Abstract
Antibiotic-resistant bacteria are major contributors to food spoilage, animal diseases, and the emergence of multidrug-resistant (MDR) bacteria in healthcare, highlighting the urgent need for effective treatments. Bacteriocins produced by lactic acid bacteria (LAB) have gained attention for their non-toxic nature and strong antimicrobial properties. LAB-derived bacteriocins have been successfully applied in food preservation and are classified by the U.S. Food and Drug Administration (FDA) as ‘food-grade’ or ‘generally recognized as safe’ (GRAS). This review summarizes recent progress in the production, purification, and emerging applications of LAB bacteriocins. It emphasizes their versatility in food preservation, agriculture, and medicine, providing insights into their role in antimicrobial development and functional food innovation.
1. Introduction
Pathogenic bacteria present significant challenges to agriculture, healthcare, and the food industry [1]. These bacterial pathogens are responsible for millions of cases of food poisoning annually and some of them like rice bacterial blight can reduce crop yields by up to 50%, leading to billions of dollars in economic losses [2,3]. Particularly concerning is the threat of antibiotic-resistant bacteria, which account for over 700,000 deaths globally each year—a number that could escalate to 10 million deaths annually by 2050 if effective measures are not implemented [4]. Various strategies have been proposed to mitigate these bacterial threats, including improved hygiene practices, phage therapy, probiotics and prebiotics, antimicrobial peptides, and vaccination [5]. Among these alternatives, bacteriocins have emerged as promising candidates due to their natural safety, low potential for resistance development, and unique targeted antimicrobial capabilities [6,7]. In general, bacteriocins typically exhibit antimicrobial activity against species closely related to the producing strain, resulting in a relatively narrow spectrum of action. However, nisin, a bacteriocin approved by the FDA as “food-grade” or GRAS (Generally Recognized as Safe), demonstrates a broader antimicrobial spectrum, highlighting the potential versatility of certain bacteriocins.
Lactic acid bacteria (LAB) are a group of Gram-positive, non-spore-forming microorganisms characterized by their acid tolerance, heat resistance, and the absence of a noticeable odor. Morphologically, they can be rod-shaped (e.g., Lactobacillus) or spherical (e.g., Lactococcus) and are classified as facultative anaerobes [8]. The major genera of LAB include Lactiplantibacillus, Latilactobacillus, Levilactobacillus, Lactococcus, etc. [9]. LAB bacteriocins, which are commonly found in fermented foods, are known for their prolific production. They are non-toxic, exhibit a low propensity for inducing resistance, and possess potent antimicrobial activity [10]. The antimicrobial action of LAB bacteriocins involves disrupting cell membrane integrity and inhibiting protein and nucleic acid synthesis, making the development of resistance challenging [11,12]. LAB bacteriocins can retain their activity for extended periods under freeze-drying or refrigeration conditions. Many bacteriocins have narrow activity spectra, targeting primarily strains closely related to their producers. In contrast, some exhibit broad-spectrum antimicrobial activity against diverse genera. The regulation of bacteriocin production is often complex and can be influenced by various environmental factors, including pH, temperature, and the composition of the growth medium [13]. As proteinaceous compounds, bacteriocins are susceptible to enzymatic degradation after absorption [14]. Magnusson et al. isolated over 1200 strains of LAB from various environments. Some of the strains showed strong to moderate antimicrobial activity against Aspergillus fumigatus, Aspergillus nidulans, Penicillium commune, etc. And antifungal cyclic dipeptides produced by lactic acid bacteria were identified [15]. These characteristics highlight their potential as viable alternatives to commercial antibiotics and chemical preservatives. The application potential of LAB bacteriocins spans multiple fields. In the food industry, they serve as natural preservatives, effectively inhibiting common foodborne pathogens such as Listeria monocytogenes, Salmonella Typhimurium, and Staphylococcus aureus [16]. By enhancing food safety while reducing reliance on chemical preservatives, LAB bacteriocins align with consumer demand for natural and clean-label products [17]. Beyond food preservation, LAB bacteriocins also demonstrate potential as eco-friendly biocontrol agents in agriculture, suppressing plant pathogens and promoting sustainable farming practices [18]. In the biomedical field, LAB bacteriocins show promise as antibiotic alternatives for combating multidrug-resistant bacteria, highlighting their critical role in addressing the global antibiotic resistance crisis [6].
This review provides an overview of the fundamental types of LAB bacteriocins and the strategies employed for their discovery, isolation, purification, and identification. It also summarizes the synthesis, structural forms, and antibacterial activities of bacteriocins. Particular emphasis is placed on recent advances in production strategies, purification techniques, and emerging applications of bacteriocins.
With their unique properties and broad application potential, LAB bacteriocins are poised to become essential tools in enhancing food safety, promoting green agriculture, and driving biomedical innovations. Supported by technological advancements, favorable policies, and international collaboration, LAB bacteriocins are expected to make significant contributions to achieving global sustainability goals.
2. Fundamental Characteristics and Classification of Bacteriocins
2.1. Key Developments of Bacteriocins
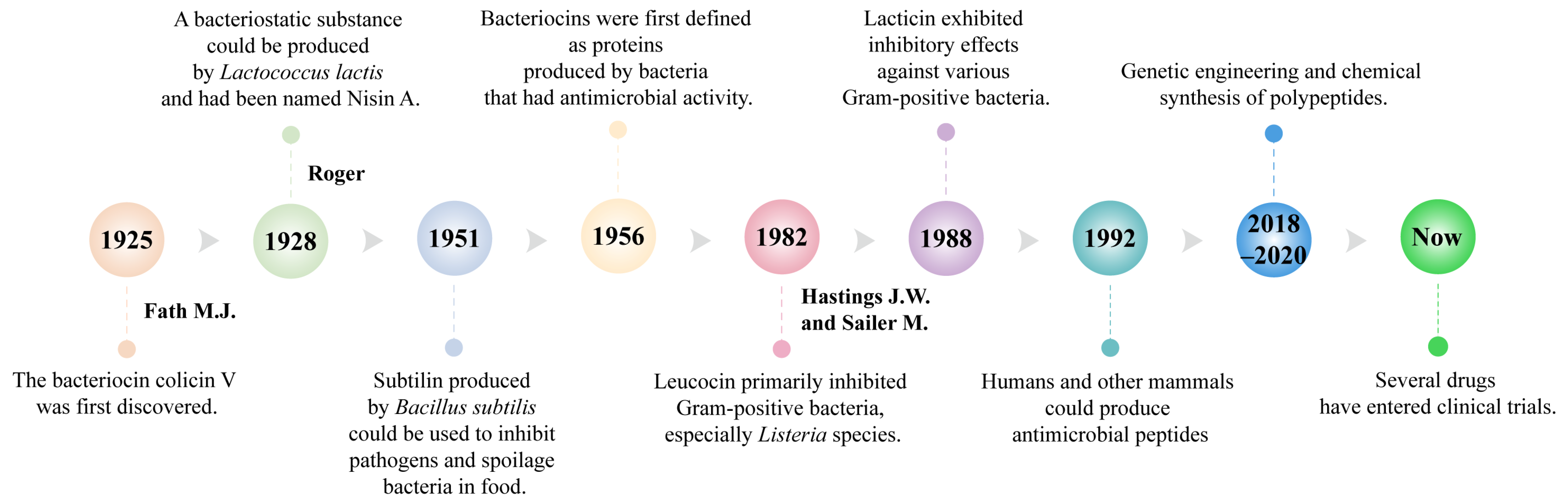
Bacteriocins, natural antimicrobial peptides synthesized by bacteria during the logarithmic growth phase, are garnering attention for their safety, efficacy, and biodegradability. These small molecules play a crucial role in microbial communities by inhibiting the growth of harmful bacteria, thereby providing a competitive advantage to the producing bacteria within their environment [19,20]. Research on bacteriocins began in 1925 when Fath first discovered colicin V in the fermented broth cultures of Escherichia coli [21]. In 1928, Rogers identified a substance produced by Lactococcus lactis with broad-spectrum inhibitory activity against Gram-positive bacteria, naming it Nisin A. Nisin A became the first bacteriocin widely used for food preservation [22,23,24]. In 1951, researchers identified Subtilin, produced by Bacillus subtilis, which was primarily used to inhibit pathogenic and spoilage bacteria in food [25]. In 1956, bacteriocins were first defined as proteins with antimicrobial activity produced by bacteria, laying the foundation for subsequent research and classification [23].
Over the years, researchers have discovered an increasing number of bacteriocins (Figure 1). Qiao et al. found that BMP32r, a type of bacteriocin, disrupts mature biofilms by killing bacteria in the biofilm and its effectiveness in killing Listeria monocytogenes persisters makes it a promising potential anti-biofilm agent against Listeria monocytogenes [26]. In 1992, Lacticin 481, produced by Lactococcus lactis, was identified as an inhibitor of various Gram-positive bacteria [27]. In 2016, Zipperer et al. found that lugdunin produced by Staphylococcus. lugdunensis had a broad spectrum of biological activity against Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus, vancomycin, and glycopeptide-intermediate-resistant Staphylococcus aureus strains [28]. Li et al. discovered that sublancin produced by Bacillus subtilis can act directly on the host, selectively triggering the innate immune response and modulating the structural and functional composition of the microbiota to protect mice from pathogens [29]. Khorshidian et al. discovered that pediocin produced by Pediococcus could be effective against many Gram-positive bacteria, particularly Listeria, and is commonly used for preserving meat and meat products [30].
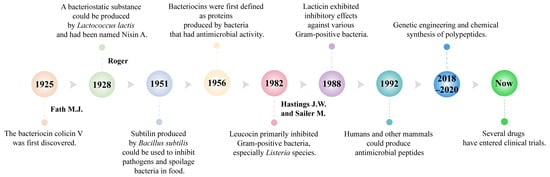
Figure 1.
Key milestones of antimicrobial peptides.
Since Klaenhammer first proposed a classification method for bacteriocins in 1936, advancements in high-throughput technologies have significantly progressed the field [31]. Detailed investigations into the structure, function, and mechanisms of bacteriocins have led to the ongoing identification and characterization of new variants, resulting in the evolution of the initial classification scheme over time [8,32]. Currently, after an extensive literature review, four main categories of bacteriocins have been identified and confirmed [33,34]. The following will be a detailed discussion of the classification and basic characteristics of LAB bacteriocins, and will not be further expanded here.
2.2. Fundamental Characteristics of LAB Bacteriocins
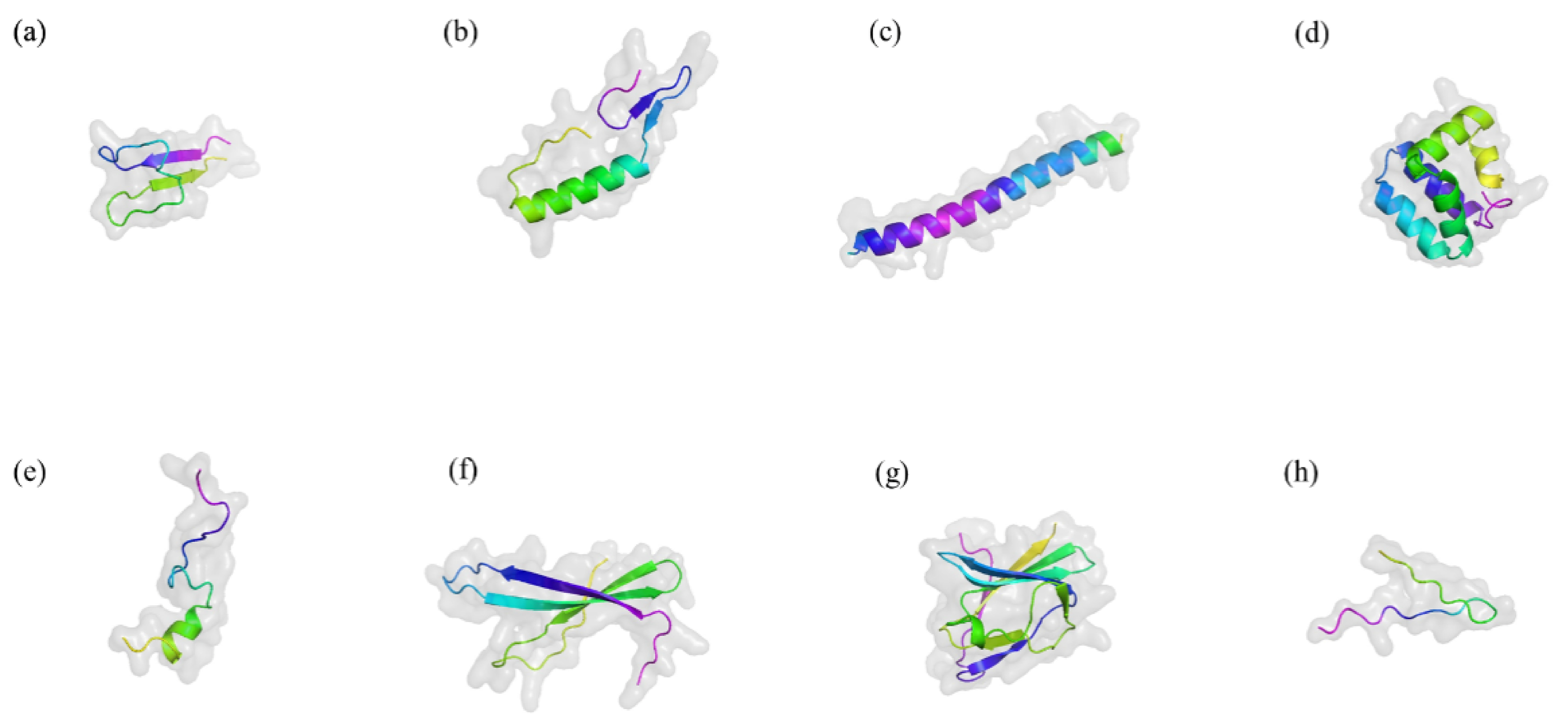
LAB, first reported in 1982, have been isolated from a variety of environments, including fermented cucumbers, silage, bioethanol production plants, dairy products, and brewing wort [35]. They are Gram-positive, non-sporulating microorganisms that produce bacteriocins—proteinaceous or peptide compounds with significant antimicrobial activity. They are classified as obligate homofermentative, obligate heterofermentative and facultative heterofermentative. LAB bacteriocins are known for their remarkable thermal stability and tolerance to acidic and alkaline conditions, allowing them to remain active in diverse environments [36]. Notably, the antimicrobial activity of LAB bacteriocins exhibits significant specificity [8]. Some bacteriocins target closely related bacterial strains with high precision, while others demonstrate a broader spectrum, effectively inhibiting pathogens across multiple genera [16]. Moreover, LAB are easy to culture and store, offering benefits such as regulating gut microbiota, modulating the immune system, antagonizing pathogens, and promoting animal growth [37]. They not only improve gut microbiota but also positively impact food production and fermentation processes [10,38]. They have a DNA G+C content of less than 50 mol% and can produce a wide range of digestive enzymes and small bioactive compounds under both homolactic and heterolactic fermentation conditions [39]. Figure 2 illustrates the structures of several representative bacteriocins [40].
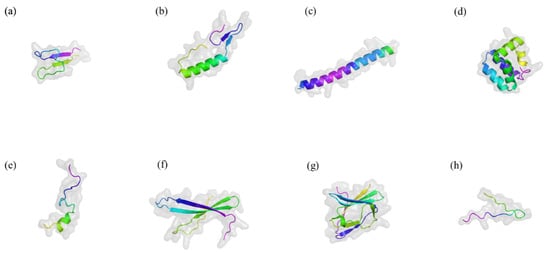
Figure 2.
Typical three-dimensional structure of several bacteriocins. Drawn by Alphold (https://alphafoldserver.com/ accessed on 20 October 2024). (a) Nisin A. (b) Pediocin PA-1. (c) Lactococcin G. (d) Enterocin AS-48. (e) Bacteriocin 31. (f) Helveticin J-11. (g) Enterolysin A. (h) MccJ25. The figure illustrates the classic three-dimensional structures of antimicrobial peptides, including α-helix, β-sheet, extended structure, and loop and extended regions. These structures interact with bacterial membranes, penetrating and disrupting their integrity, thereby exerting antimicrobial effects.
