Autoantibodies Targeting the Hypothalamic-Pituitary-Ovarian Axis in Polycystic Ovary Syndrome: Emerging Key Players in Pathogenesis?
Abstract
1. Introduction
2. PCOS Pathogenesis and Pathophysiology
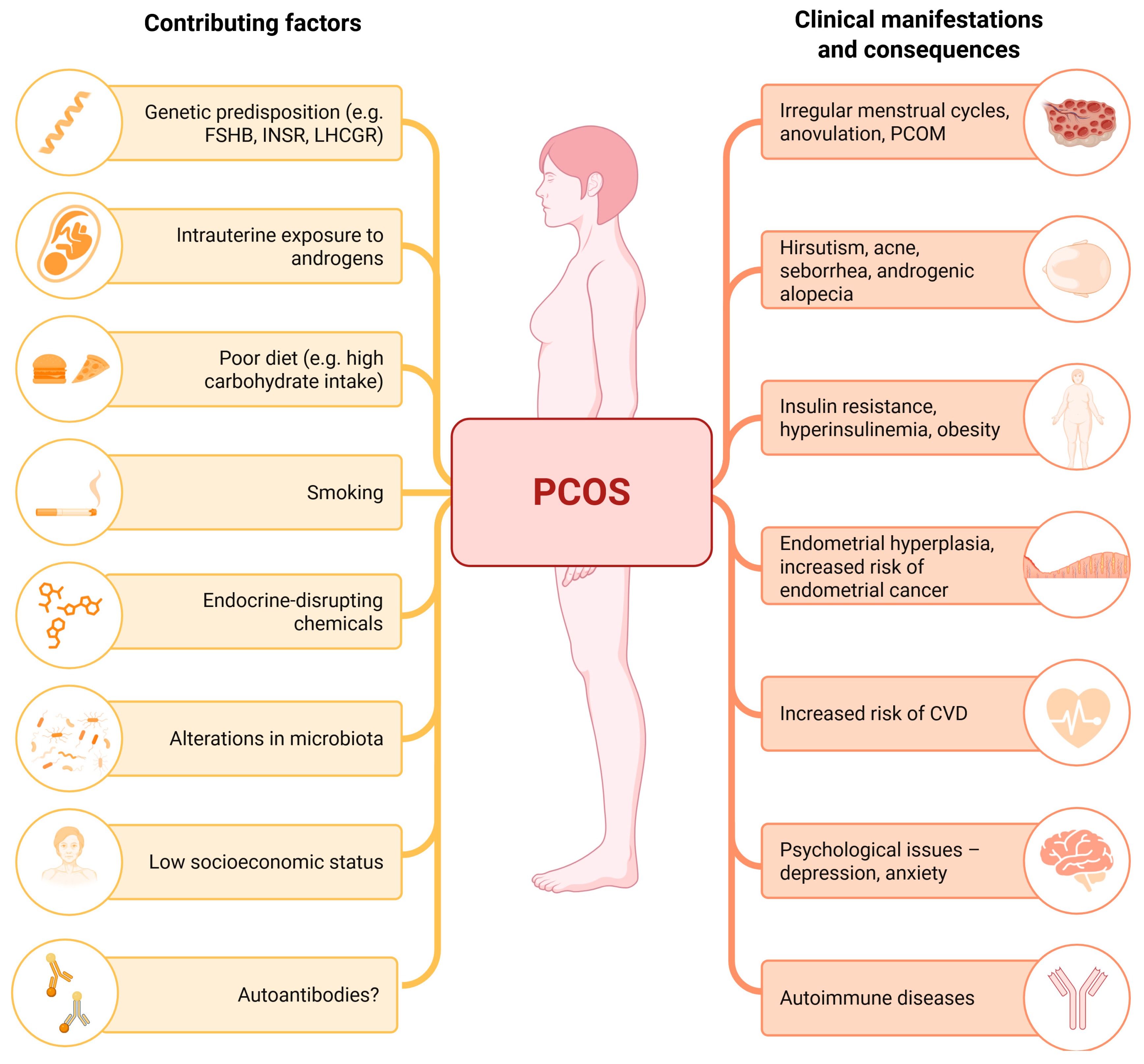
2.1. Factors Contributing to PCOS Etiology
2.1.1. Genetic Factors
2.1.2. Intrauterine and Perinatal Factors
2.1.3. Postnatal and Environmental Contributors
2.2. Hormonal Imbalance in PCOS
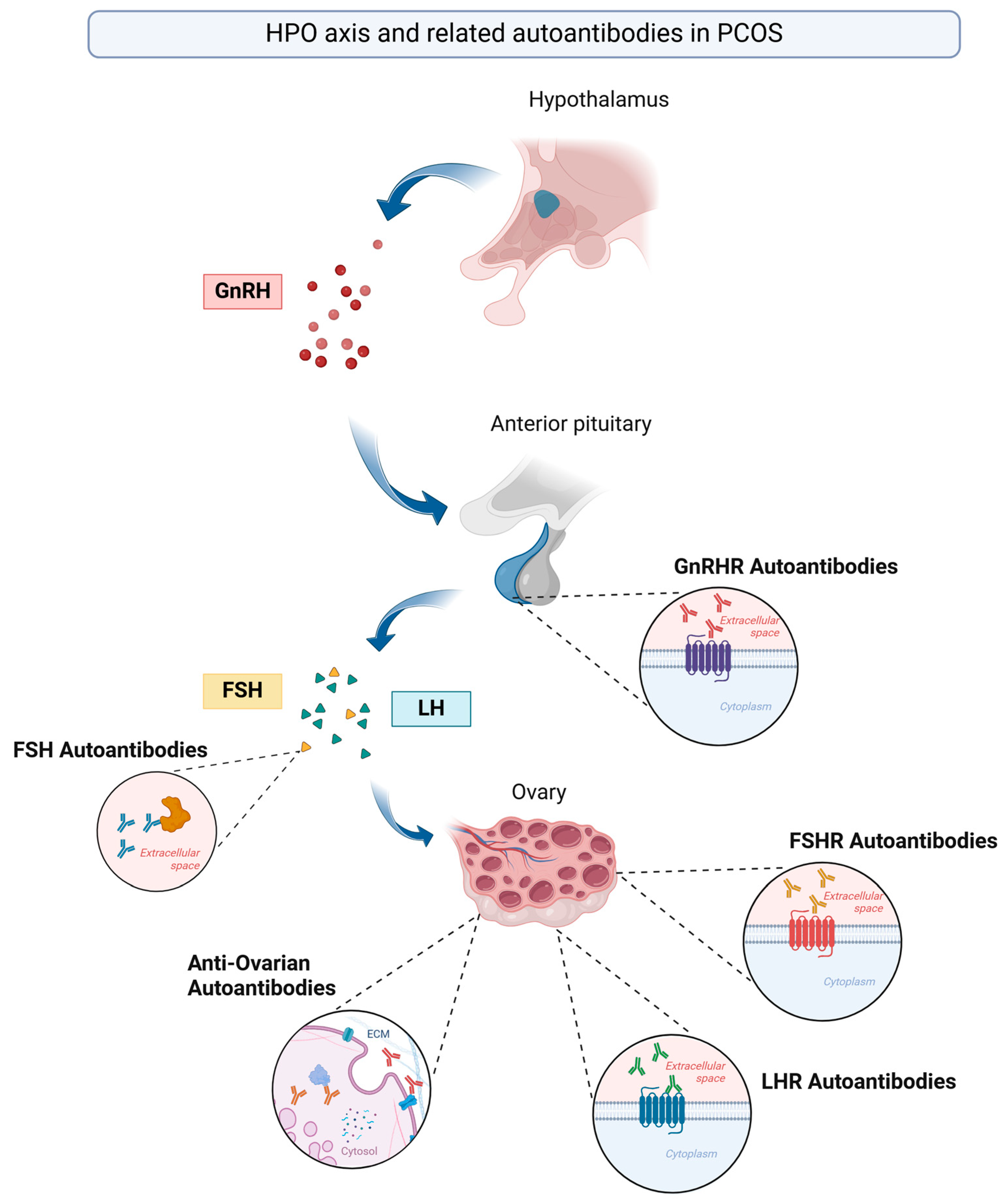
3. Autoantibodies in the Pathogenesis of PCOS—Current Evidence
3.1. Anti-FSH Autoantibodies
3.2. Anti-FSHR and Anti-LHR Autoantibodies
3.3. Anti-Ovarian Autoantibodies (AOAs)
3.4. GnRHR Autoantibodies
3.5. Beyond Autoantibodies: Role of T-Cell-Mediated Immunity
4. Is There a Place for an Autoimmune Cause of PCOS?
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 17-OHP | 17-Hydroxyprogesterone |
| ACTH | Adrenocorticotropic hormone |
| AMH | Anti-Müllerian hormone |
| anti-FSH | Antibodies against follicle-stimulating hormone |
| anti-FSHR | Antibodies against follicle-stimulating hormone receptor |
| anti-LHR | Antibodies against luteinizing hormone receptor |
| anti-V14D IgA | IgA antibodies against 78–93 region (V14D) of the human FSH β-chain |
| AOAs | Anti-ovarian antibodies |
| ASRM | American Society for Reproductive Medicine |
| BMI | Body mass index |
| CVD | Cardiovascular disease |
| DAST | Dexamethasone androgen-suppression test |
| DHEA | Dehydroepiandrosterone |
| ECM | Extracellular matrix |
| EDCs | Endocrine-disrupting chemicals |
| FAH | Functional adrenal hyperandrogenism |
| FOH | Functional ovarian hyperandrogenism |
| FSH | Follicle-stimulating hormone |
| FSHB | Beta subunit of follicle-stimulating hormone |
| FSHR | Follicle-stimulating hormone receptor |
| GnRH | Gonadotropin-releasing hormone |
| GnRHR | Gonadotropin-releasing hormone receptor |
| GnRHR-ECL2-AAbs | Activating autoantibodies (AAbs) to the second extracellular loop (ECL2) of gonadotropin-releasing hormone receptor (GnRHR) |
| GPCR | G protein-coupled receptor |
| GWASs | Genome-wide association studies |
| hCG | Human chorionic gonadotropin |
| HPO | Hypothalamic–pituitary–ovarian |
| IFN-γ | Interferon gamma |
| IgA | Immunoglobulin A |
| IGF-1 | Insulin-like growth factor 1 |
| IgG | Immunoglobulin G |
| IgM | Immunoglobulin M |
| IL-2 | Interleukin-2 |
| IL-2Rγ | Interleukin-2 receptor gamma-chain |
| IL-6 | Interleukin-6 |
| INSR | Insulin receptor |
| IU/ml | International units per milliliter |
| IVF | In vitro fertilization |
| LH | Luteinizing hormone |
| LHCGR | Luteinizing hormone/choriogonadotropin receptor |
| LHR | Luteinizing hormone receptor |
| NCAH | Non-classical congenital adrenal hyperplasia |
| OD | Optical density |
| OR | Odds ratio |
| PBMCs | Peripheral blood mononuclear cells |
| PCOM | Polycystic ovarian morphology |
| PCOS | Polycystic ovary syndrome |
| POF | Premature ovarian failure |
| SHBG | Sex hormone-binding globulin |
| TNF-α | Tumor necrosis factor alpha |
| Tregs | Regulatory T cells |
References
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef] [PubMed]
- Ganie, M.A.; Vasudevan, V.; Wani, I.A.; Baba, M.S.; Arif, T.; Rashid, A. Epidemiology, pathogenesis, genetics & management of polycystic ovary syndrome in India. Indian J. Med. Res. 2019, 150, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Glueck, C.J.; Goldenberg, N. Characteristics of obesity in polycystic ovary syndrome: Etiology, treatment, and genetics. Metabolism 2019, 92, 108–120. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef] [PubMed]
