starkey, J.D.; Yamamoto, M.; Yamamoto, S.; Goldhamer, D.J. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J. Histochem. Cytochem. 2011, 59, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Pesall, J.E.; Gilkerson, K.K.; McFarland, D.C. The effect of hepatocyte growth factor on turkey satellite cell proliferation and differentiation. Poult. Sci. 2002, 81, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Strzalka, W.; Ziemienowicz, A. Proliferating cell nuclear antigen (PCNA): A key factor in DNA replication and cell cycle regulation. Ann. Bot. 2011, 107, 1127–1140. [Google Scholar] [CrossRef]
- Guan, X.; Lei, Q.; Yan, Q.; Li, X.; Zhou, J.; Du, G.; Chen, J. Trends and ideas in technology, regulation and public acceptance of cultured meat. Future Foods 2021, 3, 100032. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, H.J.; Lee, D.Y.; Kang, J.H.; Ramani, S.; Park, S.; Hur, S.J. Principal protocols for the processing of cultured meat. J. Anim. Sci. Technol. 2021, 63, 673–680. [Google Scholar] [CrossRef]
- Rodriguez Escobar, M.I.; Cadena, E.; Nhu, T.T.; Cooreman-Algoed, M.; De Smet, S.; Dewulf, J. Analysis of the Cultured Meat Production System in Function of Its Environmental Footprint: Current Status, Gaps and Recommendations. Foods 2021, 10, 2941. [Google Scholar] [CrossRef]
- Martins, B.; Bister, A.; Dohmen, R.G.J.; Gouveia, M.A.; Hueber, R.; Melzener, L.; Messmer, T.; Papadopoulos, J.; Pimenta, J.; Raina, D.; et al. Advances and Challenges in Cell Biology for Cultured Meat. Annu. Rev. Anim. Biosci. 2024, 12, 345–368. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, M.; Jeong, J.; Lee, Y.S.; Yoon, J.W.; An, M.J.; Jung, H.Y.; Kim, C.H.; Ahn, Y.; Choi, K.H.; et al. Unlocking the potential of stem cells: Their crucial role in the production of cultivated meat. Curr. Res. Food Sci. 2023, 7, 100551. [Google Scholar] [CrossRef]
- Soice, E.; Johnston, J. Immortalizing Cells for Human Consumption. Int. J. Mol. Sci. 2021, 22, 11660. [Google Scholar] [CrossRef] [PubMed]
- Zehorai, E.; Maor-Shoshani, A.; Molotski, N.; Dorojkin, A.; Marelly, N.; Dvash, T.; Lavon, N. From fertilised oocyte to cultivated meat–harnessing bovine embryonic stem cells in the cultivated meat industry. Reprod. Fertil. Dev. 2023, 36, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Jan, A.T.; Baig, M.H.; Ahmad, K.; Malik, A.; Rabbani, G.; Kim, T.; Lee, I.K.; Lee, Y.H.; Park, S.Y.; et al. Fibromodulin and regulation of the intricate balance between myoblast differentiation to myocytes or adipocyte-like cells. FASEB J. 2018, 32, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Malik, A.; Pokharel, S.; Ahmad, S.; Mir, B.A.; Cho, K.H.; Kim, J.; Kong, J.C.; Lee, D.M.; Chung, K.Y.; et al. Identification of genes differentially expressed in myogenin knock-down bovine muscle satellite cells during differentiation through RNA sequencing analysis. PLoS ONE 2014, 9, e92447. [Google Scholar] [CrossRef]
- Kolkmann, A.M.; Van Essen, A.; Post, M.J.; Moutsatsou, P. Development of a Chemically Defined Medium for in vitro Expansion of Primary Bovine Satellite Cells. Front. Bioeng. Biotechnol. 2022, 10, 895289. [Google Scholar] [CrossRef]