2.3. Classification of LAB Bacteriocins
Since Klaenhammer introduced the first classification of LAB bacteriocins in 1993 [31], numerous bacteriocins have been isolated from LAB. Although LAB bacteriocins fit the definition of antimicrobial peptides, the newly identified RiPP (ribosomally synthesized and post-translationally modified peptides, a class of bioactive peptides produced through ribosomal synthesis of precursor peptides followed by enzymatic post-translational modifications) subgroups and their activities deviate from traditional bacteriocin classifications. Class IV bacteriocins are complex cyclic peptides composed of lipids and carbohydrates [41]. However, to date, no LAB bacteriocins have clearly demonstrated these properties, leaving this class relatively underexplored [42]. Based on the revised suggestions proposed by Cotter et al. in 2005, LAB bacteriocins have been simplified into two categories [42]: Class I (modified bacteriocins) and Class II (unmodified bacteriocins), as distinguished in the revised classification. The original Class III was renamed “lysozymes,” and Class IV was eliminated. While this section focuses on bacteriocins isolated from LAB, the classification scheme is also applicable to known antimicrobial peptides isolated from other ecological environments. Based on molecular weight, chemical structure, thermal stability, and post-translational modifications, LAB bacteriocins are primarily classified into three categories [33,43,44].
Class I (less than 10 kDa): Class I bacteriocins are small peptides that undergo post-translational modifications during biosynthesis. These bacteriocins feature a leader peptide, which plays a role in recognition, transport, and maintaining the activity of the core peptide, and is subsequently fused with the core peptide [45]. Medema et al. provided key characteristics for the systematic definition of Class I bacteriocins. Lanthipeptides, the most extensively studied within Class I, contain special amino acids and typically include residues like lanthionine, heterocyclic structures, head-to-tail cyclization, and glycosylation [46,47]. The genes involved in the maturation of lanthipeptides are typically located within the same operon. Type I (Lan-BC modification) and Type II (LanM-modified) are considered antibiotics [48]. Types III and IV lack known antimicrobial activity and are not further discussed here.
Class II (greater than 10 kDa): Class II bacteriocins are non-modified, heat-stable, non-thiol peptides. Most of them are cationic peptides, and their antimicrobial activity is primarily exerted by enhancing membrane permeability [49,50]. They are further classified into four subclasses based on their structural features. Class IIa bacteriocins, characterized by a double glycine-type signal sequence, are exemplified by Pediocin PA-1, which shows strong inhibitory activity against Listeria monocytogenes in a model gut environment [51]. Class IIb bacteriocins consist of two peptides that synergistically enhance activity through disulfide bonds formed by cysteine residues, such as Lactococcin G [52]. Class IIc bacteriocins are cyclic peptides with covalently bonded N- and C-termini, typically exceeding 30 kDa in molecular weight and exhibiting a narrow antimicrobial spectrum [53]. Class IId bacteriocins are linear, single-chain peptides that may contain thioether bonds or lack cysteine residues, often displaying broad-spectrum antimicrobial activity [54].
Class III bacteriocins are high-molecular-weight (30–80 kDa), heat-sensitive proteins primarily derived from lactic acid bacteria and other Gram-negative bacteria [55]. Their heat sensitivity limits their application in food preservation.
In 2016, researchers analyzed 238 complete Lactobacillus genomes in a database, revealing that the numbers of gene clusters encoding presumed Class I, II, and III bacteriocins were 137, 514, and 97, respectively. This study found that Class II bacteriocins are the most abundant antimicrobial peptides in Lactobacillus [8]. According to bacterial taxonomy, there are six genera of LAB, with their names and ecological origins presented in Table 1.
Most bacteriocins studied in LAB belong to Class I or Class II due to their heat stability, which makes them particularly suitable for food preservation. These bacteriocins typically exhibit high selectivity, targeting specific bacterial species [56,57,58,59].

Table 1.
Origins and representative bacteriocins of LAB [60].
Table 1.
Origins and representative bacteriocins of LAB [60].
| Genus | Ecological Origin | Examples |
|---|---|---|
| Lactococcus | Fermented food, dairy products | Nisin A, Lactococcin Z |
| Lactobacillus | Fermented foods, plants | Bacteriocin ST69BZ, Sakacins D98a |
| Streptococcus | Animal gastrointestinal tract | Lactostrepcin, Sb15 |
| Pediococcus | Fermented foods, plants | Pediocin PA-1, Pediocin ST18 |
| Carnobacterium | Refrigerated food, ocean | Piscicolin 61, Divergicin 750 |
| Enterococcus | Human and animal gastrointestinal tract | Enterocin A, Cytolysin |
3. Biosynthesis of Bacteriocins
Bacteriocins are ribosomally synthesized peptide compounds that are initially biologically inactive and require post-translational modifications to become functionally active [61]. The genes encoding bacteriocins are typically clustered within operons, which may be located on chromosomes, plasmids, or sometimes inserted into chromosomes via transposons [61]. The biosynthetic pathway of bacteriocins is relatively conserved, encompassing the synthesis of precursor bacteriocins, cleavage of the leader sequence at specific processing sites, and secretion of the precursors into the extracellular environment [62]. During this process, the operons include multiple functional modules that regulate the synthesis, processing, and secretion of bacteriocins [63,64]. Prior to secretion, precursor bacteriocins undergo further modifications, such as the formation of thioether cross-links (e.g., lanthionine or methyllanthionine) through the degradation of serine or threonine residues, or the addition of cysteine residues to unsaturated amino acids, enhancing their structural stability and biological activity.
3.1. Key Components and Signal Transduction in Bacteriocin Synthesis
The synthesis of bacteriocins involves five main types of genes. Structural genes encode the precursor proteins of bacteriocins, typically carrying an N-terminal leader sequence (such as double glycine-type or signal peptide-type) [65]. The two conserved glycine residues at the C-terminus are recognized by ABC transporters, which cleave the leader sequence and secrete the mature bacteriocin. During this process, the signal peptide sequence plays a crucial role in the processing and secretion of bacteriocins via the general secretory pathway [66]. Immunity genes encode small proteins comprising 51–154 amino acids, which protect the producing bacteria from the toxic effects of their own bacteriocins [67]. Transport and processing genes encode proteins responsible for the processing, transport, and secretion of precursor bacteriocins, ensuring their proper release [68]. Modification genes encode enzymes that carry out post-translational modifications, converting precursor bacteriocins into their active, mature forms [69]. Lastly, regulatory genes control the expression of bacteriocin synthesis genes by regulating signaling pathways, thereby enabling efficient bacteriocin production under appropriate conditions [70].
The production and secretion of bacteriocins are regulated by a signal transduction system comprising three key components: induction peptide, response regulatory protein, and sensor histidine protein kinase (HPK). The induction peptide is a small cationic molecule that forms an amphipathic α-helix and serves as a signaling molecule for the regulatory system, also referred to as a “quorum-sensing” signal [71]. It plays a crucial role in controlling the synthesis of specific bacteriocins. The response regulatory protein transmits the signal, acting as a key mediator in the signal transduction process, while HPK detects the signal and initiates the corresponding regulatory processes [72,73].
Two primary models have been proposed to explain the induction mechanism for bacteriocin synthesis. The first model suggests that induction peptides are continuously produced in small quantities during bacterial growth and gradually accumulate. When their concentration reaches a specific threshold, the expression of bacteriocin-related genes is triggered [74]. The second model proposes that the production of induction peptides can temporarily increase under certain environmental conditions. Once the concentration surpasses the self-induction threshold, the expression of bacteriocin gene clusters is activated, accelerating bacteriocin synthesis [74].
3.2. Characterization of Synthesis of Different Classes of Bacteriocins
The biosynthesis of bacteriocins involves the ribosomal synthesis of precursor proteins, followed by various post-translational modifications, cleavage, and maturation processes [75]. These processes vary across the four classes of LAB bacteriocins, resulting in diverse structures and modes of action.
Class I bacteriocins, such as Nisin and Lacticin 3147, undergo post-translational modifications involving dehydratases and cyclases. Nisin, produced by Lactococcus lactis, was discovered and commercialized in the 1950s [76]. The precursor protein and its functional domains (NisB, NisC, and NisP) are synthesized ribosomally. The substrate NisA undergoes dehydration of serine and threonine residues by the dehydratase NisB and the cyclase NisC. This process results in the formation of dehydrated amino acids, which subsequently interact with cysteine thiols to form thioether bridges, completing the cyclization. Specific proteases, such as NisP, then cleave the leader sequence, releasing the mature bacteriocin [77]. In addition to Nisin, other lantibiotics, such as Lacticin 481, undergo similar modifications [78]. Non-lantibiotic Class I bacteriocins do not undergo extensive modifications. They possess mature head-to-tail structures, and their cyclic conformation confers stability, allowing them to withstand various environmental stresses [79].
Class II bacteriocins are divided into four subclasses, each with distinct formation mechanisms. Generally, the genes associated with Class II bacteriocins include those encoding precursor proteins, immunity proteins, transporter proteins, and membrane-bound auxiliary proteins [80]. The biosynthesis process begins with the synthesis of precursor proteins containing mature antimicrobial peptide sequences and leader peptides. Leader peptides, which are recognized and cleaved by signal peptidases, are crucial for the proper folding and maturation of antimicrobial peptides. The cleaved precursor proteins undergo further processing by immunity proteins, typically proteases, to release the mature antimicrobial peptides [81].
Class III bacteriocins, which include lysozymes (IIIa) and non-lysozymes (IIIb), have different formation mechanisms compared to Classes I and II [43]. Their synthesis is tightly regulated at the transcriptional level, involving specific promoters and transcription factors. These high-molecular-weight, heat-labile proteins undergo post-translational modifications, such as folding, assembly, or glycosylation, before being released into the environment.
Class IV bacteriocins, also known as complex bacteriocins, contain lipid or carbohydrate groups [82]. During their synthesis, precursor proteins bind with these lipid or carbohydrate groups, resulting in structures with a high content of hydrophobic residues. Specific proteases then cleave the leader sequence to release the mature bacteriocin. These modifications can increase hydrophobicity and stability, thereby enhancing the bacteriocins’ effectiveness in diverse environmental conditions. These complex bacteriocins serve as potent antimicrobials, particularly in nutrient-rich environments where competition is high [82].
4. Antimicrobial Mechanisms of LAB Bacteriocins
Antibiotics have traditionally been the cornerstone of bacterial infection treatment. However, their widespread use has led to significant issues with bacterial resistance. Unlike antibiotics, most bacteriocins exhibit a narrower spectrum of activity but are valued for their excellent stability, structural diversity, and low toxicity. Additionally, their specific antimicrobial mechanisms and biosynthesis processes reduce the likelihood of resistance development.
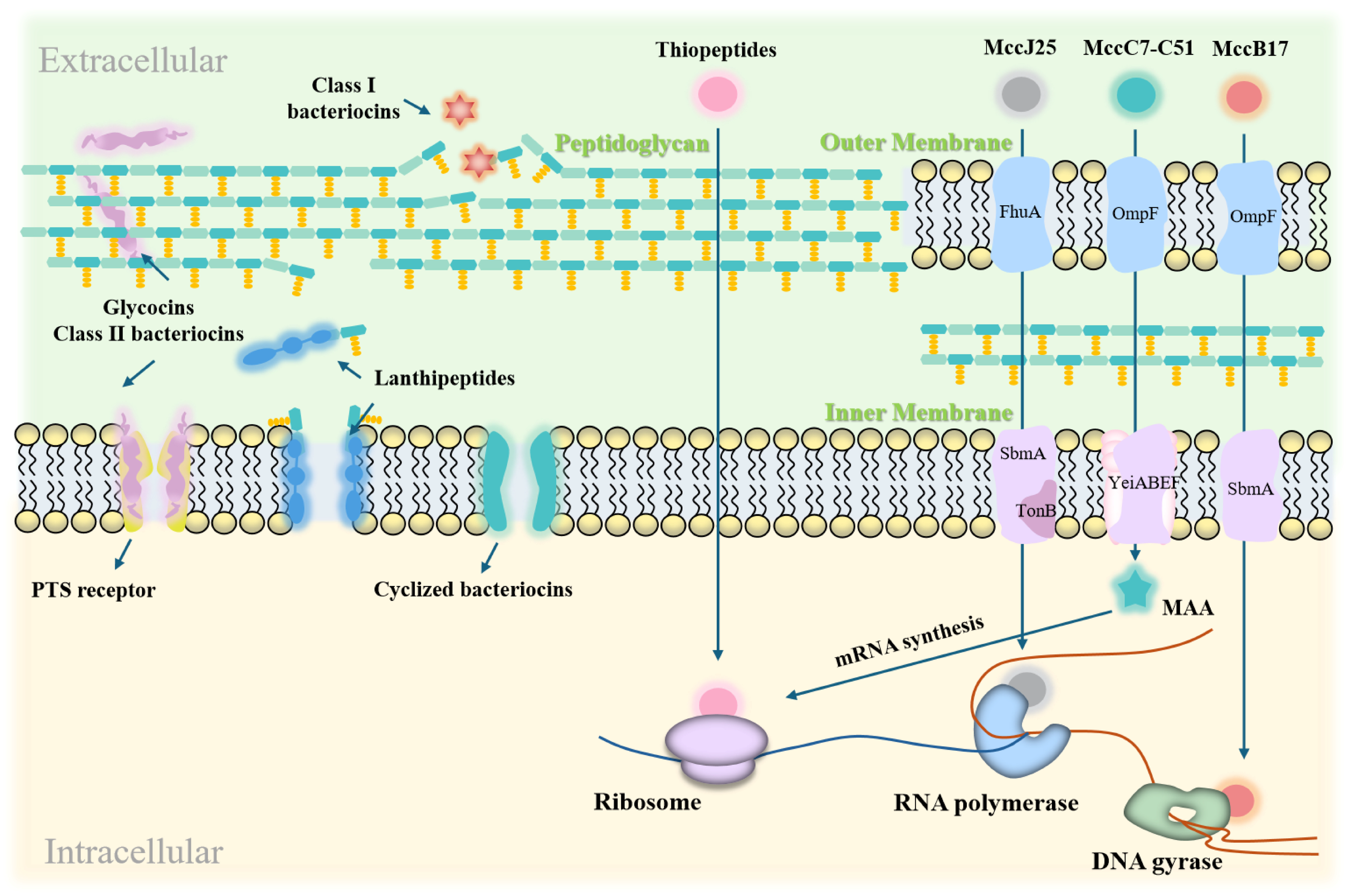
LAB bacteriocins are natural antimicrobial substances that have garnered significant attention in both the food industry and the medical field [83,84]. Their primary mechanism of action involves disrupting bacterial cell membranes, leading to bacterial cell death [85]. Figure 3 illustrates the modes of action of LAB bacteriocins against Gram-positive and Gram-negative targets.
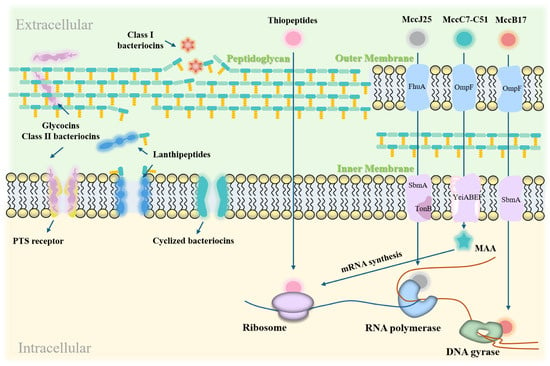
Figure 3.
Action modes of LAB bacteriocins on Gram-positive and Gram-negative bacterial targets. LAB bacteriocins exert their effects by disrupting cell membranes, forming pores, and interfering with gene expression and protein synthesis. In Gram-positive bacteria, they inhibit growth by forming membrane pores and blocking peptidoglycan synthesis. In Gram-negative bacteria, they inhibit growth by damaging cell membranes and disrupting cellular functions.