- Piltonen, T.T.; Komsi, E.; Morin-Papunen, L.C.; Korhonen, E.; Franks, S.; Jarvelin, M.R.; Arffman, R.K.; Ollila, M.M. AMH as part of the diagnostic PCOS workup in large epidemiological studies. Eur. J. Endocrinol. 2023, 188, 547–554. [Google Scholar] [CrossRef]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K.; Endocrine, S. Diagnosis and treatment of polycystic ovary syndrome: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef]
- Shahid, R.; Iahtisham Ul, H.; Mahnoor; Awan, K.A.; Iqbal, M.J.; Munir, H.; Saeed, I. Diet and lifestyle modifications for effective management of polycystic ovarian syndrome (PCOS). J. Food Biochem. 2022, 46, e14117. [Google Scholar] [CrossRef]
- Sadeghi, H.M.; Adeli, I.; Calina, D.; Docea, A.O.; Mousavi, T.; Daniali, M.; Nikfar, S.; Tsatsakis, A.; Abdollahi, M. Polycystic Ovary Syndrome: A Comprehensive Review of Pathogenesis, Management, and Drug Repurposing. Int. J. Mol. Sci. 2022, 23, 583. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Bednarska, S.; Siejka, A. The pathogenesis and treatment of polycystic ovary syndrome: What’s new? Adv. Clin. Exp. Med. 2017, 26, 359–367. [Google Scholar] [CrossRef]
- Dapas, M.; Dunaif, A. Deconstructing a Syndrome: Genomic Insights Into PCOS Causal Mechanisms and Classification. Endocr. Rev. 2022, 43, 927–965. [Google Scholar] [CrossRef]
- Kem, D.C.; Li, H.; Yu, X.; Weedin, E.; Reynolds, A.C.; Forsythe, E.; Beel, M.; Fischer, H.; Hines, B.; Guo, Y.; et al. The Role of GnRH Receptor Autoantibodies in Polycystic Ovary Syndrome. J. Endocr. Soc. 2020, 4, bvaa078. [Google Scholar] [CrossRef] [PubMed]
- Petrikova, J.; Lazurova, I. Ovarian failure and polycystic ovary syndrome. Autoimmun. Rev. 2012, 11, A471–A478. [Google Scholar] [CrossRef]
- Zhao, Y.; Pang, J.; Fang, X.; Yan, Z.; Yang, H.; Deng, Q.; Ma, T.; Lv, M.; Li, Y.; Tu, Z.; et al. Causal relationships between modifiable risk factors and polycystic ovary syndrome: A comprehensive Mendelian randomization study. Front Endocrinol. 2024, 15, 1348368. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.M.; Sadrzadeh, S.; Lambalk, C.B.; Boomsma, D.I. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J. Clin. Endocrinol. Metab. 2006, 91, 2100–2104. [Google Scholar] [CrossRef]
- Dapas, M.; Dunaif, A. The contribution of rare genetic variants to the pathogenesis of polycystic ovary syndrome. Curr. Opin. Endocr. Metab. Res. 2020, 12, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kobaly, K.; Vellanki, P.; Sisk, R.K.; Armstrong, L.; Lee, J.Y.; Lee, J.; Hayes, M.G.; Urbanek, M.; Legro, R.S.; Dunaif, A. Parent-of-origin effects on glucose homeostasis in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2014, 99, 2961–2966. [Google Scholar] [CrossRef]
- Rosenfield, R.L. Current concepts of polycystic ovary syndrome pathogenesis. Curr. Opin. Pediatr. 2020, 32, 698–706. [Google Scholar] [CrossRef]
- Demissie, M.; Lazic, M.; Foecking, E.M.; Aird, F.; Dunaif, A.; Levine, J.E. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E262–E268. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Abbott, D.H.; Eisner, J.R.; Goy, R.W. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil. Steril. 1997, 67, 155–163. [Google Scholar] [CrossRef]
- Tata, B.; Mimouni, N.E.H.; Barbotin, A.L.; Malone, S.A.; Loyens, A.; Pigny, P.; Dewailly, D.; Catteau-Jonard, S.; Sundstrom-Poromaa, I.; Piltonen, T.T.; et al. Elevated prenatal anti-Mullerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat. Med. 2018, 24, 834–846. [Google Scholar] [CrossRef]