- Yu, I.S.; Choi, J.; Kim, M.K.; Kim, M.J. The Comparison of Commercial Serum-Free Media for Hanwoo Satellite Cell Proliferation and the Role of Fibroblast Growth Factor 2. Food Sci. Anim. Resour. 2023, 43, 1017–1030. [Google Scholar] [CrossRef]
- Stout, A.J.; Mirliani, A.B.; Rittenberg, M.L.; Shub, M.; White, E.C.; Yuen, J.S.K., Jr.; Kaplan, D.L. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 2022, 5, 466. [Google Scholar] [CrossRef]
- Kolkmann, A.M.; Post, M.J.; Rutjens, M.A.M.; van Essen, A.L.M.; Moutsatsou, P. Serum-free media for the growth of primary bovine myoblasts. Cytotechnology 2020, 72, 111–120. [Google Scholar] [CrossRef]
- Fu, X.; Xiao, J.; Wei, Y.; Li, S.; Liu, Y.; Yin, J.; Sun, K.; Sun, H.; Wang, H.; Zhang, Z.; et al. Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion. Cell Res. 2015, 25, 655–673. [Google Scholar] [CrossRef]
- Joanisse, S.; Parise, G. Cytokine Mediated Control of Muscle Stem Cell Function. Adv. Exp. Med. Biol. 2016, 900, 27–44. [Google Scholar] [CrossRef]
- Relaix, F.; Bencze, M.; Borok, M.J.; Der Vartanian, A.; Gattazzo, F.; Mademtzoglou, D.; Perez-Diaz, S.; Prola, A.; Reyes-Fernandez, P.C.; Rotini, A.; et al. Perspectives on skeletal muscle stem cells. Nat. Commun. 2021, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Syverud, B.C.; VanDusen, K.W.; Larkin, L.M. Growth Factors for Skeletal Muscle Tissue Engineering. Cells Tissues Organs 2016, 202, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Waldemer-Streyer, R.J.; Kim, D.; Chen, J. Muscle cell-derived cytokines in skeletal muscle regeneration. FEBS J. 2022, 289, 6463–6483. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Ahmad, K.; Lee, E.J.; Lee, Y.H.; Choi, I. Implications of Insulin-Like Growth Factor-1 in Skeletal Muscle and Various Diseases. Cells 2020, 9, 1773. [Google Scholar] [CrossRef]
- Mourkioti, F.; Rosenthal, N. IGF-1, inflammation and stem cells: Interactions during muscle regeneration. Trends Immunol. 2005, 26, 535–542. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.; Xu, Y.; Yu, D.; Li, D.; Liu, X.; Du, W. Insulin-like growth factor-1 (IGF-1) promotes myoblast proliferation and skeletal muscle growth of embryonic chickens via the PI3K/Akt signalling pathway. Cell Biol. Int. 2015, 39, 910–922. [Google Scholar] [CrossRef]
- Weinstein, R.B.; Eleid, N.; LeCesne, C.; Durando, B.; Crawford, J.T.; Heffner, M.; Layton, C.; O’Keefe, M.; Robinson, J.; Rudinsky, S.; et al. Differential half-maximal effects of human insulin and its analogs for in situ glucose transport and protein synthesis in rat soleus muscle. Metabolism 2002, 51, 1065–1070. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Rohling, M.; Martin, S. Insulin: Too much of a good thing is bad. BMC Med. 2020, 18, 224. [Google Scholar] [CrossRef]
- Leitner, B.P.; Siebel, S.; Akingbesote, N.D.; Zhang, X.; Perry, R.J. Insulin and cancer: A tangled web. Biochem. J. 2022, 479, 583–607. [Google Scholar] [CrossRef]
- von Maltzahn, J.; Jones, A.E.; Parks, R.J.; Rudnicki, M.A. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. USA 2013, 110, 16474–16479. [Google Scholar] [CrossRef]
- Conejo, R.; Lorenzo, M. Insulin signaling leading to proliferation, survival, and membrane ruffling in C2C12 myoblasts. J. Cell Physiol. 2001, 187, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Conejo, R.; Valverde, A.M.; Benito, M.; Lorenzo, M. Insulin produces myogenesis in C2C12 myoblasts by induction of NF-kappaB and downregulation of AP-1 activities. J. Cell Physiol. 2001, 186, 82–94. [Google Scholar] [CrossRef]