4.1. Mechanisms of Cell Membrane Disruption
As illustrated below, the most common mechanism by which bacteriocins exert their effects is through specific binding to receptors on the bacterial cell membrane. This binding leads to structural disruption of the membrane. This disruption creates pores in the membrane, causing leakage of cellular contents and ultimately leading to cell death. Bacteriocins achieve this through the following mechanisms:
Pore Formation: Class II bacteriocins create pores in the cytoplasmic membrane, leading to increased permeability [81]. The net charge and hydrophobic properties are the primary factors driving the binding of bacteriocins to bacterial cell membranes. Cationic bacteriocins interact with the anionic bacterial cell membrane, promoting the aggregation of bacteriocins on its surface. Hydrophilic groups of the bacteriocins embed within the lipid molecules, while the hydrophobic groups face the external environment. This orientation leads to the formation of ion channels, known as pores and gates [86]. For example, bacteriocin BM1122, produced by Lentilactobacillus crustorum MN047, creates channels in the cell membrane, increasing permeability. This causes leakage of cytoplasmic components, ultimately leading to bacterial death [87]. Additionally, other Class II bacteriocins like nisin A and pediocin PA-1 bound to the mannose phosphotransferase system (Man-PTS) on the cell membrane, leading to pore formation [81].
Increased Membrane Permeability: The action mechanism of Class I bacteriocins, such as lanthipeptides, increases membrane permeability. These bacteriocins possess hydrophobic and positively charged surfaces that interact electrostatically with negatively charged phospholipids. Ion channels cause cell membrane rupture or leakage of intracellular substances, increasing membrane permeability and disrupting normal metabolic functions. This membrane disruption ultimately leads to irreversible cellular collapse and bacterial lethality. For instance, the novel bacteriocin LSB1 from Lactiplantibacillus disrupts the physical barrier of Streptococcus mutans cells and disturbs the balance of intracellular and extracellular substances in vitro [88].
Compared to traditional antibiotics, LAB bacteriocins are generally less prone to inducing resistance. Nonetheless, bacteriocins that target bacterial nucleic acids may still carry a potential risk of resistance development. In vivo studies have indicated that resistance mechanisms in Staphylococcus aureus to nisin often involve alterations in membrane lipid composition or the activation of efflux pumps [89]. Moreover, environmental stressors, such as triclosan (TCS), have been directly linked to increased resistance, with TCS resistance being associated with heightened resistance to other antibiotics [90]. To mitigate these risks, it is crucial to explore the combination of LAB bacteriocins with other antimicrobial agents and further investigate the genetic mechanisms underlying resistance, aiming to reduce the likelihood of LAB bacteriocin resistance.
4.2. Interference with Gene Expression and Protein Synthesis
Bacteriocins can also inhibit bacterial growth by interfering with gene expression and protein synthesis through specific mechanisms:
Disruption of DNA and RNA replication: Bacteriocins block the transfer of genetic information, thereby inhibiting gene expression [91]. The exceptional activity of Microcin J25 (MccJ25) against pathogenic enterotoxigenic Escherichia coli (ETEC) is attributed to its ability to permeate bacterial membranes and its strong affinity for the secondary channel of RNA polymerase, which inhibits the transcription process. MccJ25 exhibits high endotoxin-neutralizing activity both in vitro and in vivo, and significantly suppresses the secretion and expression of pro-inflammatory factors. Importantly, MccJ25 does not exhibit a significant mutation rate, reducing the likelihood of developing antibiotic resistance [92].
Inhibition of Protein Synthesis: Bacteriocins can target components involved in protein synthesis, disrupting bacterial protein production. For instance, the bacteriocin MccC7-C51 inhibits aspartyl-tRNA synthetase, thereby blocking mRNA synthesis [93]. Bacteriocin MccB17 interferes with DNA replication by inhibiting DNA gyrase-mediated supercoiling [94]. Additionally, bacteriocins can alter cell membrane permeability, inhibit cellular respiration and spore germination, and disrupt intracellular enzyme activity [43]. These effects interfere with normal bacterial functions and ultimately lead to cell death.
4.3. Differential Mechanisms of Action Against Gram-Positive and Gram-Negative Bacteria
Bacteriocins employ different mechanisms of action against Gram-positive and Gram-negative bacteria:
Gram-Positive Bacteria: Bacteriocins primarily inhibit these bacteria by forming membrane pores and blocking peptidoglycan synthesis. For example, Nisin binds to lipid II molecules, inhibiting peptidoglycan synthesis and forming pores in the cell membrane, which leads to cell lysis in vitro [95]. Class III bacteriocins, including lysozyme-like bacteriocins, are crucial for degrading bacterial cell walls by hydrolyzing β-1,4-glycosidic bonds in peptidoglycan, making them particularly effective against Gram-positive bacteria [43].
Gram-Negative Bacteria: Bacteriocins inhibit these bacteria by disrupting the functions of both the outer and inner membranes. For example, pediocin alters outer membrane permeability and interferes with protein synthesis, thereby inhibiting bacterial growth [30].
5. Production of LAB Bacteriocins
The production and optimization of LAB bacteriocins involve meticulous processes including isolation, purification, and identification, as well as strategies to enhance production efficiency [96]. These antimicrobial peptides are derived from various natural sources and undergo rigorous testing and refinement to achieve high purity and functional characterization. Advanced techniques, such as chromatography and gene sequencing, are employed to analyze the structures and properties of these antimicrobial peptides [97]. Additionally, innovative approaches such as heterologous expression, optimization of cultivation conditions, and genetic modifications are pivotal for maximizing bacteriocin yield and effectiveness [96,98].
5.1. Challenges in the Production of LAB Bacteriocins
The isolation, purification, and characterization of bacteriocins produced by LAB is a complex process requiring a series of advanced techniques to ensure high purity and functional analysis. Several challenges arise during the separation and purification stages. First, fermentation broths contain numerous secondary metabolites, and the actual concentration of bacteriocins is typically low. This not only increases the difficulty of separation but also potentially compromises detection accuracy during characterization. Second, compared to general proteins, LAB bacteriocins have relatively small molecular weights, making their sizes similar to certain peptide fragments present in the culture medium, thereby adding to the complexity of the purification process [99]. Common methods for the isolation and purification of LAB bacteriocins include ammonium sulfate precipitation, membrane filtration, and various chromatographic techniques [100]. Among these, ammonium sulfate precipitation is the most widely used salting-out method [101]. However, the ammonium sulfate precipitation method has limitations, as high salt concentrations can cause bacteriocin denaturation, thereby reducing its antimicrobial activity [102]. Ion-exchange chromatography exploits the positively charged nature of bacteriocins under specific pH conditions to separate proteins with different properties. During the purification of LAB bacteriocins, NaCl gradients are typically employed for elution to isolate the target bacteriocins effectively [27,102]. For example, Callewaert et al. employed a strong cation exchanger to directly isolate amylovorin L471, a bacteriocin from Lactobacillus amylovorus DCE 471, from the fermentation medium [103]. Membrane filtration technology, on the other hand, separates target peptides based on molecular weight cutoffs. Fricourt et al. successfully isolated the bacteriocin Plantaricin F from the fermentation supernatant of Lactiplantibacillus BF001 using ultrafiltration with a molecular weight cutoff of 10,000 Da [104].
It is established that the main method of bacteriocin research is PCR analysis, which makes it possible to quickly and easily identify the presence of bacteriocin encoding genes [105]. At the protein level, SDS-PAGE is a commonly employed analytical method used to evaluate the molecular weight and purity of bacteriocins. Zamfir et al. employed liquid chromatography to determine the amino acid sequence of a bacteriocin from Acidophilus IBB 801 [106]. Zafar et al. used the SDS-PAGE technique to characterize a bacteriocin produced by a strain of Bifidobacterium [107]. In addition, mass spectrometry techniques, such as LC-MS/MS, serve as standard methods for confirming molecular structure and purity, offering detailed information on molecular weight and composition [108]. More advanced structural analysis techniques further enhance the understanding of bacteriocin molecular structures. For example, nuclear magnetic resonance (NMR) spectroscopy enables precise molecular structure determination, while infrared (IR) spectroscopy identifies key functional groups within the molecule [109,110]. In addition to experimental techniques, bioinformatics and genomic tools have played a crucial role in the discovery and study of bacteriocins. Genomic mining methods allow for the rapid identification of new bacteriocin gene clusters. For instance, researchers have discovered novel bacteriocin gene clusters, including lactone peptides, in 224 species of rumen bacteria and 5 species of rumen archaea [111]. These tools not only accelerate the discovery of bacteriocins but also provide a foundation for functional modification and performance optimization [91,97,112].
While these techniques significantly advance bacteriocin research, challenges remain in achieving cost-efficient and scalable production. High-purity purification methods, such as RP-HPLC, are resource-intensive and may not be suitable for industrial-scale applications. Future efforts should focus on developing sustainable and cost-effective methods to bridge laboratory feasibility and practical implementation.
5.2. Strategies for Enhancing Production Efficiency
Researchers have employed various strategies to enhance the efficiency of bacteriocin production. As shown in Table 2, these approaches include heterologous expression, solid-phase synthesis, chemical or physical mutagenesis, optimization of production conditions, microbial co-culture techniques, gene editing, and computational modeling. Traditional culturing methods, although effective, often result in low yields and cumbersome extraction processes [113], prompting researchers to develop various strategies to enhance bacteriocin production efficiency. Heterologous expression is a common method in which bacteriocin genes are cloned into different host organisms to significantly increase yields. For example, Sommer et al. achieved the extracellular expression of Colicin E1 and Cloacin DF13 bacteriocins by co-expressing bacteriocin release proteins (BRPs) [114]. Jiménez et al. expressed Enterocin A in Komagataella phaffii and Kluyveromyces lactis [115]. Meanwhile, Li et al. heterologously expressed the hybrid peptide EF-1 in Komagataella phaffii cells [116]. Hayward et al. used high-throughput screening to discover and express the Nek2 cancer inhibitor from a bacteriocin associated with a Toxoplasma-like protozoan [117]. Chemical reagents such as 40% ammonium sulfate and chloroform/methanol are used for the crude extraction of bacteriocin proteins, followed by reversed-phase high-performance liquid chromatography (RP-HPLC) for purifying antimicrobial peptides [53]. Jiménez et al. also cloned the enterocin A gene into Latilactobacillus sakei Lb 790 for expression [118]. Solid-phase peptide synthesis (SPPS) is used to synthesize specific bacteriocin peptides. Additionally, chemical or physical mutagenesis is used to modify production strains, which enhances bacteriocin yield [119,120].
Heterologous expression has been widely utilized for the large-scale production of antimicrobial peptides. However, this process involves significant costs associated with the development of expression systems, culture maintenance, and downstream processing. For instance, the commonly used Komagataella phaffii expression system requires a substantial amount of carbon sources [116]. Similarly, advanced purification techniques such as RP-HPLC and affinity chromatography can achieve excellent purity. However, the high costs of chromatography materials and solvents significantly limit their feasibility for large-scale industrial production [107]. To address these challenges, researchers are actively exploring innovative approaches. Optimizing production conditions is crucial for increasing bacteriocin yield. Factors such as medium composition, pH, temperature, and microbial growth kinetics significantly impact bacteriocin biosynthesis [121]. For example, Dündar et al. improved bacteriocin extraction efficiency through pH-mediated cell adsorption [122]. Pediocin production is influenced by a number of nutritional parameters. Anastasiadou et al. found that glucose was the best carbon source for pediocin production in Lactococcus lactis, while glycerol was the strongest inhibitor of pediocin production [123]. Researchers also optimized fermentation technology by controlling factors such as oxygen supply, temperature, and pH. These adjustments increased bacteriocin yield and activity [124]. Latilactobacillus curvatus/Pediococcus acidilactici produced the highest BLIS (Bacteriocin-Like Inhibitory Substances, a protein or peptide substance produced by a bacterium that has antimicrobial activity) at a growth temperature of 28 °C and pH 5, and the optimal conditions for BLIS production by Latilactobacillus sakei were 24 °C and pH 6.5. BLIS production by Latilactobacillus curvatus/Pediococcus acidilactici bacteria was strongly influenced by carbon and nitrogen sources [125]. Qiao et al. increased the production of Lactococcus lactis by producing bacterial cellulose membranes with different properties and using a co-culture approach [126]. Castro et al. assessed the effect of several factors on BLIS production and found that BLIS produced by the LAB strain was strongly influenced by NaCl concentration and the presence of surfactant [127]. Microbial co-culture techniques are employed to enhance bacteriocin production. For instance, researchers established a co-culture strategy involving bacterial cellulose (BC) and Lactococcus lactis, which significantly increased lacticin 3147 yield and activity to 6260 IU/mL [128]. Gene editing technologies, such as CRISPR-Cas9, are employed to modify lactic acid bacteria, thereby enhancing bacteriocin production capacity. These technologies are currently applied in the production of fermented foods and nutritional products [129]. Precise gene modifications enable researchers to optimize metabolic pathways in lactic acid bacteria, directing more precursor substances toward bacteriocin synthesis [130]. This approach increases synthesis efficiency [131]. Additionally, computational modeling and systems biology approaches offer significant potential for optimizing bacteriocin production strategies. Computer models simulate bacterial growth and metabolism, while molecular dynamics (MD) simulations are used to verify structures and interactions [132,133]. Researchers employed systems biology to construct and screen a semi-library of Phyper-spank variants, gaining insights into bacterial metabolic networks. This work provides theoretical support for optimizing bacteriocin production [134]. These strategies not only significantly increase bacteriocin yield and purity but also enhance efficiency and cost control in the production process.

Table 2.
Strategies for enhancing production of LAB bacteriocins.
Table 2.
Strategies for enhancing production of LAB bacteriocins.
| Category | Strategies and Examples | References |
|---|---|---|
| Heterologous Expression | Expression in Pichia—Kluyveromyces | [118,124] |
| Solid-Phase Peptide Synthesis (SPPS) | Synthesis of specific bacteriocin peptides | [119] |
| Mutagenesis | Chemical or physical modification of production strains | [120] |
| Production Conditions | Control of pH, temperature, and oxygen during fermentation | [121,124] |
| Microbial Co-culture Techniques | Co-culture strategies to enhance bacteriocin production | [126] |
| Gene Editing (CRISPR-Cas9) | Modification of LAB to enhance bacteriocin production capacity | [128,130] |
| Computational Modeling | Simulation of bacterial growth and metabolism | [131,132] |
| Systems Biology Approaches | Human and animal gastrointestinal tract | [133] |
6. Applications of LAB Bacteriocins in Various Industries
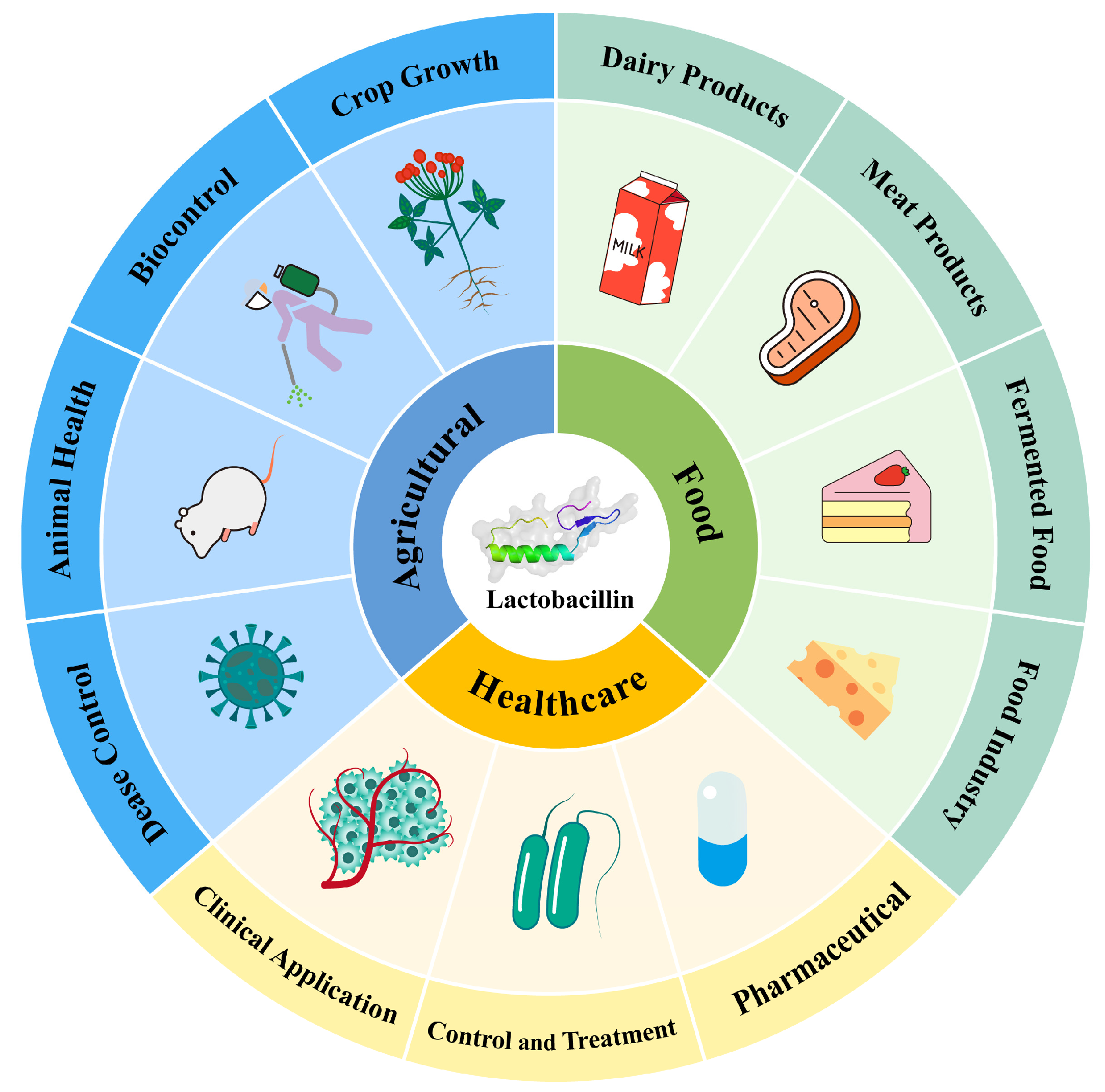
Traditional methods in food preservation, agriculture, and medicine face significant challenges, including chemical residues, resistance, and environmental pollution [128]. LAB bacteriocins offer safer and more sustainable alternatives. In food preservation, they extend shelf life and enhance quality. In agriculture, they control plant diseases and promote animal health. In medicine, they combat resistant pathogens and bolster immune responses. Figure 4 illustrates the applications and advantages of bacteriocins in these areas.