- Sir-Petermann, T.; Maliqueo, M.; Angel, B.; Lara, H.E.; Perez-Bravo, F.; Recabarren, S.E. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: Possible implications in prenatal androgenization. Hum. Reprod. 2002, 17, 2573–2579. [Google Scholar] [CrossRef] [PubMed]
- Franks, S.; Berga, S.L. Does PCOS have developmental origins? Fertil. Steril. 2012, 97, 2–6. [Google Scholar] [CrossRef]
- Melo, A.S.; Vieira, C.S.; Barbieri, M.A.; Rosa, E.S.A.C.; Silva, A.A.; Cardoso, V.C.; Reis, R.M.; Ferriani, R.A.; Silva-de-Sa, M.F.; Bettiol, H. High prevalence of polycystic ovary syndrome in women born small for gestational age. Hum. Reprod. 2010, 25, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Sadrzadeh, S.; Klip, W.A.; Broekmans, F.J.; Schats, R.; Willemsen, W.N.; Burger, C.W.; Van Leeuwen, F.E.; Lambalk, C.B.; Group, O.P. Birth weight and age at menarche in patients with polycystic ovary syndrome or diminished ovarian reserve, in a retrospective cohort. Hum. Reprod. 2003, 18, 2225–2230. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, M.O.; Dumesic, D.A.; Chazenbalk, G.; Azziz, R. Polycystic ovary syndrome: Etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 2011, 7, 219–231. [Google Scholar] [CrossRef]
- Welt, C.K.; Gudmundsson, J.A.; Arason, G.; Adams, J.; Palsdottir, H.; Gudlaugsdottir, G.; Ingadottir, G.; Crowley, W.F. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: The impact of weight on phenotype and metabolic features. J. Clin. Endocrinol. Metab. 2006, 91, 4842–4848. [Google Scholar] [CrossRef]
- Barrea, L.; Frias-Toral, E.; Verde, L.; Ceriani, F.; Cucalon, G.; Garcia-Velasquez, E.; Moretti, D.; Savastano, S.; Colao, A.; Muscogiuri, G. PCOS and nutritional approaches: Differences between lean and obese phenotype. Metab. Open 2021, 12, 100123. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.; Zhao, J.; Jiao, P.; Zeng, L.; Zhang, H.; Zhao, Y.; Shi, L.; Hu, H.; Luo, L.; et al. Relationship between body composition, insulin resistance, and hormonal profiles in women with polycystic ovary syndrome. Front. Endocrinol. 2022, 13, 1085656. [Google Scholar] [CrossRef]
- Legro, R.S.; Kunselman, A.R.; Dodson, W.C.; Dunaif, A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J. Clin. Endocrinol. Metab. 1999, 84, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, S.; Haberle, L.; Dittrich, R.; Oppelt, P.G.; Reissmann, C.; Kronawitter, D.; Beckmann, M.W.; Mueller, A. Smoking is associated with increased free testosterone and fasting insulin levels in women with polycystic ovary syndrome, resulting in aggravated insulin resistance. Fertil. Steril. 2010, 94, 673–677. [Google Scholar] [CrossRef]
- Vagi, S.J.; Azziz-Baumgartner, E.; Sjodin, A.; Calafat, A.M.; Dumesic, D.; Gonzalez, L.; Kato, K.; Silva, M.J.; Ye, X.; Azziz, R. Exploring the potential association between brominated diphenyl ethers, polychlorinated biphenyls, organochlorine pesticides, perfluorinated compounds, phthalates, and bisphenol A in polycystic ovary syndrome: A case-control study. BMC Endocr. Disord. 2014, 14, 86. [Google Scholar] [CrossRef]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, Y.; Qiu, X.; Zhang, C.; Li, R.; Qiao, J. Association of serum levels of typical organic pollutants with polycystic ovary syndrome (PCOS): A case-control study. Hum. Reprod. 2015, 30, 1964–1973. [Google Scholar] [CrossRef]