- Youssef, A.; Aboalola, D.; Han, V.K. The Roles of Insulin-Like Growth Factors in Mesenchymal Stem Cell Niche. Stem Cells Int. 2017, 2017, 9453108. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Lee, E.J.; Ali, S.; Han, K.S.; Hur, S.J.; Lim, J.H.; Choi, I. Licochalcone A and B enhance muscle proliferation and differentiation by regulating Myostatin. Phytomedicine 2024, 125, 155350. [Google Scholar] [CrossRef] [PubMed]
- Skrivergaard, S.; Young, J.F.; Sahebekhtiari, N.; Semper, C.; Venkatesan, M.; Savchenko, A.; Stogios, P.J.; Therkildsen, M.; Rasmussen, M.K. A simple and robust serum-free media for the proliferation of muscle cells. Food Res. Int. 2023, 172, 113194. [Google Scholar] [CrossRef]
- Tai, P.W.; Fisher-Aylor, K.I.; Himeda, C.L.; Smith, C.L.; Mackenzie, A.P.; Helterline, D.L.; Angello, J.C.; Welikson, R.E.; Wold, B.J.; Hauschka, S.D. Differentiation and fiber type-specific activity of a muscle creatine kinase intronic enhancer. Skelet. Muscle 2011, 1, 25. [Google Scholar] [CrossRef]
- Romani, M.; Auwerx, J. Phalloidin Staining of Actin Filaments for Visualization of Muscle Fibers in Caenorhabditis elegans. Bio Protoc. 2021, 11, e4183. [Google Scholar] [CrossRef]
- Gunasekara, H.; Perera, T.; Chao, C.J.; Bruno, J.; Saed, B.; Anderson, J.; Zhao, Z.; Hu, Y.S. Phalloidin-PAINT: Enhanced quantitative nanoscale imaging of F-actin. Biophys. J. 2024, 123, 3051–3064. [Google Scholar] [CrossRef]
- Lee, E.J.; Shaikh, S.; Ahmad, K.; Ahmad, S.S.; Lim, J.H.; Park, S.; Yang, H.J.; Cho, W.K.; Park, S.J.; Lee, Y.H.; et al. Isolation and Characterization of Compounds from Glycyrrhiza uralensis as Therapeutic Agents for the Muscle Disorders. Int. J. Mol. Sci. 2021, 22, 876. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.J.; Shaikh, S.; Ahmad, S.S.; Lim, J.H.; Baral, A.; Hur, S.J.; Sohn, J.H.; Choi, I. The Role of Insulin in the Proliferation and Differentiation of Bovine Muscle Satellite (Stem) Cells for Cultured Meat Production. Int. J. Mol. Sci. 2025, 26, 4109. https://doi.org/10.3390/ijms26094109
Lee EJ, Shaikh S, Ahmad SS, Lim JH, Baral A, Hur SJ, Sohn JH, Choi I. The Role of Insulin in the Proliferation and Differentiation of Bovine Muscle Satellite (Stem) Cells for Cultured Meat Production. International Journal of Molecular Sciences. 2025; 26(9):4109. https://doi.org/10.3390/ijms26094109
Chicago/Turabian StyleLee, Eun Ju, Sibhghatulla Shaikh, Syed Sayeed Ahmad, Jeong Ho Lim, Ananda Baral, Sun Jin Hur, Jung Hoon Sohn, and Inho Choi. 2025. "The Role of Insulin in the Proliferation and Differentiation of Bovine Muscle Satellite (Stem) Cells for Cultured Meat Production" International Journal of Molecular Sciences 26, no. 9: 4109. https://doi.org/10.3390/ijms26094109
APA StyleLee, E. J., Shaikh, S., Ahmad, S. S., Lim, J. H., Baral, A., Hur, S. J., Sohn, J. H., & Choi, I. (2025). The Role of Insulin in the Proliferation and Differentiation of Bovine Muscle Satellite (Stem) Cells for Cultured Meat Production. International Journal of Molecular Sciences, 26(9), 4109. https://doi.org/10.3390/ijms26094109