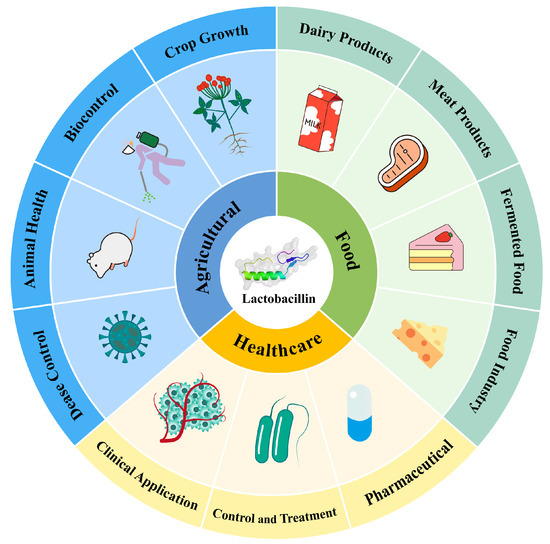
Figure 4.
Application of LAB bacteriocin in various industries.
6.1. Food Preservation and Antimicrobial Packaging
Food is the most basic substance on which people depend for their survival. In recent years, the risks to human health posed by the use of chemical preservatives in food have become increasingly well known. As a result, there is a growing need to find new safe preservatives and methods of preservation [135]. LAB bacteriocins have been utilized in traditional food processing for over 50 years and have been recognized as Generally Regarded as Safe (GRAS) by the U.S. Food and Drug Administration (FDA) [135]. As natural preservatives, LAB bacteriocins can extend food shelf life and improve safety and quality. They are utilized in fermented dairy products as well as in antimicrobial packaging and biodegradable materials [17].
The application of LAB bacteriocins in food preservation overcomes significant limitations of traditional methods. They offer a natural and effective way to extend shelf life, enhance food safety, and reduce chemical usage. Nisin functions as a functional additive, enhancing the nutritional value and health benefits of food. Nisin has been shown to have no significant effect on Vero cells at concentrations below 11.4 μg/mL after 48 h of exposure. Additionally, it exhibits no notable toxicity to vaginal epithelial cells at a concentration of 318 μg/mL [136]. These findings highlight its excellent biosafety profile, alongside its ability to maintain food freshness and inhibit the growth of food spoilage microorganisms. Extensive research has been conducted on the application of nisin in food products [137]. Oladunjoye et al. demonstrated that treating fresh-cut tomatoes with 5000 IU/mL of nisin significantly inhibited the growth of Listeria monocytogenes [138]. Lee et al. treated beef jerky with 500 IU/g of nisin after 3 days of storage, finding that Bacillus cereus growth only began after 21 days. This treatment effectively prolonged shelf life and inhibited microbial growth [139]. Gharsallaoui et al. incorporated 2.5 to 12.5 mg/kg of nisin into vacuum-packed Korean seasoned beef and found that it strongly inhibited the growth of Bacillus subtilis [140]. There is also considerable research on other LAB bacteriocins in food. For example, enterocin AS-48 has been studied in egg liquid, targeting Bacillus cereus and Staphylococcus aureus. The minimum inhibitory concentrations at 4 °C and 28 °C were found to be 5 μg/mL and 0.05 μg/mL, respectively. Furthermore, electron microscopy revealed a synergistic effect between enterocin AS-48 and lysozyme [141]. Additionally, studies have found that pediocin PA-1/AcH applied in meat products inhibits the growth of Listeria monocytogenes more effectively than nisin, without affecting the growth of other bacteria [142]. Zhao et al. developed biodegradable polysaccharide substrates combined with natural antimicrobials for sustainable food packaging [17]. In meat products, pediocin demonstrated excellent antimicrobial effects, preventing contamination by Staphylococcus aureus and Listeria while maintaining activity under low-temperature conditions, making it suitable for various meat product preservation methods [26]. Narrow-spectrum bacteriocins, such as lactococcin 3147 and enterocin AS-48, have been used to selectively inhibit high-risk bacteria. Scannell et al. used immobilized Lactococcus lactis protein 3147 for in situ protection of cultures, resulting in a reduction in Staphylococcus aureus viable counts of approximately 1.5 log units in cheese and 2.8 log units in ham [143]. Enterocin TJUQ1, a novel class II bacteriocin, is effective against foodborne pathogens such as Staphylococcus aureus, Listeria monocytogenes, Escherichia coli, and Salmonella. The MIC against Listeria monocytogenes CMCC 1595 was found to be 5.26 ± 0.24 μg/mL [11]. Additionally, LAB bacteriocins have potential as food additives to enhance food functionality. LAB strains in traditional fermented dairy products, such as Bulgarian yogurt and feta cheese, contribute to microbial diversity, promoting digestion and nutrient absorption [144]. The diverse applications of bacteriocins—from fermented products to antimicrobial packaging—underscore their potential to revolutionize food preservation.
6.2. Agricultural Applications
The use of chemical pesticides and antibiotics in agriculture has led to significant issues, including environmental pollution, antibiotic residues, and the development of resistant pathogens [145]. These problems necessitate the exploration of natural and sustainable alternatives. LAB bacteriocins show promise in disease control, animal health management, and environmental protection, offering eco-friendly solutions that reduce reliance on synthetic chemicals and antibiotics [146].
In agriculture, LAB bacteriocins have shown significant effectiveness in disease control and animal health management. For plant disease control, LAB bacteriocins inhibit various pathogenic bacteria, including Ralstonia solanacearum and Pseudomonas syringae. They achieve this by inducing systemic resistance and competing for ecological niches, which reduces disease incidence [146]. Moreover, Gerez et al. found that certain LAB bacteriocins also inhibited plant pathogenic fungi, aiding in the control of fungal diseases such as powdery mildew and downy mildew [147]. In freshwater environments, Streptococcus and Lactococcus are considered pathogenic bacteria in aquaculture. Gatesoupe reported that adding probiotics such as Carnobacterium and Enterococcus to fish feed effectively stimulated the fish immune system and inhibited the proliferation of pathogens like Streptococcus and Lactococcus [148]. These bacteria are major pathogens in freshwater fish and play a critical role in the pathogenic dynamics of the aquatic microbiome. Wyszyńska investigated the use of LAB as an oral vaccine delivery system to control Campylobacter infections in chickens, which improved poultry health [149]. LAB adhere to intestinal epithelial cells, effectively inhibiting pathogens such as Salmonella and E. coli, and preventing enteric infections [150]. Millette et al. found that LAB bacteriocins, by inhibiting harmful bacteria, improved the intestinal microbial environment, promoted nutrient absorption, and enhanced animal growth rates [151]. As natural antimicrobials, LAB bacteriocins reduce the need for antibiotics, thereby lowering antibiotic residues and resistance issues, and supporting long-term disease management in animals [63]. In biocontrol and environmental protection, LAB bacteriocins reduce the need for chemical pesticides, decrease soil and water pollution, and contribute to environmental protection. Studies showed that LAB (such as Lacticaseibacillus, Lactococcus, and Enterococcus) could replace some herbicides, reducing environmental pollution and pesticide residues [152]. Due to their unique mechanisms of action, bacteriocins do not easily induce resistance in pathogens, thereby effectively addressing pesticide resistance issues [153]. LAB bacteriocins target harmful soil pathogens such as Listeria, Actinomyces, Proteus, and Bacteroides. Additionally, they foster beneficial microbial growth, enhance the soil microbial environment, improve soil fertility, and promote crop growth [154,155]. LAB bacteriocins offer an eco-friendly solution to agricultural challenges, overcoming the limitations of chemical pesticides and antibiotics. They effectively control plant and animal pathogens, enhance soil fertility, and promote plant growth while minimizing environmental impact.
6.3. Medical Applications
The widespread use of antibiotics in medicine has led to the emergence of resistant pathogens, making infections increasingly difficult to treat. Additionally, antibiotics can disrupt the human microbiota, leading to various health issues. LAB bacteriocins present promising alternatives or supplements to antibiotics. Their unique mechanisms minimize the risk of resistance development and selectively target pathogens without harming beneficial bacteria [156]. LAB bacteriocins hold significant potential in disease treatment and prevention. Notable examples include nisin Z, nisin A variants, and gallidermin, which are known to enhance immune responses [157]. Research suggests that combining bacteriocins with Pseudomonas aeruginosa lipopolysaccharides enhances innate immune responses without triggering septic reactions [158,159]. LAB bacteriocins, with their distinct mechanisms compared to antibiotics, are less likely to induce resistance, making them promising alternatives or supplements. Studies demonstrate that bacteriocins effectively inhibit a range of resistant pathogens, including Methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant Enterococcus (VRE), and Clostridioides difficile infections (CDI) [160,161]. For antibiotic-resistant Gram-negative pathogens, studies have shown that Acidophilus STRAIN 52 and Limosilactobacillus fermentum STRAIN 45 inhibit Cephalosporin-resistant Escherichia coli strains with a 100% inhibition rate [162]. In a cell lysis experiment, the addition of 6400 Au/mL of bacteriocin inhibited the growth activity of Pseudomonas aeruginosa, reducing its activity from 8.7 × 106 CFU/mL to 1.3 × 107 CFU/mL [163]. Acidocin exhibited a moderate antibacterial effect against drug-resistant Pseudomonas aeruginosa, with an inhibition zone of 15.00 mm [164]. Lü et al. isolated Lactocin MXJ 32A from the Loigolactobacillus coryniformis MXJ 32 strain, which strongly inhibited the growth of Salmonella (inhibition zone > 20 mm). The antibacterial activity against Salmonella strains 87T4, 1006D, 36T, 557D, and 798D was measured at 4112.4, 3812.1, 4288.4, 4422.5, and 3770.0 AU/mL, respectively [165]. For antibiotic-resistant Gram-positive pathogens, the bacteriocin MXJ 32A produced by Loigolactobacillus coryniformis showed an inhibition zone of 23 mm against Staphylococcus aureus, with an antibacterial activity of 4650.0 AU/mL [165]. For antibiotic-resistant Gram-positive pathogens, the bacteriocin MXJ 32A produced by Loigolactobacillus coryniformis showed an inhibition zone of 23 mm against Staphylococcus aureus, with an antibacterial activity of 4650.0 AU/mL [166]. For antibiotic-resistant Gram-positive pathogens, the bacteriocin MXJ 32A produced by Loigolactobacillus coryniformis showed an inhibition zone of 23 mm against Staphylococcus aureus, with an antibacterial activity of 4650.0 AU/mL [145,167]. Additionally, bacteriocins EF478, BLIS, and Gallocin D demonstrated antibacterial activities against VRE with values of 320.0 AU/mL, 3000.0 AU/mL, and 1.56 µg/mL, respectively [168,169].
In clinical applications, LAB bacteriocins have garnered significant attention. LAB bacteriocins and their probiotic active cell substances exert numerous beneficial effects in the gastrointestinal tract, preventing pathogen adhesion, establishment, and replication through multiple antimicrobial mechanisms [170]. Products containing nisin effectively inhibit oral pathogens, helping to prevent gingivitis and oral ulcers [171,172]. For instance, Limosilactobacillus fermentum HV6b MTCC produces an Lla-class bacteriocin peptide, HV6b, which effectively inhibits various pathogens associated with human vaginal infections, including Bacteroides, Gardnerella vaginalis, Staphylococcus, and Streptococcus in vitro [173]. Additionally, nisin shows potential for targeted cancer therapy [174]. Advances in nanodrug delivery systems and genetic engineering have positioned bacterium-based diagnostic and therapeutic delivery systems as a major research focus in chemical biology [98,175]. LAB bacteriocins can target cell membranes to avoid immune responses and induce cell death directly through pore formation, thereby reducing systemic drug toxicity. Researchers have developed natural biomimetic delivery systems using these bacteriocins to address antibiotic resistance. LAB also exhibit antitumor effects by inhibiting mutagenic activity and reducing the production of carcinogens and associated enzymes, demonstrating potential for cancer prevention and treatment [176,177,178]. Studies have shown that specific cellular components of LAB strains can induce strong adjuvant effects, regulate cell-mediated immune responses, activate the reticuloendothelial system, enhance cytokine pathways, and modulate interleukins and tumor necrosis factors [179,180,181,182]. For example, plantaricin effectively inhibits pathogens causing skin infections, thereby accelerating wound healing through its antimicrobial properties. Reuterin exhibits broad-spectrum antimicrobial activity, effectively targeting a wide range of pathogenic bacteria in the gastrointestinal tract. Clinical studies have evaluated its efficacy in modulating gut microbiota, reducing gastrointestinal infections, and alleviating inflammatory bowel disease symptoms. The probiotic potential of Limosilactobacillus and its reuterin production underscore its role in maintaining gut health and preventing gastrointestinal disorders. Additionally, LAB bacteriocins can reduce reliance on synthetic antibiotics and help manage emerging immunocompromised populations [183].
LAB bacteriocins offer a viable solution to challenges posed by antibiotic resistance and human microbiota disruption. Their unique mechanisms and targeted action enable the effective treatment and prevention of infections. Additionally, LAB bacteriocins enhance immune responses and provide therapeutic benefits in cancer and gastrointestinal disorders. Integrating bacteriocins into clinical applications reduces reliance on synthetic antibiotics, mitigates resistance, and offers safer, more effective treatments. The potential antiviral properties of LAB bacteriocins have garnered significant attention from researchers. For instance, a peptide produced by Enterococcus mundtii ST4V has been demonstrated to exhibit notable antiviral activity [73], effectively inactivating multiple strains of herpes simplex virus (HSV), poliovirus, and an attenuated measles virus strain. Similarly, Todorov et al. investigated a pediocin-like peptide produced by Enterococcus faecium ST5Ha, which showed a strong inhibitory effect against herpes simplex virus type 1 (HSV-1) in vitro [184]. Despite these findings, the precise mechanisms through which bacteriocins act against viruses remain unclear. Wachsman et al. proposed that these mechanisms may involve blocking viral receptor binding sites or inhibiting viral replication [185].