- Merkin, S.S.; Phy, J.L.; Sites, C.K.; Yang, D. Environmental determinants of polycystic ovary syndrome. Fertil. Steril. 2016, 106, 16–24. [Google Scholar] [CrossRef]
- Rebar, R.; Judd, H.L.; Yen, S.S.; Rakoff, J.; Vandenberg, G.; Naftolin, F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J. Clin. Investig. 1976, 57, 1320–1329. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, L.; Hall, J.E.; Potau, N.; Carrascosa, A.; Prat, N.; Taylor, A.E. Ovarian 17-hydroxyprogesterone hyperresponsiveness to gonadotropin-releasing hormone (GnRH) agonist challenge in women with polycystic ovary syndrome is not mediated by luteinizing hormone hypersecretion: Evidence from GnRH agonist and human chorionic gonadotropin stimulation testing. J. Clin. Endocrinol. Metab. 1996, 81, 4103–4107. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Mortensen, M.; Wroblewski, K.; Littlejohn, E.; Ehrmann, D.A. Determination of the source of androgen excess in functionally atypical polycystic ovary syndrome by a short dexamethasone androgen-suppression test and a low-dose ACTH test. Hum. Reprod. 2011, 26, 3138–3146. [Google Scholar] [CrossRef]
- McCartney, C.R.; Campbell, R.E.; Marshall, J.C.; Moenter, S.M. The role of gonadotropin-releasing hormone neurons in polycystic ovary syndrome. J. Neuroendocr. 2022, 34, e13093. [Google Scholar] [CrossRef] [PubMed]
- Ruddenklau, A.; Campbell, R.E. Neuroendocrine Impairments of Polycystic Ovary Syndrome. Endocrinology 2019, 160, 2230–2242. [Google Scholar] [CrossRef]
- Purwar, A.; Nagpure, S. Insulin Resistance in Polycystic Ovarian Syndrome. Cureus 2022, 14, e30351. [Google Scholar] [CrossRef]
- Houston, E.J.; Templeman, N.M. Reappraising the relationship between hyperinsulinemia and insulin resistance in PCOS. J. Endocrinol. 2025, 265, e240269. [Google Scholar] [CrossRef] [PubMed]
- Visser, J.A.; de Jong, F.H.; Laven, J.S.; Themmen, A.P. Anti-Mullerian hormone: A new marker for ovarian function. Reproduction 2006, 131, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cimino, I.; Casoni, F.; Liu, X.; Messina, A.; Parkash, J.; Jamin, S.P.; Catteau-Jonard, S.; Collier, F.; Baroncini, M.; Dewailly, D.; et al. Novel role for anti-Mullerian hormone in the regulation of GnRH neuron excitability and hormone secretion. Nat. Commun. 2016, 7, 10055. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Wang, R.; Xue, S.; Ying, Q.; Jin, L. Roles of estrogen and its receptors in polycystic ovary syndrome. Front. Cell Dev. Biol. 2024, 12, 1395331. [Google Scholar] [CrossRef]
- Boutzios, G.; Karalaki, M.; Zapanti, E. Common pathophysiological mechanisms involved in luteal phase deficiency and polycystic ovary syndrome. Impact on fertility. Endocrine 2013, 43, 314–317. [Google Scholar] [CrossRef]
- Fanta, M. Is polycystic ovary syndrome, a state of relative estrogen excess, a real risk factor for estrogen-dependant malignancies? Gynecol. Endocrinol. 2013, 29, 145–147. [Google Scholar] [CrossRef]
- Hickey, M.; Higham, J.M.; Fraser, I. Progestogens with or without oestrogen for irregular uterine bleeding associated with anovulation. Cochrane Database Syst. Rev. 2012, 2012, CD001895. [Google Scholar] [CrossRef]
- Mobeen, H.; Afzal, N.; Kashif, M. Polycystic Ovary Syndrome May Be an Autoimmune Disorder. Scientifica 2016, 2016, 4071735. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Zhang, G.; Deng, J.; Fischer, H.; Craig, L.B.; Yu, X.; Kem, D.C. Gonadotrophin-releasing hormone receptor autoantibodies induce polycystic ovary syndrome-like features in a rat model. Exp. Physiol. 2021, 106, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Weedin, E.A.; Burks, H.R.; Yu, X.; Li, H.L.; Aston, C.E.; Kem, D.C.; Craig, L.B. Elevated activity levels of activating autoantibodies to the GnRH receptor in patients with polycystic ovary syndrome. FS Rep. 2020, 1, 299–304. [Google Scholar] [CrossRef]
- Haller, K.; Mathieu, C.; Rull, K.; Matt, K.; Bene, M.C.; Uibo, R. IgG, IgA and IgM antibodies against FSH: Serological markers of pathogenic autoimmunity or of normal immunoregulation? Am. J. Reprod. Immunol. 2005, 54, 262–269. [Google Scholar] [CrossRef]
- Dolfin, E.; Guani, B.; Lussiana, C.; Mari, C.; Restagno, G.; Revelli, A. FSH-receptor Ala307Thr polymorphism is associated to polycystic ovary syndrome and to a higher responsiveness to exogenous FSH in Italian women. J. Assist. Reprod. Genet. 2011, 28, 925–930. [Google Scholar] [CrossRef]
- Francois, C.M.; Petit, F.; Giton, F.; Gougeon, A.; Ravel, C.; Magre, S.; Cohen-Tannoudji, J.; Guigon, C.J. A novel action of follicle-stimulating hormone in the ovary promotes estradiol production without inducing excessive follicular growth before puberty. Sci. Rep. 2017, 7, 46222. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.S.; Roy, B.S.; Mahale, S.D. Mutations and polymorphisms in FSH receptor: Functional implications in human reproduction. Reproduction 2013, 146, R235–R248. [Google Scholar] [CrossRef]
- Meyer, W.R.; Lavy, G.; DeCherney, A.H.; Visintin, I.; Economy, K.; Luborsky, J.L. Evidence of gonadal and gonadotropin antibodies in women with a suboptimal ovarian response to exogenous gonadotropin. Obs. Gynecol. 1990, 75, 795–799. [Google Scholar]
- Schniewind, H.A.; Sattler, L.M.; Haudum, C.W.; Munzker, J.; Minich, W.B.; Obermayer-Pietsch, B.; Schomburg, L. Autoimmunity to the Follicle-Stimulating Hormone Receptor (FSHR) and Luteinizing Hormone Receptor (LHR) in Polycystic Ovarian Syndrome. Int. J. Mol. Sci. 2021, 22, 13667. [Google Scholar] [CrossRef]
- Dal Pra, C.; Chen, S.; Furmaniak, J.; Smith, B.R.; Pedini, B.; Moscon, A.; Zanchetta, R.; Betterle, C. Autoantibodies to steroidogenic enzymes in patients with premature ovarian failure with and without Addison’s disease. Eur. J. Endocrinol. 2003, 148, 565–570. [Google Scholar] [CrossRef]
- Falorni, A.; Laureti, S.; Candeloro, P.; Perrino, S.; Coronella, C.; Bizzarro, A.; Bellastella, A.; Santeusanio, F.; De Bellis, A. Steroid-cell autoantibodies are preferentially expressed in women with premature ovarian failure who have adrenal autoimmunity. Fertil. Steril. 2002, 78, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Gobert, B.; Barbarino-Monnier, P.; Guillet-May, F.; Bene, M.C.; Faure, G.C. Anti-ovary antibodies after attempts at human in vitro fertilization induced by follicular puncture rather than hormonal stimulation. J. Reprod. Fertil. 1992, 96, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Fénichel, P.; Gobert, B.; Carré, Y.; Barbarino-Monnier, P.; Hiéronimus, S. Polycystic o vary syndrome in autoimmune disease. Lancet 1999, 353, 2210. [Google Scholar] [CrossRef] [PubMed]
- Shoukry, M.M.; Amer, H.A.; El-Kabarity, R.H.; Wahba, N.S. The role of anti-ovarian autoantibodies in Polycystic Ovary Syndrome. Egypt. J. Hosp. Med. 2020, 81, 1326–1329. [Google Scholar] [CrossRef]
- Geng, X.; He, Z.; Bao, Z.; Di, W.; Gu, Z. Aberrant HPO Axis Alterations and Autoimmune Abnormalities in PCOS Patients with DOR: A Retrospective Analysis. J. Clin. Med. 2023, 12, 5212. [Google Scholar] [CrossRef]