The activity of LAB bacteriocins observed during in vitro studies offers exciting new insights into the development of cancer treatment strategies. Chiu et al. reported that certain factors produced by Lactobacillus rhamnosus and Lacticaseibacillus significantly induced apoptosis pathways in monocytic leukemia cell lines, effectively suppressing cancer cell proliferation [186]. Additionally, an experimental study in mice demonstrated that feeding Lacticaseibacillus helveticus R389 delayed tumor progression [187]. Further investigations revealed that this effect might be attributed to reduced levels of pro-inflammatory cytokines such as IL-6 [187]. These groundbreaking findings not only highlight the potential of bacteriocins in the fight against cancer but also emphasize their value as promising adjuvants in cancer therapy. Their combined immunomodulatory and pro-apoptotic properties open new avenues for integrating bacteriocins into advanced cancer treatment strategies [188].
7. Future Directions and Prospects
LAB bacteriocins are widely acknowledged for their safety and high antimicrobial efficiency, making them valuable as natural food preservatives [189]. Their applications extend to preserving animal-derived foods, with commercial examples like nisin and pediocin successfully inhibiting spoilage bacteria and pathogens in several countries [190]. Additionally, bacteriocins such as Enterocin AS-48 [191], Bovicin HC5 [192], Enterocin 416K1 [61], pediocin, and Bifidin C6165 [193] have demonstrated potential in extending the shelf life of fruit-based products. Despite substantial advancements in LAB bacteriocin research, their clinical and industrial applications encounter persistent challenges. Key obstacles include low production efficiency, high purification costs, and stringent regulatory requirements [20]. Current methods, such as chemical synthesis and heterologous expression, often yield low quantities and inadequate purity, undermining economic feasibility and application scalability [194,195]. The incomplete understanding of bacteriocin mechanisms represents another significant barrier to their practical application [195,196]. This knowledge gap delays industrialization and raises the standards for further development. Bacteriocin stability and activity can be compromised under challenging environmental conditions, such as high temperatures, extreme pH levels, or complex food matrices. For example, nisin stability decreases in acidic environments, limiting its application in certain food preservation scenarios. These limitations restrict their use in specific industrial processes and highlight the need for stability-enhancing modifications. Moreover, their relatively weaker inhibitory effects on Gram-negative bacteria fail to meet the demands for broad-spectrum antimicrobial applications. These limitations highlight the importance of developing more stable and active bacteriocins or their derivatives as a critical pathway to overcoming application bottlenecks.
In both agriculture and medicine, LAB bacteriocins, as natural antimicrobial agents, have shown great potential for various applications. Particularly in agriculture, LAB bacteriocins can be used as biopesticides and plant growth promoters to control crop diseases and enhance plant growth. However, the in vitro activity of LAB bacteriocins does not always translate effectively in complex agricultural environments. LAB are ubiquitous members of many plant microbiomes, but there have been no reports on how LAB interact with hosts to exert antimicrobial effects. Moreover, LAB bacteriocins exhibit relatively weak inhibitory activity against plant pathogenic fungi and bacteria. For instance, in soil and the rhizosphere microbial communities of plants, the antimicrobial efficacy of bacteriocins may be influenced by factors such as soil composition, environmental temperature, and humidity [197,198]. LAB bacteriocins are present in very low concentrations in organic agricultural soils. Currently, they exert antimicrobial effects primarily through exogenous application, such as spraying or coating, rather than through natural production in the soil [199]. Research on these environmental factors is still in its early stages. Future studies should include more field trials to evaluate the practical application of LAB bacteriocins in different agricultural contexts.
The mechanisms of LAB bacteriocins in clinical applications are complex. Compared to conventional antibiotics, the clinical potential of LAB bacteriocins is mainly limited by their narrow antimicrobial spectrum and inability to directly target bacterial infection sites. Additionally, their presumed antigenicity and potential bio-toxicity further restrict their use. In the medical field, LAB, as intestinal probiotics, contribute to gut health by preventing and treating related disorders, thus supporting the overall health of the host. Research has shown that lactic acid bacteriocins can impact gut health and immune system function. This occurs either by increasing the proportion of beneficial bacteria in the gut microbiota or by directly influencing host metabolism [200,201]. Their inhibitory effects on antibiotic-resistant strains, particularly Gram-positive bacteria, have been partially validated. However, the absorption, distribution, metabolism, and therapeutic effects of bacteriocins in the human body remain insufficiently understood. Further exploration through in vivo experiments and preclinical studies is essential to elucidate their activity and potential therapeutic value. Despite evidence from animal studies demonstrating the ability of LAB bacteriocins to inhibit resistant bacterial strains, their long-term impact on the human microbiome remains unclear. Therefore, medical applications must carefully assess their long-term effects on human health, particularly on the immune system and microbiome. To minimize potential risks, future research should focus on the safety assessment of LAB bacteriocins and conduct long-term preclinical and clinical trials.
To address these challenges, the development of efficient and cost-effective separation and purification technologies is critical. Such advancements could not only reduce production costs but also expand the application fields of bacteriocins [195]. For instance, key technological breakthroughs may be achieved through protein engineering, genetic modification, or the chemical synthesis of novel antimicrobial peptides [202,203]. These technological advancements could promote growth in the food industry and pave the way for broader applications in animal feed, organic fertilizers, environmental protection, and personal care products [204,205]. While genetic and metabolic engineering technologies can enhance the production yield of bacteriocins to some extent, their adoption remains constrained by cost pressures and regulatory limitations, particularly in food and pharmaceutical sectors where stricter safety standards are required. These challenges suggest that, beyond technological breakthroughs, supportive regulations and policies are equally essential. From a future development perspective, the sustained progress of LAB bacteriocins requires dual drivers of technological innovation and regulatory support. On the one hand, the application of modern biotechnologies, such as heterologous expression systems, intelligent bioreactors, and metabolic engineering, will significantly improve production efficiency and reduce costs. On the other hand, close collaboration with regulatory agencies through systematic safety and efficacy validation studies can provide guarantees for the marketization of bacteriocin products. Simultaneously, their application fields are expected to expand further, such as combining with biodegradable packaging materials in the food industry, developing composite biopesticides in agriculture, and exploring personalized therapeutic strategies for specific diseases in the medical field.
Future development priorities should focus on several aspects. First, enhancement of high-throughput screening technologies should be prioritized to discover new bacteriocins with higher antimicrobial activity and broader-spectrum effects. Predictive models based on computational biology and artificial intelligence can significantly accelerate this process, substantially shortening research cycles. Additionally, leveraging synthetic biology to design and optimize bacteriocin structures can further enhance their stability and functionality. For example, modifications to amino acid sequences could lead to the development of bacteriocins that are more heat-resistant, pH-stable, or adaptable to complex environments, offering solutions for specialized processing environments and complex applications.
Second, in terms of production processes, exploring the combination of continuous fermentation technologies and microbial co-culture systems could improve production efficiency and reduce resource consumption. By precisely controlling fermentation conditions and metabolic pathways, production yields could be significantly increased while reducing the accumulation of by-products, enhancing the overall sustainability of production processes. Additionally, purification techniques based on green chemistry and environmentally friendly processes, such as membrane separation and nanotechnology, could enable efficient and cost-effective bacteriocin extraction and purification.
Third, the multifunctional applications of LAB bacteriocins merit further exploration. For example, in food safety, antimicrobial packaging materials with real-time monitoring capabilities could be developed using Internet of Things (IoT) technologies to dynamically monitor and control bacterial contamination. In agriculture, integrated solutions combining bacteriocins with eco-friendly pesticides could minimize the use of chemical pesticides while improving crop yield and quality. In medicine, by integrating with precision medicine and gene therapy technologies, bacteriocins could be explored for applications in specific pathogenic infections, tumor immunotherapy, and even gut microbiota regulation.
Moreover, the role of bacteriocins in ecological balance and environmental protection also warrants attention. Through ecological engineering, bacteriocins could be used to regulate environmental microbial communities to remediate polluted soils or improve water quality. Additionally, combining bacteriocins with biodegradable materials could lead to the development of sustainable, eco-friendly products to support carbon neutrality goals.
Finally, international collaboration and policy development are crucial for driving the advancement of bacteriocins. Strengthening international technological cooperation and data sharing could accelerate the promotion of bacteriocin applications worldwide. Simultaneously, refining regulatory frameworks for bacteriocin-related products could pave the way for their commercialization on a global scale.
Through multidimensional technological innovations and cross-disciplinary resource integration, LAB bacteriocins are poised to overcome existing limitations and play a larger role in food safety, green agriculture, environmental protection, and precision medicine. Looking ahead, LAB bacteriocins are not only tools to address current challenges but also potential engines driving the bioeconomy and sustainable development.
8. Conclusions
In conclusion, although lactic acid bacteriocins have advanced as natural food preservatives, their broader application still faces several challenges. Addressing high purification costs, optimizing synthesis methods, exploring new applications, and securing regulatory approval are essential steps. With technological innovation and regulatory support, the future prospects for bacteriocins are promising, offering new opportunities across various industries.
Author Contributions
Conceptualization, H.P. and Z.L.; data curation, writing—original draft preparation, X.C.; writing—review and editing, H.B. and W.M.; visualization, Y.Y., X.Z., Q.S., H.C., Z.C. and Y.L.; project administration, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangxi Key Research and Development Plan (Grant Nos.: Guinongke AB241484045, GuiKe AB23075145); Laibin Scientific Research and Technology Development Program (Grant No.: 240108); the Guangxi Department of Science and Technology (Grant No.: GuiKe AB21238003); Guangxi Agriculture Department Science and Technology Project (grant No.: Z202214); Fangchenggang Science and Technology Programme (Grant No.: fangke AB24002021); the Qingxiu District Science and Technology Bureau (Project No.: 2022004); the Liangqing District Major Special Project (Project No.: 202118); the GuiKe Special Project (Nos.: 24-14, 24-12, 24-2, 24-10); and the Department of Science and Technology of Jilin Province (20240305059YY to Z.Z.).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Doyle, M.E. Multidrug-resistant pathogens in the food supply. Foodborne Pathog. Dis. 2015, 12, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yan, J.; Liang, Y.; Shi, Y.; He, Z.; Wu, Y.; Zeng, Q.; Liu, X.; Peng, J. Resistance Genes and their Interactions with Bacterial Blight/Leaf Streak Pathogens (Xanthomonas oryzae) in Rice (Oryza sativa L.)-an Updated Review. Rice 2020, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, K.; Krishna, S.; Majumdar, S.; Nath, U.; Nataraj, K.N.; Prakash, N.B. Co-cultivation of Beta vulgaris limits the pre-harvest colonization of foodborne pathogen (Salmonella spp.) on tomato. Int. J. Food Microbiol. 2020, 332, 108768. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.; Davies, S.C. Tackling antimicrobial resistance globally. Med. J. Aust. 2017, 207, 371–373. [Google Scholar] [CrossRef]
- Vikram, A.; Callahan, M.T.; Woolston, J.W.; Sharma, M.; Sulakvelidze, A. Phage biocontrol for reducing bacterial foodborne pathogens in produce and other foods. Curr. Opin. Biotechnol. 2022, 78, 102805. [Google Scholar] [CrossRef]
- Murugaiyan, J.; Kumar, P.A.; Rao, G.S.; Iskandar, K.; Hawser, S.; Hays, J.P.; Mohsen, Y.; Adukkadukkam, S.; Awuah, W.A.; Jose, R.A.M.; et al. Progress in Alternative Strategies to Combat Antimicrobial Resistance: Focus on Antibiotics. Antibiotics 2022, 11, 200. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Qiao, X.X.; Du, R.P.; Wang, Y.; Han, Y.; Zhou, Z.J. Purification, characterization and mode of action of enterocin, a novel bacteriocin produced by Enterococcus faecium TJUQ1. Int. J. Biol. Macromol. 2020, 144, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Venema, K.; Haverkort, R.E.; Abee, T.; Haandrikman, A.J.; Leenhouts, K.J.; de Leij, L.; Venema, G.; Kok, J. Mode of action of LciA, the lactococcin A immunity protein. Mol. Microbiol. 1994, 14, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Kasimin, M.E.; Shamsuddin, S.; Molujin, A.M.; Sabullah, M.K.; Gansau, J.A.; Jawan, R. Enterocin: Promising Biopreservative Produced by Enterococcus sp. Microorganisms 2022, 10, 684. [Google Scholar] [CrossRef]