- Lonsdale, R.N.; Roberts, P.F.; Trowell, J.E. Autoimmune oophoritis associated with polycystic ovaries. Histopathology 1991, 19, 77–81. [Google Scholar] [CrossRef]
- van Gelderen, C.J.; Gomes dos Santos, M.L. Polycystic ovarian syndrome. Evidence for an autoimmune mechanism in some cases. J. Reprod. Med. 1993, 38, 381–386. [Google Scholar]
- Luborsky, J.L.; Shatavi, S.; Adamczyk, P.; Chiong, C.; Llanes, B.; Lafniztzegger, J.; Soltes, B.; McGovern, P.; Santoro, N. Polycystic ovary syndrome and ovarian autoimmunity--assessment of ovarian antibodies by EIA. J. Reprod. Immunol. 1999, 42, 79–84. [Google Scholar] [CrossRef]
- Rojansky, N.; Roll, D.; Meirow, D. Polycystic ovary syndrome: An autoimmune disease? J. Reprod. Med. 1997, 42, 325–328. [Google Scholar]
- Li, H.; Guo, Y.; Deng, J.; Fischer, H.; Weedin, E.A.; Burks, H.R.; Craig, L.B.; Yu, X. Increased testosterone and proinflammatory cytokines in patients with polycystic ovary syndrome correlate with elevated GnRH receptor autoantibody activity assessed by a fluorescence resonance energy transfer-based bioassay. Endocrine 2021, 74, 163–171. [Google Scholar] [CrossRef]
- McCartney, C.R.; Campbell, R.E. Abnormal GnRH Pulsatility in Polycystic Ovary Syndrome: Recent Insights. Curr. Opin. Endocr. Metab. Res. 2020, 12, 78–84. [Google Scholar] [CrossRef]
- Tsutsumi, R.; Webster, N.J. GnRH pulsatility, the pituitary response and reproductive dysfunction. Endocr. J. 2009, 56, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.; Cariboni, A.M.; Marelli, M.M.; Moretti, R.M.; Andre, V.; Marzagalli, M.; Limonta, P. GnRH and GnRH receptors in the pathophysiology of the human female reproductive system. Hum. Reprod. Update 2016, 22, 358–381. [Google Scholar] [CrossRef] [PubMed]
- Bliss, S.P.; Navratil, A.M.; Xie, J.; Roberson, M.S. GnRH signaling, the gonadotrope and endocrine control of fertility. Front. Neuroendocr. 2010, 31, 322–340. [Google Scholar] [CrossRef]
- Millar, R.P.; Lu, Z.L.; Pawson, A.J.; Flanagan, C.A.; Morgan, K.; Maudsley, S.R. Gonadotropin-releasing hormone receptors. Endocr. Rev. 2004, 25, 235–275. [Google Scholar] [CrossRef]
- Davies, T.F.; Andersen, S.; Latif, R.; Nagayama, Y.; Barbesino, G.; Brito, M.; Eckstein, A.K.; Stagnaro-Green, A.; Kahaly, G.J. Graves’ disease. Nat. Rev. Dis. Primers 2020, 6, 52. [Google Scholar] [CrossRef]
- Sattler, L.M.; Schniewind, H.A.; Minich, W.B.; Haudum, C.W.; Niklowitz, P.; Munzker, J.; Kovacs, G.L.; Reinehr, T.; Obermayer-Pietsch, B.; Schomburg, L. Natural autoantibodies to the gonadotropin-releasing hormone receptor in polycystic ovarian syndrome. PLoS ONE 2021, 16, e0249639. [Google Scholar] [CrossRef]
- Chen, H.F.; Jeung, E.B.; Stephenson, M.; Leung, P.C. Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor gamma-chain messenger ribonucleic acids that are regulated by GnRH in vitro. J. Clin. Endocrinol. Metab. 1999, 84, 743–750. [Google Scholar] [CrossRef][Green Version]
- Rose, N.R.; Bona, C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited). Immunol. Today 1993, 14, 426–430. [Google Scholar] [CrossRef]
- Doti, N.; Mardirossian, M.; Sandomenico, A.; Ruvo, M.; Caporale, A. Recent Applications of Retro-Inverso Peptides. Int. J. Mol. Sci. 2021, 22, 8677. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Deng, J.; Gali, H.; Weedin, E.A.; Burks, H.R.; Craig, L.B.; Yu, X. GnRH receptor-activating autoantibodies in polycystic ovary syndrome: Identification of functional epitopes and development of epitope mimetic inhibitors. Endocrine 2022, 75, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.C.; Min, S.H.; Freeh, S.M.; Michael, S.D. The estrogen-injected female mouse: New insight into the etiology of PCOS. Reprod. Biol. Endocrinol. 2009, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Yellayi, S.; Naaz, A.; Szewczykowski, M.A.; Sato, T.; Woods, J.A.; Chang, J.; Segre, M.; Allred, C.D.; Helferich, W.G.; Cooke, P.S. The phytoestrogen genistein induces thymic and immune changes: A human health concern? Proc. Natl. Acad. Sci. USA 2002, 99, 7616–7621. [Google Scholar] [CrossRef] [PubMed]
- Hefler-Frischmuth, K.; Walch, K.; Huebl, W.; Baumuehlner, K.; Tempfer, C.; Hefler, L. Serologic markers of autoimmunity in women with polycystic ovary syndrome. Fertil. Steril. 2010, 93, 2291–2294. [Google Scholar] [CrossRef]
- Buteva-Hristova, I.; Lazarov, V.; Lozanov, V.; Gateva, A.; Bechev, B.; Kavaldzieva, K.; Mladenov, N.; Trifonova, N.; Dimitrova-Dikanarova, D.; Kamenov, Z. Serum anti-α-crystallin antibodies in women with endocrine disorders. Biotechnol. Biotechnol. Equip. 2017, 31, 574–580. [Google Scholar] [CrossRef]
- Palacio, J.R.; Iborra, A.; Ulcova-Gallova, Z.; Badia, R.; Martinez, P. The presence of antibodies to oxidative modified proteins in serum from polycystic ovary syndrome patients. Clin. Exp. Immunol. 2006, 144, 217–222. [Google Scholar] [CrossRef]
| Autoantibody | Study | Year | Results | Conclusions |
|---|---|---|---|---|
| GnRHR-AAbs | Kem et al. [13] | 2020 | OD (405 nm) value (exact values not provided) p = 0.000036 | GnRHR-ECL2-AAbs are significantly elevated in patients with PCOS compared with healthy controls and controls with ovulatory infertility. The GnRHR antagonist cetrorelix significantly suppressed the elevated GnRHR activity induced by IgG from patients with PCOS. |
| Weedin et al. [54] | 2020 | PCOS 3.66 ± 0.84 vs. controls 3.45 ± 0.99 GnRHR activity (fold increase over buffer baseline) adjusted 1 p = 0.0029 | The baseline GnRHR-AAb activity level was significantly higher in patients with PCOS than in control subjects. The addition of cetrorelix resulted in the significant suppression of AAb activity levels in patients with PCOS as a group, whereas control subjects were unaffected. | |
| Li et al. [71] | 2021 | PCOS 39% vs. controls 0% p < 0.01 GnRHR activation (fold increase over buffer baseline; exact values not provided) p < 0.01 | Serum GnRHR-AAb activity and positivity were significantly higher in patients with PCOS than in normal controls. Elevated GnRHR-AAb activity was associated with increased testosterone and proinflammatory cytokines (IL-2, IL-6, IFN-γ, TNF-α) in PCOS. | |
| Sattler et al. [78] | 2021 | PCOS 2.0% vs. controls 0.5% p > 0.05 | Natural GnRHR-AAbs are present in a very small fraction of adult control subjects and subjects with PCOS of European decent. The results do not support the hypothesis that GnRHR constitutes a relevant autoantigen in PCOS. | |
| anti-FSH | Haller et al. [55] | 2005 | anti-FSH-IgA level adjusted 2 OR 2.15 p = 0.015 anti-V14D-IgA level adjusted 2 OR 24.68 p = 0.015 | The model showed anti-FSH IgA and anti-V14D IgA as significant risk factors for PCOS and endometriosis. Naturally occurring anti-FSH IgA could be a marker for infertility in ovarian disorders. |
| anti-FSHR | Schniewind et al. [60] | 2018 | PCOS 2.0% vs. controls 0.9% p > 0.05 | The prevalence of anti-FSHR is low, both in control subjects and in women with PCOS. It is therefore unlikely that autoimmunity to FSHR constitutes a frequent cause of hyperandrogenemia or ovulatory dysfunction in PCOS. |