- Todorov, S.D.; Popov, I.; Weeks, R.; Chikindas, M.L. Use of Bacteriocins and Bacteriocinogenic Beneficial Organisms in Food Products: Benefits, Challenges, Concerns. Foods 2022, 11, 3145. [Google Scholar] [CrossRef]
- Magnusson, J.; Ström, K.; Roos, S.; Sjögren, J.; Schnürer, J. Broad and complex antifungal activity among environmental isolates of lactic acid bacteria. FEMS Microbiol. Lett. 2003, 219, 129–135. [Google Scholar] [CrossRef]
- Mohanty, D.; Suar, M.; Panda, S.K. Nanotechnological interventions in bacteriocin formulations advances, and scope for challenging food spoilage bacteria and drug-resistant foodborne pathogens. Crit. Rev. Food Sci. Nutr. 2025, 65, 1126–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; An, J.J.; Su, H.X.; Li, B.; Liang, D.W.; Huang, C.X. Antimicrobial food packaging integrating polysaccharide-based substrates with green antimicrobial agents: A sustainable path. Food Res. Int. 2022, 155, 111096. [Google Scholar] [CrossRef]
- Settanni, L.; Corsetti, A. Application of bacteriocins in vegetable food biopreservation. Int. J. Food Microbiol. 2008, 121, 123–138. [Google Scholar] [CrossRef]
- Daba, G.M.; Elkhateeb, W.A. Ribosomally synthesized bacteriocins of lactic acid bacteria: Simplicity yet having wide potentials-A review. Int. J. Biol. Macromol. 2024, 256, 128325. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial production of bacteriocins: Latest research development and applications. Biotechnol. Adv. 2018, 36, 2187–2200. [Google Scholar] [CrossRef]
- Fath, M.J.; Zhang, L.H.; Rush, J.; Kolter, R. Purification and characterization of colicin V from Escherichia coli culture supernatants. Biochemistry 1994, 33, 6911–6917. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.; Morell, J.L. Nisin. The assignment of sulfide bridges of beta-methyllanthionine to a novel bicyclic structure of identical ring size. J. Am. Chem. Soc. 1970, 92, 2919–2920. [Google Scholar] [CrossRef] [PubMed]
- Mattick, A.T.; Hirsch, A. Further observations on an inhibitory substance (nisin) from Lactic streptococci. Lancet 1947, 2, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.A. The inhibiting effect of Streptococcus lactis on Lactobacillus bulgaricus. J. Bacteriol. 1928, 16, 321–325. [Google Scholar] [CrossRef]
- Babasaki, K.; Takao, T.; Shimonishi, Y.; Kurahashi, K. Subtilosin A, a new antibiotic peptide produced by Bacillus subtilis 168: Isolation, structural analysis, and biogenesis. J. Biochem. 1985, 98, 585–603. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, L.; Wang, X.; Liu, B.; Shan, Y.; Yi, Y.; Zhou, Y.; Lü, X. Antibiofilm Effects of Bacteriocin BMP32r on Listeria monocytogenes. Probiotics Antimicrob. Proteins 2022, 14, 1067–1076. [Google Scholar] [CrossRef]
- Piard, J.C.; Muriana, P.M.; Desmazeaud, M.J.; Klaenhammer, T.R. Purification and Partial Characterization of Lacticin 481, a Lanthionine-Containing Bacteriocin Produced by Lactococcus lactis subsp. lactis CNRZ 481. Appl. Environ. Microbiol. 1992, 58, 279–284. [Google Scholar] [CrossRef]
- Zipperer, A.; Konnerth, M.C.; Laux, C.; Berscheid, A.; Janek, D.; Weidenmaier, C.; Burian, M.; Schilling, N.A.; Slavetinsky, C.; Marschal, M.; et al. Human commensals producing a novel antibiotic impair pathogen colonization. Nature 2016, 535, 511–516. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Yang, G.; Tao, L. Sublancin protects against methicillin-resistant Staphylococcus aureus infection by the combined modulation of innate immune response and microbiota. Peptides 2021, 141, 170533. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Mohammadi, M.; Mortazavian, A.M.; Yousefi, M. Antibacterial Activity of Pediocin and Pediocin-Producing Bacteria Against Listeria monocytogenes in Meat Products. Front. Microbiol. 2021, 12, 709959. [Google Scholar] [CrossRef]
- Klaenhammer, T.R. Genetics of bacteriocins produced by lactic acid bacteria. FEMS Microbiol. Rev. 1993, 12, 39–85. [Google Scholar] [CrossRef] [PubMed]
- Reuben, R.C.; Torres, C. Bacteriocins: Potentials and prospects in health and agrifood systems. Arch. Microbiol. 2024, 206, 233. [Google Scholar] [CrossRef]
- Negash, A.W.; Tsehai, B.A. Current Applications of Bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.K.; Devi, P.B.; Reddy, G.B.; Jaiswal, A.K.; Kavitake, D.; Shetty, P.H. Biosynthesis, classification, properties, and applications of Weissella bacteriocins. Front. Microbiol. 2024, 15, 1406904. [Google Scholar] [CrossRef] [PubMed]
- Selle, K.; Klaenhammer, T.R. Genomic and phenotypic evidence for probiotic influences of Lactobacillus gasseri on human health. FEMS Microbiol. Rev. 2013, 37, 915–935. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Maske, B.L.; De Dea Lindner, J.; Vale, A.S.; Favero, G.R.; Viesser, J.; de Carvalho, J.C.; Góes-Neto, A.; Soccol, C.R. An updated review on bacterial community composition of traditional fermented milk products: What next-generation sequencing has revealed so far. Crit. Rev. Food Sci. Nutr. 2022, 62, 1870–1889. [Google Scholar] [CrossRef]
- Garbacz, K. Anticancer activity of lactic acid bacteria. Semin. Cancer Biol. 2022, 86, 356–366. [Google Scholar] [CrossRef]
- Harper, A.R.; Dobson, R.; Morris, V.K.; Moggré, G.J. Fermentation of plant-based dairy alternatives by lactic acid bacteria. Microb. Biotechnol. 2022, 15, 1404–1421. [Google Scholar] [CrossRef]
- Tian, L.; Hu, S.; Jia, J.; Tan, W.; Yang, L.; Zhang, Q.; Liu, X.; Duan, X. Effects of short-term fermentation with lactic acid bacteria on the characterization, rheological and emulsifying properties of egg yolk. Food Chem. 2021, 341, 128163. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; Montalbán-López, M.; Cebrián, R.; Valdivia, E.; Martínez-Bueno, M.; Maqueda, M. AS-48 bacteriocin: Close to perfection. Cell. Mol. Life Sci. 2011, 68, 2845–2857. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing innate immunity for food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Fact. 2014, 13 (Suppl. S1), S3. [Google Scholar] [CrossRef] [PubMed]
- Arnison, P.G.; Bibb, M.J.; Bierbaum, G.; Bowers, A.A.; Bugni, T.S.; Bulaj, G.; Camarero, J.A.; Campopiano, D.J.; Challis, G.L.; Clardy, J.; et al. Ribosomally synthesized and post-translationally modified peptide natural products: Overview and recommendations for a universal nomenclature. Nat. Prod. Rep. 2013, 30, 108–160. [Google Scholar] [CrossRef]
- Medema, M.H.; Kottmann, R.; Yilmaz, P.; Cummings, M.; Biggins, J.B.; Blin, K.; de Bruijn, I.; Chooi, Y.H.; Claesen, J.; Coates, R.C.; et al. Minimum Information about a Biosynthetic Gene cluster. Nat. Chem. Biol. 2015, 11, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Phelan, R.W.; Barret, M.; Cotter, P.D.; O’Connor, P.M.; Chen, R.; Morrissey, J.P.; Dobson, A.D.; O’Gara, F.; Barbosa, T.M. Subtilomycin: A new lantibiotic from Bacillus subtilis strain MMA7 isolated from the marine sponge Haliclona simulans. Mar. Drugs 2013, 11, 1878–1898. [Google Scholar] [CrossRef]
- Knerr, P.J.; van der Donk, W.A. Discovery, biosynthesis, and engineering of lantipeptides. Annu. Rev. Biochem. 2012, 81, 479–505. [Google Scholar] [CrossRef]
- Nissen-Meyer, J.; Rogne, P.; Oppegård, C.; Haugen, H.S.; Kristiansen, P.E. Structure-function relationships of the non-lanthionine-containing peptide (class II) bacteriocins produced by gram-positive bacteria. Curr. Pharm. Biotechnol. 2009, 10, 19–37. [Google Scholar] [CrossRef]
- Moll, G.N.; Konings, W.N.; Driessen, A.J. Bacteriocins: Mechanism of membrane insertion and pore formation. Antonie Leeuwenhoek 1999, 76, 185–198. [Google Scholar] [CrossRef]
- Kuniyoshi, T.M.; O’Connor, P.M.; Lawton, E.; Thapa, D.; Mesa-Pereira, B.; Abulu, S.; Hill, C.; Ross, R.P.; Oliveira, R.P.S.; Cotter, P.D. An oxidation resistant pediocin PA-1 derivative and penocin A display effective anti-Listeria activity in a model human gut environment. Gut Microbes 2022, 14, 2004071. [Google Scholar] [CrossRef]
- Cotter, P.D. An ‘Upp’-turn in bacteriocin receptor identification. Mol. Microbiol. 2014, 92, 1159–1163. [Google Scholar] [CrossRef]
- Ghosh, B.; Sukumar, G.; Ghosh, A.R. Purification and characterization of pediocin from probiotic Pediococcus pentosaceus GS4, MTCC 12683. Folia Microbiol. 2019, 64, 765–778. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.M.; O’Shea, E.F.; Cotter, P.D.; Hill, C.; Ross, R.P. The potency of the broad spectrum bacteriocin, bactofencin A, against staphylococci is highly dependent on primary structure, N-terminal charge and disulphide formation. Sci. Rep. 2018, 8, 11833. [Google Scholar]
- Vaičikauskaitė, M.; Ger, M.; Valius, M.; Maneikis, A.; Lastauskienė, E.; Kalėdienė, L.; Kaunietis, A. Geobacillin 26 high molecular weight bacteriocin from a thermophilic bacterium. Int. J. Biol. Macromol. 2019, 141, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, O.G.; Derendorf, H.; Hillman, J.D. Pharmacodynamic activity of the lantibiotic MU1140. Int. J. Antimicrob. Agents 2009, 33, 70–74. [Google Scholar] [CrossRef]
- Tresnak, D.T.; Hackel, B.J. Mining and Statistical Modeling of Natural and Variant Class IIa Bacteriocins Elucidate Activity and Selectivity Profiles across Species. Appl. Environ. Microbiol. 2020, 86, e01646-20. [Google Scholar] [CrossRef]
- Yaghoubi, A.; Ghazvini, K.; Hasanian, S.M.; Avan, A.; Soleimanpour, S.; Khazaei, M. Bacterial Peptides and Bacteriocins as a Promising Therapy for Solid Tumor. Curr. Pharm. Des. 2022, 28, 3105–3113. [Google Scholar] [CrossRef]
- Zhao, X.; Kuipers, O.P. Nisin- and Ripcin-Derived Hybrid Lanthipeptides Display Selective Antimicrobial Activity against Staphylococcus aureus. ACS Synth. Biol. 2021, 10, 1703–1714. [Google Scholar] [CrossRef]
- van Belkum, M.J.; Stiles, M.E. Nonlantibiotic antibacterial peptides from lactic acid bacteria. Nat. Prod. Rep. 2000, 17, 323–335. [Google Scholar] [CrossRef]
- Fernandes, A.; Jobby, R. Bacteriocins from lactic acid bacteria and their potential clinical applications. Appl. Biochem. Biotechnol. 2022, 194, 4377–4399. [Google Scholar] [CrossRef]
- Simons, A.; Alhanout, K.; Duval, R.E. Bacteriocins, Antimicrobial Peptides from Bacterial Origin: Overview of Their Biology and Their Impact against Multidrug-Resistant Bacteria. Microorganisms 2020, 8, 639. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ramos, A.; Madi-Moussa, D.; Coucheney, F.; Drider, D. Current Knowledge of the Mode of Action and Immunity Mechanisms of LAB-Bacteriocins. Microorganisms 2021, 9, 2107. [Google Scholar] [CrossRef] [PubMed]
- Wirawan, R.E.; Swanson, K.M.; Kleffmann, T.; Jack, R.W.; Tagg, J.R. Uberolysin: A novel cyclic bacteriocin produced by Streptococcus uberis. Microbiology 2007, 153, 1619–1630. [Google Scholar] [CrossRef]
- Miceli de Farias, F.; O’Connor, P.M.; Buttimer, C.; Kamilari, E.; Soria, M.C.; Johnson, C.N.; Deliephan, A.; Hill, D.; Fursenko, O.; Wiese, J.; et al. Raffinocyclicin is a novel plasmid-encoded circular bacteriocin produced by Lactococcus raffinolactis with broad-spectrum activity against many gram-positive food pathogens. Appl. Environ. Microbiol. 2024, 90, e0080924. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Halami, P.M.; Tamang, J.P. Novel pathways in bacteriocin synthesis by lactic acid bacteria with special reference to ethnic fermented foods. Food Sci. Biotechnol. 2022, 31, 1–16. [Google Scholar] [CrossRef]
- Vermeulen, R.; Deane, S.; Dicks, L.; Rohwer, J.; van Staden, A. Manganese Privation-Induced Transcriptional Upregulation of the Class IIa Bacteriocin Plantaricin 423 in Lactobacillus plantarum Strain 423. Appl. Environ. Microbiol. 2021, 87, e0097621. [Google Scholar] [CrossRef]
- Borrero, J.; Kelly, E.; O’Connor, P.M.; Kelleher, P.; Scully, C.; Cotter, P.D.; Mahony, J.; van Sinderen, D. Plantaricyclin A, a Novel Circular Bacteriocin Produced by Lactobacillus plantarum NI326: Purification, Characterization, and Heterologous Production. Appl. Environ. Microbiol. 2018, 84, e01801–e01817. [Google Scholar] [CrossRef]
- Martínez-Cuesta, M.C.; Buist, G.; Kok, J.; Hauge, H.H.; Nissen-Meyer, J.; Peláez, C.; Requena, T. Biological and molecular characterization of a two-peptide lantibiotic produced by Lactococcus lactis IFPL105. J. Appl. Microbiol. 2000, 89, 249–260. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Circular and Leaderless Bacteriocins: Biosynthesis, Mode of Action, Applications, and Prospects. Front. Microbiol. 2018, 9, 2085. [Google Scholar] [CrossRef]
- Straume, D.; Kjos, M.; Nes, I.F.; Diep, D.B. Quorum-sensing based bacteriocin production is down-regulated by N-terminally truncated species of gene activators. Mol. Genet. Genom. 2007, 278, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Molecules 2017, 22, 1255. [Google Scholar] [CrossRef]
- Qian, X.; Tian, P.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Quorum Sensing of Lactic Acid Bacteria: Progress and Insights. Food Rev. Int. 2023, 39, 4781–4792. [Google Scholar] [CrossRef]
- Todorov, S.D.; Wachsman, M.B.; Knoetze, H.; Meincken, M.; Dicks, L.M. An antibacterial and antiviral peptide produced by Enterococcus mundtii ST4V isolated from soya beans. Int. J. Antimicrob. Agents 2005, 25, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Draper, L.A.; Cotter, P.D.; Hill, C.; Ross, R.P. Lantibiotic resistance. Microbiol. Mol. Biol. Rev. 2015, 79, 171–191. [Google Scholar] [CrossRef]
- Zeng, L.P.; Fan, A.P.; Yang, G.M.; Nong, Y.P.; Lu, Y.F.; Yang, R.P. Nisin and ε-polylysine combined treatment enhances quality of fresh-cut jackfruit at refrigerated storage. Front. Nutr. 2024, 11, 1299810. [Google Scholar] [CrossRef]
- Wu, J.J.; Zang, M.W.; Wang, S.W.; Zhao, B.; Bai, J.; Xu, C.C.; Shi, Y.X.; Qiao, X.L. Nisin: From a structural and meat preservation perspective. Food Microbiol. 2023, 111, 104207. [Google Scholar] [CrossRef]
- McAuliffe, O.; Hill, C.; Ross, R.P. Each peptide of the two-component lantibiotic lacticin 3147 requires a separate modification enzyme for activity. Microbiology 2000, 146 Pt 9, 2147–2154. [Google Scholar] [CrossRef][Green Version]
- Masuda, Y.; Zendo, T.; Sonomoto, K. New type non-lantibiotic bacteriocins: Circular and leaderless bacteriocins. Benef. Microbes 2012, 3, 3–12. [Google Scholar] [CrossRef]
- Antoshina, D.V.; Balandin, S.V.; Ovchinnikova, T.V. Structural Features, Mechanisms of Action, and Prospects for Practical Application of Class II Bacteriocins. Biochemistry 2022, 87, 1387–1403. [Google Scholar] [CrossRef]
- Diep, D.B.; Skaugen, M.; Salehian, Z.; Holo, H.; Nes, I.F. Common mechanisms of target cell recognition and immunity for class II bacteriocins. Proc. Natl. Acad. Sci. USA 2007, 104, 2384–2389. [Google Scholar] [CrossRef] [PubMed]