| anti-LHR | Schniewind et al. [60] | 2010 | PCOS 0.4% vs. controls 1.2% p > 0.05 | The prevalence of anti-LHR is low, both in control subjects and in women with PCOS. It is therefore unlikely that autoimmunity to LHR constitutes a frequent cause of hyperandrogenemia or ovulatory dysfunction in PCOS. |
| AOAs | Shoukry et al. [65] | 2020 | PCOS 7.4 (6.7–8) 3 vs. controls 6.6 (6.5–7.3) 3 IU/mL p = 0.001 | The presence of an autoimmune response against ovarian tissue in female patients with PCOS suggests a potential autoimmune mechanism in the pathogenesis of the condition. |
| Fénichel et al. [64] | 1999 | OD value (exact values not provided) IgG p < 0.0001 IgA p < 0.003 IgM p < 0.0003 | High concentrations of AOAs found in a group with PCOS suggest that an immune reaction is associated with PCOS. Positive AOAs for at least one isotype were present in 15 (44%) of 34 women with PCOS. | |
| Luborsky et al. [69] | 1999 | PCOS 25.0% vs. controls 19.0% p > 0.05 PCOS 0.52 (0.15–1.46) 4 vs. controls 0.47 (0.24–0.93) 4 OD (405 nm) p = 0.845 | The frequency of AOA was similar among the controls and those with PCOS. Thus, the prevalence of ovarian antibodies in patients with PCOS is not significantly different to in controls. |
| Autoantibody | Autoantibody Level in Women with PCOS | Summary of Study Results |
|---|---|---|
| GnRHR-AAbs | Probably increased | The vast majority of studies show increased levels of autoantibodies in women with PCOS, and the importance of autoantibodies has been demonstrated in preclinical studies [13,53,54,71,78]. |
| AOAs | Possibly increased | The results are heterogeneous, but many studies show higher levels of autoantibodies in women with PCOS [64,65,69,70]. |
| anti-FSH | Possibly increased | There is little evidence, but what exists does suggest a higher level of autoantibodies in women with PCOS [55]. |
| anti-FSHR | Possibly not increased | There is little evidence, but what exists does not support a higher concentration of autoantibodies in women with PCOS [60]. |
| anti-LHR | Possibly not increased | There is little evidence, but what exists does not support a higher concentration of autoantibodies in women with PCOS [60]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akpang, N.; Kwiatkowski, J.; Zaborowska, L.; Ludwin, A. Autoantibodies Targeting the Hypothalamic-Pituitary-Ovarian Axis in Polycystic Ovary Syndrome: Emerging Key Players in Pathogenesis? Int. J. Mol. Sci. 2025, 26, 4121. https://doi.org/10.3390/ijms26094121
Akpang N, Kwiatkowski J, Zaborowska L, Ludwin A. Autoantibodies Targeting the Hypothalamic-Pituitary-Ovarian Axis in Polycystic Ovary Syndrome: Emerging Key Players in Pathogenesis? International Journal of Molecular Sciences. 2025; 26(9):4121. https://doi.org/10.3390/ijms26094121
Chicago/Turabian StyleAkpang, Nicole, Jakub Kwiatkowski, Lucja Zaborowska, and Artur Ludwin. 2025. "Autoantibodies Targeting the Hypothalamic-Pituitary-Ovarian Axis in Polycystic Ovary Syndrome: Emerging Key Players in Pathogenesis?" International Journal of Molecular Sciences 26, no. 9: 4121. https://doi.org/10.3390/ijms26094121
APA StyleAkpang, N., Kwiatkowski, J., Zaborowska, L., & Ludwin, A. (2025). Autoantibodies Targeting the Hypothalamic-Pituitary-Ovarian Axis in Polycystic Ovary Syndrome: Emerging Key Players in Pathogenesis? International Journal of Molecular Sciences, 26(9), 4121. https://doi.org/10.3390/ijms26094121