- Gabrielsen, C.; Brede, D.A.; Nes, I.F.; Diep, D.B. Circular bacteriocins: Biosynthesis and mode of action. Appl. Environ. Microbiol. 2014, 80, 6854–6862. [Google Scholar] [CrossRef] [PubMed]
- Kavita; Om, H.; Chand, U.; Kushawaha, P.K. Postbiotics: An alternative and innovative intervention for the therapy of inflammatory bowel disease. Microbiol. Res. 2024, 279, 127550. [Google Scholar]
- Peng, Z.; Xiong, T.; Huang, T.; Xu, X.Y.; Fan, P.R.; Qiao, B.L.; Xie, M.Y. Factors affecting production and effectiveness, performance improvement and mechanisms of action of bacteriocins as food preservative. Crit. Rev. Food Sci. Nutr. 2023, 63, 12294–12307. [Google Scholar] [CrossRef]
- Xin, W.G.; Wu, G.; Ying, J.P.; Xiang, Y.Z.; Jiang, Y.H.; Deng, X.Y.; Lin, L.B.; Zhang, Q.L. Antibacterial activity and mechanism of action of bacteriocin LFX01 against Staphylococcus aureus and Escherichia coli and its application on pork model. Meat Sci. 2023, 196, 109045. [Google Scholar] [CrossRef]
- Wiedemann, I.; Böttiger, T.; Bonelli, R.R.; Wiese, A.; Hagge, S.O.; Gutsmann, T.; Seydel, U.; Deegan, L.; Hill, C.; Ross, P.; et al. The mode of action of the lantibiotic lacticin 3147--a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 2006, 61, 285–296. [Google Scholar] [CrossRef]
- Lu, Y.Y.; Yan, H.; Li, X.; Gu, Y.X.; Wang, X.; Yi, Y.L.; Shan, Y.Y.; Liu, B.F.; Zhou, Y.; Lü, X. Physicochemical properties and mode of action of a novel bacteriocin BM1122 with broad antibacterial spectrum produced by Lactobacillus crustorum MN047. J. Food Sci. 2020, 85, 1523–1535. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Jiang, Y.H.; Li, H.W.; Li, X.Z.; Zhang, Q.L. Purification and characterization of Lactobacillus plantarum-derived bacteriocin with activity against Staphylococcus argenteus planktonic cells and biofilm. J. Food Sci. 2022, 87, 2718–2731. [Google Scholar] [CrossRef]
- Zhou, H.; Fang, J.; Tian, Y.; Lu, X.Y. Mechanisms of nisin resistance in Gram-positive bacteria. Ann. Microbiol. 2014, 64, 413–420. [Google Scholar] [CrossRef]
- Yang, Q.E.; Ma, X.; Li, M.; Zhao, M.; Zeng, L.; He, M.; Deng, H.; Liao, H.; Rensing, C.; Friman, V.P.; et al. Evolution of triclosan resistance modulates bacterial permissiveness to multidrug resistance plasmids and phages. Nat. Commun. 2024, 15, 3654. [Google Scholar] [CrossRef]
- Gu, X.Y.; Zhao, J.C.; Zhang, R.L.; Yu, R.H.; Guo, T.T.; Kong, J. Molecular Analysis of Glutamate Decarboxylases in Enterococcus avium. Front. Microbiol. 2021, 12, 691968. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shang, L.; Yang, G.; Dai, Z.; Zeng, X.; Qiao, S. Biosynthetic Microcin J25 Exerts Strong Antibacterial, Anti-Inflammatory Activities, Low Cytotoxicity Without Increasing Drug-Resistance to Bacteria Target. Front. Immunol. 2022, 13, 811378. [Google Scholar] [CrossRef] [PubMed]
- Severinov, K.; Nair, S.K. Microcin C: Biosynthesis and mechanisms of bacterial resistance. Future Microbiol. 2012, 7, 281–289. [Google Scholar] [CrossRef]
- Yorgey, P.; Lee, J.; Kördel, J.; Vivas, E.; Warner, P.; Jebaratnam, D.; Kolter, R. Posttranslational modifications in microcin B17 define an additional class of DNA gyrase inhibitor. Proc. Natl. Acad. Sci. USA 1994, 91, 4519–4523. [Google Scholar] [CrossRef] [PubMed]
- Panina, I.; Krylov, N.; Nolde, D.; Efremov, R.; Chugunov, A. Environmental and dynamic effects explain how nisin captures membrane-bound lipid II. Sci. Rep. 2020, 10, 8821. [Google Scholar] [CrossRef]
- Cui, Y.L.; Luo, L.L.; Wang, X.; Lu, Y.Y.; Yi, Y.; Shan, Y.L.; Liu, B.F.; Zhou, Y.; Lü, X. Mining, heterologous expression, purification, antibactericidal mechanism, and application of bacteriocins: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 863–899. [Google Scholar] [CrossRef]
- Medeiros-Silva, J.; Jekhmane, S.; Paioni, A.L.; Gawarecka, K.; Baldus, M.; Swiezewska, E.; Breukink, E.; Weingarth, M. High-resolution NMR studies of antibiotics in cellular membranes. Nat. Commun. 2018, 9, 3963. [Google Scholar] [CrossRef]
- Hussain, W.; Yang, X.; Ullah, M.; Wang, H.; Aziz, A.; Xu, F.; Asif, M.; Ullah, M.W.; Wang, S. Genetic engineering of bacteriophages: Key concepts, strategies, and applications. Biotechnol. Adv. 2023, 64, 108116. [Google Scholar] [CrossRef]
- Garsa, A.K.; Kumariya, R.; Sood, S.K.; Kumar, A.; Kapila, S. Bacteriocin production and different strategies for their recovery and purification. Probiotics Antimicrob. Proteins 2014, 6, 47–58. [Google Scholar] [CrossRef]
- Burgess, R.R. Protein precipitation techniques. Methods Enzymol. 2009, 463, 331–342. [Google Scholar]
- Remedios, M.; Moreno, F.; Callewaert, R.; De Vuyst, L. Isolation of bacteriocins through expanded bed adsorption using a hydrophobic interaction medium. Bioseparation 2001, 10, 45–50. [Google Scholar]
- Bizani, D.; Dominguez, A.P.; Brandelli, A. Purification and partial chemical characterization of the antimicrobial peptide cerein 8A. Lett. Appl. Microbiol. 2005, 41, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Callewaert, R.; De Vuyst, L. Expanded bed adsorption as a unique unit operation for the isolation of bacteriocins from fermentation media. Bioseparation 1999, 8, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Fricourt, B.V.; Barefoot, S.F.; Testin, R.F.; Hayasaka, S.S. Detection and Activity of Plantaricin F an Antibacterial Substance from Lactobacillus plantarum BF001 Isolated from Processed Channel Catfish. J. Food Prot. 1994, 57, 698–702. [Google Scholar] [CrossRef]
- Zimina, M.; Babich, O.; Prosekov, A.; Sukhikh, S.; Ivanova, S.; Shevchenko, M.; Noskova, S. Overview of Global Trends in Classification, Methods of Preparation and Application of Bacteriocins. Antibiotics 2020, 28, 553. [Google Scholar] [CrossRef]
- Zamfir, M.; Callewaert, R.; Cornea, P.C.; Savu, L.; Vatafu, I.; De Vuyst, L. Purification and characterization of a bacteriocin produced by Lactobacillus acidophilus IBB 801. J. Appl. Microbiol. 1999, 87, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Alam, S.; Sabir, M.; Saba, N.; Din, A.U.; Ahmad, R.; Khan, M.R.; Muhammad, A.; Dayisoylu, K.S. Isolation, characterization, bacteriocin production and biological potential of Bifidobacteria of ruminants. Anal. Biochem. 2022, 658, 114926. [Google Scholar] [CrossRef]
- Furmanek, B.; Kaczorowski, T.; Bugalski, R.; Bielawski, K.; Bohdanowicz, J.; Podhajska, A.J. Identification, characterization and purification of the lantibiotic staphylococcin T, a natural gallidermin variant. J. Appl. Microbiol. 1999, 87, 856–866. [Google Scholar] [CrossRef]
- Ansari, A.; Zohra, R.R.; Tarar, O.M.; Qader, S.; Aman, A. Screening, purification and characterization of thermostable, protease resistant Bacteriocin active against methicillin resistant Staphylococcus aureus (MRSA). BMC Microbiol. 2018, 18, 192. [Google Scholar] [CrossRef]
- Duraisamy, S.; Sathyan, A.; Balakrishnan, S.; Subramani, P.; Prahalathan, C.; Kumarasamy, A. Bactericidal and non-cytotoxic activity of bacteriocin produced by Lacticaseibacillus paracasei F9-02 and evaluation of its tolerance to various physico-chemical conditions. Environ. Microbiol. 2023, 25, 2882–2896. [Google Scholar] [CrossRef]
- Azevedo, A.C.; Bento, C.B.; Ruiz, J.C.; Queiroz, M.V.; Mantovani, H.C. Distribution and Genetic Diversity of Bacteriocin Gene Clusters in Rumen Microbial Genomes. Appl. Environ. Microbiol. 2015, 81, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Zhi, Z.J.; Duan, M.X.; Sun, J.H.; Jiang, H.X.; Pang, J. Insights into the formation of carboxymethyl chitosan-nisin nanogels for sustainable antibacterial activity. Food Chem. 2023, 402, 134260. [Google Scholar] [CrossRef] [PubMed]
- Zendo, T.; Nakayama, J.; Fujita, K.; Sonomoto, K. Bacteriocin detection by liquid chromatography/mass spectrometry for rapid identification. J. Appl. Microbiol. 2008, 104, 499–507. [Google Scholar] [CrossRef]
- Sommer, B.; Friehs, K.; Flaschel, E. Efficient production of extracellular proteins with Escherichia coli by means of optimized coexpression of bacteriocin release proteins. J. Biotechnol. 2010, 145, 350–358. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Borrero, J.; Diep, D.B.; Gútiez, L.; Nes, I.F.; Herranz, C.; Cintas, L.M.; Hernández, P.E. Cloning, production, and functional expression of the bacteriocin sakacin A (SakA) and two SakA-derived chimeras in lactic acid bacteria (LAB) and the yeasts Pichia pastoris and Kluyveromyces lactis. J. Ind. Microbiol. Biotechnol. 2013, 40, 977–993. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Cheng, Q.; Guo, H.N.; Zhang, R.J.; Si, D.Y. Expression of Hybrid Peptide EF-1 in Pichia pastoris, Its Purification, and Antimicrobial Characterization. Molecules 2020, 25, 5538. [Google Scholar] [CrossRef]
- Hayward, D.G.; Newbatt, Y.; Pickard, L.; Byrne, E.; Mao, G.; Burns, S.; Sahota, N.K.; Workman, P.; Collins, I.; Aherne, W.; et al. Identification by high-throughput screening of viridin analogs as biochemical and cell-based inhibitors of the cell cycle-regulated nek2 kinase. J. Biomol. Screen. 2010, 15, 918–927. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Diep, D.B.; Borrero, J.; Gútiez, L.; Arbulu, S.; Nes, I.F.; Herranz, C.; Cintas, L.M.; Hernández, P.E. Cloning strategies for heterologous expression of the bacteriocin enterocin A by Lactobacillus sakei Lb790, Lb. plantarum NC8 and Lb. casei CECT475. Microb. Cell Fact. 2015, 14, 166. [Google Scholar] [CrossRef]
- Bisset, S.W.; Yang, S.H.; Amso, Z.; Harris, P.W.R.; Patchett, M.L.; Brimble, M.A.; Norris, G.E. Using Chemical Synthesis to Probe Structure-Activity Relationships of the Glycoactive Bacteriocin Glycocin F. ACS Chem. Biol. 2018, 13, 1270–1278. [Google Scholar] [CrossRef]
- Dhingra, H.; Kaur, K.; Singh, B. Engineering and characterization of human β-defensin-3 and its analogues and microcin J25 peptides against Mannheimia haemolytica and bovine neutrophils. Vet. Res. 2021, 52, 83. [Google Scholar] [CrossRef]
- Jawan, R.; Abbasiliasi, S.; Tan, J.S.; Mustafa, S.; Halim, M.; Ariff, A.B. Influence of Culture Conditions and Medium Compositions on the Production of Bacteriocin-Like Inhibitory Substances by Lactococcus lactis Gh1. Microorganisms 2020, 8, 1454. [Google Scholar] [CrossRef]
- Dündar, H.; Atakay, M.; Çelikbıçak, Ö.; Salih, B.; Bozoğlu, F. Comparison of two methods for purification of enterocin B, a bacteriocin produced by Enterococcus faecium W3. Prep. Biochem. Biotechnol. 2015, 45, 796–809. [Google Scholar] [CrossRef]
- Anastasiadou, S.; Papagianni, M.; Ambrosiadis, I.; Koidis, P. Rapid quantifiable assessment of nutritional parameters influencing pediocin production by Pediococcus acidilactici NRRL B5627. Bioresour. Technol. 2008, 99, 6646–6650. [Google Scholar] [CrossRef]
- Wong, F.; Ariff, A.B.; Abbasiliasi, S.; Stuckey, D.C. Recovery of a bacteriocin-like inhibitory substance from Pediococcus acidilactici Kp10 using surfactant precipitation. Food Chem. 2017, 232, 245–252. [Google Scholar] [CrossRef]
- Foudjing, G.G.D.; Sarmast, E.; Allahdad, Z.; Salmieri, S.; Lacroix, M. Influence of growth parameters on bacteriocin-like inhibitory substances (BLIS) production by lactic acid bacteria. Lett. Appl. Microbiol. 2023, 76, ovac013. [Google Scholar] [CrossRef]
- Qiao, W.J.; Qiao, Y.; Gao, G.; Liao, Z.T.; Wu, Z.Z.; Saris, P.E.J.; Xu, H.J.; Qiao, M.Q. A novel co-cultivation strategy to generate low-crystallinity bacterial cellulose and increase nisin yields. Int. J. Biol. Macromol. 2022, 202, 388–396. [Google Scholar] [CrossRef]
- Castro, M.P.; Palavecino, N.Z.; Herman, C.; Garro, O.A.; Campos, C.A. Lactic acid bacteria isolated from artisanal dry sausages: Characterization of antibacterial compounds and study of the factors affecting bacteriocin production. Meat Sci. 2011, 87, 321–329. [Google Scholar] [CrossRef]
- Abedin, M.M.; Chourasia, R.; Phukon, L.C.; Sarkar, P.; Ray, R.C.; Singh, S.P.; Rai, A.K. Lactic acid bacteria in the functional food industry: Biotechnological properties and potential applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 10730–10748. [Google Scholar] [CrossRef]
- Hossain, T.J. Functional genomics of the lactic acid bacterium Limosilactobacillus fermentum LAB-1: Metabolic, probiotic and biotechnological perspectives. Heliyon 2022, 8, e11412. [Google Scholar] [CrossRef]
- Pahalagedara, A.; Flint, S.; Palmer, J.; Brightwell, G.; Gupta, T.B. Antimicrobial production by strictly anaerobic Clostridium spp. Int. J. Antimicrob. Agents 2020, 55, 105910. [Google Scholar] [CrossRef]
- Erol, I.; Kotil, S.E.; Fidan, O.; Yetiman, A.E.; Durdagi, S.; Ortakci, F. In Silico Analysis of Bacteriocins from Lactic Acid Bacteria Against SARS-CoV-2. Probiotics Antimicrob. Proteins 2023, 15, 17–29. [Google Scholar] [CrossRef]
- Patel, M.; West, S. Microbial warfare and the evolution of symbiosis. Biol. Lett. 2022, 18, 20220447. [Google Scholar] [CrossRef]
- Fristot, E.; Cambray, G.; Bonnet, J. LactoSpanks: A Collection of IPTG Inducible Promoters for the Commensal Lactic Acid Bacteria Lactobacillus gasseri. ACS Synth. Biol. 2024, 13, 951–957. [Google Scholar] [CrossRef]
- Putri, D.A.; Lei, J.; Rossiana, N.; Syaputri, Y. Biopreservation of Food Using Bacteriocins From Lactic Acid Bacteria: Classification, Mechanisms, and Commercial Applications. Int. J. Microbiol. 2024, 2024, 8723968. [Google Scholar] [CrossRef]
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, natural antimicrobials for food preservation. Int. J. Food Microbiol. 2001, 71, 1–20. [Google Scholar] [CrossRef]
- Vaucher, R.A.; Teixeira, M.L.; Brandelli, A. Investigation of the cytotoxicity of antimicrobial peptide P40 on eukaryotic cells. Curr. Microbiol. 2010, 60, 1–5. [Google Scholar] [CrossRef]
- Barbosa, A.; de Melo, M.R.; da Silva, C.; Jain, S.; Dolabella, S.S. Nisin resistance in Gram-positive bacteria and approaches to circumvent resistance for successful therapeutic use. Crit. Rev. Microbiol. 2021, 47, 376–385. [Google Scholar] [CrossRef]
- Oladunjoye, A.O.; Singh, S.; Ijabadeniyi, O.A. Inactivation of Listeria monocytogenes ATCC 7644 on fresh-cut tomato using nisin in combinations with organic salts. Braz. J. Microbiol. 2016, 47, 757–763. [Google Scholar] [CrossRef]
- Lee, N.K.; Kim, H.W.; Lee, J.Y.; Ahn, D.U.; Kim, C.J.; Paik, H.D. Antimicrobial Effect of Nisin against Bacillus cereus in Beef Jerky during Storage. Korean J. Food Sci. Anim. Resour. 2015, 35, 272–276. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a Food Preservative: Part 1: Physicochemical Properties, Antimicrobial Activity, and Main Uses. Crit. Rev. Food Sci. Nutr. 2016, 56, 1262–1274. [Google Scholar] [CrossRef]
- Mattila, K.; Saris, P.; Työppönen, S. Survival of Listeria monocytogenes on sliced cooked sausage after treatment with pediocin AcH. Int. J. Food Microbiol. 2003, 89, 281–286. [Google Scholar] [CrossRef]
- Rasch, M.; Knøchel, S. Variations in tolerance of to nisin, pediocin PA-1 and bavaricin, A. Lett. Appl. Microbiol. 1998, 27, 275–278. [Google Scholar] [CrossRef]
- Scannell, A.G.; Hill, C.; Ross, R.P.; Marx, S.; Hartmeier, W.; Elke; Arendt, K. Development of bioactive food packaging materials using immobilised bacteriocins lacticin 3147 and nisaplin. Int. J. Food Microbiol. 2000, 60, 241–249. [Google Scholar] [CrossRef]
- Petrova, P.; Ivanov, I.; Tsigoriyna, L.; Valcheva, N.; Vasileva, E.; Parvanova-Mancheva, T.; Arsov, A.; Petrov, K. Traditional Bulgarian Dairy Products: Ethnic Foods with Health Benefits. Microorganisms 2021, 9, 480. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, C.; Guo, Q.; Zhang, J.; Ruiz-Menjivar, J. The impact of agricultural chemical inputs on environment: Global evidence from informetrics analysis and visualization. Int. J. Low-Carbon Technol. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Gerez, C.L.; Carbajo, M.S.; Rollán, G.; Torres Leal, G.; Font de Valdez, G. Inhibition of citrus fungal pathogens by using lactic acid bacteria. J. Food Sci. 2010, 75, M354–M359. [Google Scholar] [CrossRef]
- Gatesoupe, F.J. Updating the importance of lactic acid bacteria in fish farming: Natural occurrence and probiotic treatments. J. Mol. Microbiol. Biotechnol. 2008, 14, 107–114. [Google Scholar] [CrossRef]
- Wyszyńska, A.K.; Godlewska, R. Lactic Acid Bacteria—A Promising Tool for Controlling Chicken Campylobacter Infection. Front. Microbiol. 2021, 12, 703441. [Google Scholar] [CrossRef]
- van Zyl, W.F.; Deane, S.M.; Dicks, L. Bacteriocin production and adhesion properties as mechanisms for the anti-listerial activity of Lactobacillus plantarum 423 and Enterococcus mundtii ST4SA. Benef. Microbes 2019, 10, 329–349. [Google Scholar] [CrossRef]
- Millette, M.; Dupont, C.; Archambault, D.; Lacroix, M. Partial characterization of bacteriocins produced by human Lactococcus lactis and Pediococccus acidilactici isolates. J. Appl. Microbiol. 2007, 102, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Shehata, A.A.; Schrödl, W.; Rodloff, A. Glyphosate suppresses the antagonistic effect of Enterococcus spp. on Clostridium botulinum. Anaerobe 2013, 20, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Petkova, M.; Gotcheva, V.; Dimova, M.; Bartkiene, E.; Rocha, J.M.; Angelov, A. Screening of Lactiplantibacillus plantarum Strains from Sourdoughs for Biosuppression of Pseudomonas syringae pv. syringae and Botrytis cinerea in Table Grapes. Microorganisms 2022, 10, 2094. [Google Scholar] [CrossRef]
- Wang, H.Y.; Shankar, V.; Jiang, X.P. Compositional and Functional Changes in Microbial Communities of Composts Due to the Composting-Related Factors and the Presence of Listeria monocytogenes. Microbiol. Spectr. 2022, 10, e0184521. [Google Scholar] [CrossRef]
- Zhou, L.; Song, C.X.; Li, Z.B.; Kuipers, O.P. Antimicrobial activity screening of rhizosphere soil bacteria from tomato and genome-based analysis of their antimicrobial biosynthetic potential. BMC Genom. 2021, 22, 29. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef]
- Kuipers, O.P.; Bierbaum, G.; Ottenwälder, B.; Dodd, H.M.; Horn, N.; Metzger, J.; Kupke, T.; Gnau, V.; Bongers, R.; van den Bogaard, P.; et al. Protein engineering of lantibiotics. Antonie Leeuwenhoek 1996, 69, 161–169. [Google Scholar] [CrossRef]
- McCaughey, L.C.; Josts, I.; Grinter, R.; White, P.; Byron, O.; Tucker, N.P.; Matthews, J.M.; Kleanthous, C.; Whitchurch, C.B.; Walker, D. Discovery, characterization and in vivo activity of pyocin SD2, a protein antibiotic from Pseudomonas aeruginosa. Biochem. J. 2016, 473, 2345–2358. [Google Scholar] [CrossRef]
- Mei, M.; Thomas, J.; Diggle, S.P. Heterogenous Susceptibility to R-Pyocins in Populations of Pseudomonas aeruginosa Sourced from Cystic Fibrosis Lungs. mBio 2021, 12, e00458-21. [Google Scholar] [CrossRef]
- Booth, S.C.; Smith, W.; Foster, K.R. The evolution of short- and long-range weapons for bacterial competition. Nat. Ecol. Evol. 2023, 7, 2080–2091. [Google Scholar] [CrossRef]
- Wang, C.; Le, M.N.-T.; Kawada-Matsuo, M.; Hisatsune, J.; Sugawara, Y.; Arai, C.; Nakanishi, J.; Takeda, K.; Shiba, H.; Sugai, M.; et al. Ursoricin, a bacteriocin of Streptococcus ursoris, has potent activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. Appl. Environ. Microbiol. 2024, 90, e0016224. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Kashif Nawaz, S.; Hasnain, S. Bacteriocins produced by, L. fermentum and, L. acidophilus can inhibit cephalosporin resistant E. coli. Braz. J. Microbiol. 2010, 41, 643–648. [Google Scholar] [PubMed]
- Todorov, S.D.; Dicks, L.M.T. Lactobacillus plantarum isolated from molasses produces bacteriocins active against Gram-negative bacteria. Enzym. Microb. Technol. 2005, 36, 318–326. [Google Scholar] [CrossRef]
- Al-Mathkhury, H.J.; Ali, A.S.; Ghafil, J.A. Antagonistic effect of bacteriocin against urinary catheter associated Pseudomonas aeruginosa biofilm. N. Am. J. Med. Sci. 2011, 3, 367–370. [Google Scholar] [CrossRef]
- Lü, X.; Yi, L.; Dang, J.; Dang, Y.; Liu, B. Purification of novel bacteriocin produced by Lactobacillus coryniformis MXJ 32 for inhibiting bacterial foodborne pathogens including antibiotic-resistant microorganisms. Food Control 2014, 46, 264–271. [Google Scholar] [CrossRef]
- Okuda, K.; Zendo, T.; Sugimoto, S.; Iwase, T.; Tajima, A.; Yamada, S.; Sonomoto, K.; Mizunoe, Y. Effects of bacteriocins on methicillin-resistant Staphylococcus aureus biofilm. Antimicrob. Agents Chemother. 2013, 57, 5572–5579. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, H.; Zhang, C.; Lu, F.; Bie, X.; Lu, Z. Expression of a novel bacteriocin-the plantaricin Pln1-in Escherichia coli and its functional analysis. Protein Expr. Purif. 2016, 119, 85–93. [Google Scholar] [CrossRef]
- Phumisantiphong, U.; Siripanichgon, K.; Reamtong, O.; Diraphat, P. A novel bacteriocin from Enterococcus faecalis 478 exhibits a potent activity against vancomycin-resistant enterococci. PLoS ONE 2017, 12, e0186415. [Google Scholar] [CrossRef]
- Hill, D.; O’Connor, P.M.; Altermann, E.; Day, L.; Hill, C.; Stanton, C.; Ross, R.P. Extensive bacteriocin gene shuffling in the Streptococcus bovis/Streptococcus equinus complex reveals gallocin D with activity against vancomycin resistant enterococci. Sci. Rep. 2020, 10, 13431. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.M.; Tian, F.W.; Zhao, J.X.; Zhang, H.; Zhai, Q.X.; Chen, W. Surface components and metabolites of probiotics for regulation of intestinal epithelial barrier. Microb. Cell Fact. 2020, 19, 23. [Google Scholar] [CrossRef]
- Birk, S.E.; Mosgaard, M.D.; Kjeldsen, R.B.; Boisen, A.; Meyer, R.L.; Nielsen, L.H. Management of oral biofilms by nisin delivery in adhesive microdevices. Eur. J. Pharm. Biopharm. 2021, 167, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.H.; Fugaban, J.; Dioso, C.M.; Bucheli, J.; Holzapfel, W.H.; Todorov, S.D. Safety and Beneficial Properties of Bacteriocinogenic Lactococcus lactis and Pediococcus pentosaceus Strains, and Their Effect Versus Oral Cavity Related and Antibiotic-Resistant Pathogens. Probiotics Antimicrob. Proteins 2024, 16, 1–15. [Google Scholar] [CrossRef]
- Kaur, B.; Balgir, P.P.; Mittu, B.; Kumar, B.; Garg, N. Biomedical applications of fermenticin HV6b isolated from Lactobacillus fermentum HV6b MTCC10770. BioMed Res. Int. 2013, 2013, 168438. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Qin, X.J.; Yang, Z.Y.; Luo, J.M.; Zhang, J.P.; Gray, B.; Pak, K.Y.; Xu, X.P.; Cheng, J.Y.; et al. Early prediction of tumor response after radiotherapy in combination with cetuximab in nasopharyngeal carcinoma using (99m) Tc-duramycin imaging. Biomed. Pharmacother. 2020, 125, 109947. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Feng, X.L.; Mai, B.J.; Li, X.; Wang, F.; Liu, J.X.; Liu, X.; Zhang, K.; Wang, X.B. Bacterial-based cancer therapy: An emerging toolbox for targeted drug/gene delivery. Biomaterials 2021, 277, 121124. [Google Scholar] [CrossRef] [PubMed]
- Kamarajan, P.; Ateia, I.; Shin, J.M.; Fenno, J.C.; Le, C.; Zhan, L.; Chang, A.; Darveau, R.; Kapila, Y.L. Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of Integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PLoS Pathog. 2020, 16, e1008881. [Google Scholar] [CrossRef]
- Mehta, J.P.; Ayakar, S.; Singhal, R.S. The potential of paraprobiotics and postbiotics to modulate the immune system: A Review. Microbiol. Res. 2023, 275, 127449. [Google Scholar] [CrossRef]
- Naidu, A.S.; Bidlack, W.R.; Clemens, R.A. Probiotic spectra of lactic acid bacteria (LAB). Crit. Rev. Food Sci. Nutr. 1999, 39, 13–126. [Google Scholar] [CrossRef]
- Dolgushina, V.F. The immunocorrective properties of bacterial preparations (lactobacterin, bifidumbacterin) and bemitil in pregnant women with a urogenital infection. Zh. Mikrobiol. Epidemiol. Immunobiol. 1991, 4, 56–58. [Google Scholar]
- Esber, H.J.; Ganfield, D.; Rosenkrantz, H. Staphage lysate: An immunomodulator of the primary immune response in mice. Immunopharmacology 1985, 10, 77–82. [Google Scholar] [CrossRef]
- Joerger, R.D. Salmonella enterica’s “Choice”: Itaconic Acid Degradation or Bacteriocin Immunity Genes. Genes 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Ragavan, M.L.; Hemalatha, S. The functional roles of short chain fatty acids as postbiotics in human gut: Future perspectives. Food Sci. Biotechnol. 2024, 33, 275–285. [Google Scholar] [CrossRef]
- Mathur, H.; Field, D.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocin-Antimicrobial Synergy: A Medical and Food Perspective. Front. Microbiol. 2017, 8, 1205. [Google Scholar] [CrossRef]
- Todorov, S.D.; Wachsman, M.; Tomé, E.; Dousset, X.; Destro, M.T.; Dicks, L.M.; Franco, B.D.; Vaz-Velho, M.; Drider, D. Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol. 2010, 27, 869–879. [Google Scholar] [CrossRef] [PubMed]
- Wachsman, M.B.; Castilla, V.; de Ruiz Holgado, A.P.; de Torres, R.A.; Sesma, F.; Coto, C.E. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antivir. Res. 2003, 58, 17–24. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Hsieh, Y.J.; Liao, K.W.; Peng, K.C. Preferential promotion of apoptosis of monocytes by Lactobacillus casei rhamnosus soluble factors. Clin. Nutr. 2010, 29, 131–140. [Google Scholar] [CrossRef] [PubMed]
- de Moreno de LeBlanc, A.; Matar, C.; LeBlanc, N.; Perdigón, G. Effects of milk fermented by Lactobacillus helveticus R389 on a murine breast cancer model. Breast Cancer Res. 2005, 7, R477–R486. [Google Scholar] [CrossRef]
- Meade, E.; Slattery, M.A.; Garvey, M. Bacteriocins, Potent Antimicrobial Peptides and the Fight against Multi Drug Resistant Species: Resistance Is Futile. Antibiotics 2020, 9, 32. [Google Scholar] [CrossRef]
- Hernández-González, J.C.; Martínez-Tapia, A.; Lazcano-Hernández, G.; García-Pérez, B.E.; Castrejón-Jiménez, N.S. Bacteriocins from Lactic Acid Bacteria. A Powerful Alternative as Antimicrobials, Probiotics, and Immunomodulators in Veterinary Medicine. Animals 2021, 11, 979. [Google Scholar] [CrossRef]
- Martinenghi, L.D.; Leisner, J.J. Scientists’ Assessments of Research on Lactic Acid Bacterial Bacteriocins 1990–2010. Front. Microbiol. 2022, 13, 908336. [Google Scholar] [CrossRef]
- Yaacob, S.N.; Wahab, R.A.; Misson, M.; Sabullah, M.K.; Huyop, F.; Zin, N.M. Lactic acid bacteria and their bacteriocins: New potential weapons in the fight against methicillin-resistant Staphylococcus aureus. Future Microbiol. 2022, 17, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.M.; Paiva, A.D.; Cruz, A.M.; Vanetti, M.C.; Ferreira, S.O.; Mantovani, H.C. Bovicin HC5 and nisin reduce cell viability and the thermal resistance of Alicyclobacillus acidoterrestris endospores in fruit juices. J. Sci. Food Agric. 2022, 102, 3994–4002. [Google Scholar] [CrossRef]
- Barbosa, A.; Mantovani, H.C.; Jain, S. Bacteriocins from lactic acid bacteria and their potential in the preservation of fruit products. Crit. Rev. Biotechnol. 2017, 37, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Diep, D.B.; Håvarstein, L.S.; Nes, I.F. A bacteriocin-like peptide induces bacteriocin synthesis in Lactobacillus plantarum C11. Mol. Microbiol. 1995, 18, 631–639. [Google Scholar] [CrossRef]
- Schiefelbein, K.; Lang, J.; Schuster, M.; Grigglestone, C.E.; Striga, R.; Bigler, L.; Schuman, M.C.; Zerbe, O.; Li, Y.; Hartrampf, N. Merging Flow Synthesis and Enzymatic Maturation to Expand the Chemical Space of Lasso Peptides. J. Am. Chem. Soc. 2024, 146, 17261–17269. [Google Scholar] [CrossRef]
- Mukherjee, S.; van der Donk, W.A. Mechanistic studies on the substrate-tolerant lanthipeptide synthetase ProcM. J. Am. Chem. Soc. 2014, 136, 10450–10459. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Yanagida, F.; Shinohara, T. Isolation and identification of lactic acid bacteria from soil using an enrichment procedure. Lett. Appl. Microbiol. 2005, 40, 195–200. [Google Scholar] [CrossRef]
- Fhoula, I.; Najjari, A.; Turki, Y.; Jaballah, S.; Boudabous, A.; Ouzari, H. Diversity and antimicrobial properties of lactic acid bacteria isolated from rhizosphere of olive trees and desert truffles of Tunisia. BioMed Res. Int. 2013, 2013, 405708. [Google Scholar] [CrossRef]
- Duar, R.M.; Lin, X.B.; Zheng, J.; Martino, M.E.; Grenier, T.; Pérez-Muñoz, M.E.; Leulier, F.; Gänzle, M.; Walter, J. Lifestyles in transition: Evolution and natural history of the genus Lactobacillus. FEMS Microbiol. Rev. 2017, 41, S27–S48. [Google Scholar] [CrossRef]
- Walsh, M.C.; Gardiner, G.E.; Hart, O.M.; Lawlor, P.G.; Daly, M.; Lynch, B.; Richert, B.T.; Radcliffe, S.; Giblin, L.; Hill, C.; et al. Predominance of a Bacteriocin-Producing Lactobacillus Salivarius Component of a Five-Strain Probiotic in the Porcine Ileum and Effects on Host Immune Phenotype. FEMS Microbiol. Ecol. 2008, 64, 317–327. [Google Scholar] [CrossRef]
- Sturme, M.H.J.; Kleerebezem, M.; Nakayama, J.; Akkermans, A.D.L.; Vaugha, E.E.; de Vos, W.M. Cell to Cell Communication by Autoinducing Peptides in Gram-Positive Bacteria. Antonie Leeuwenhoek 2002, 81, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Chen, Y.Y.; Song, Z.Y.; Tan, Z.Z.; Cheng, J.J. Recent advances in design of antimicrobial peptides and polypeptides toward clinical translation. Adv. Drug Deliv. Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Jayaraman, A.; Van Deventer, J.A.; Lee, K. Engineering Selectively Targeting Antimicrobial Peptides. Annu. Rev. Biomed. Eng. 2021, 23, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Cesa-Luna, C.; Alatorre-Cruz, J.M.; Carreño-López, R.; Quintero-Hernández, V.; Baez, A. Emerging Applications of Bacteriocins as Antimicrobials, Anticancer Drugs, and Modulators of The Gastrointestinal Microbiota. Pol. J. Microbiol. 2021, 70, 143–159. [Google Scholar] [CrossRef]
- Garsa, A.K.; Choudhury, P.K.; Puniya, A.K.; Dhewa, T.; Malik, R.K.; Tomar, S.K. Bovicins: The Bacteriocins of Streptococci and Their Potential in Methane Mitigation. Probiotics Antimicrob. Proteins 2019, 11, 1403–1413. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).