Effect of Diethylstilbestrol on Implantation and Decidualization in Mice
Abstract
:1. Introduction
2. Results
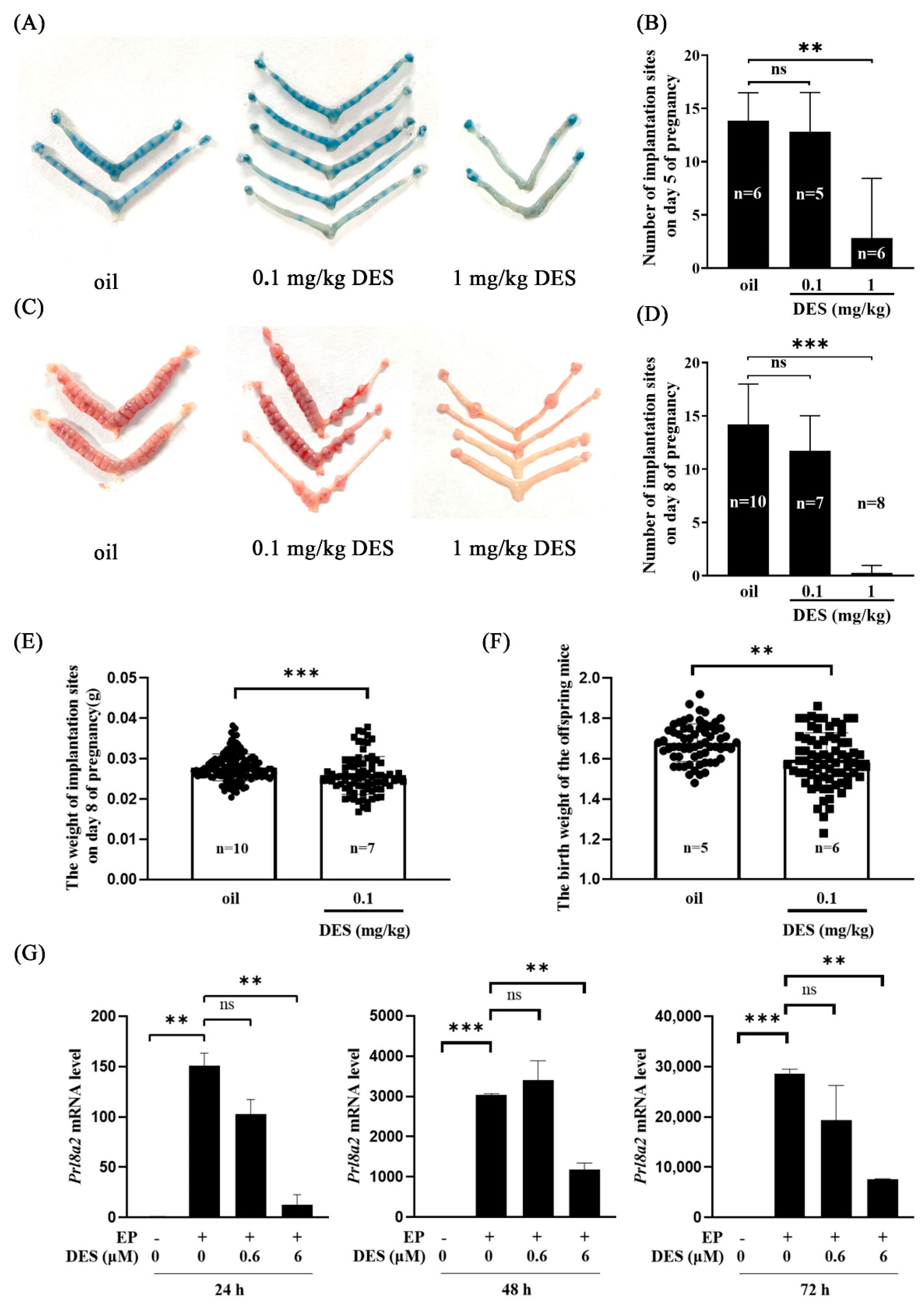
2.1. Effects of DES on Implantation and Decidualization
2.2. The Effect of DES on Preimplantation Embryo Development
2.3. DES Causes Uterine Epithelial Abnormalities
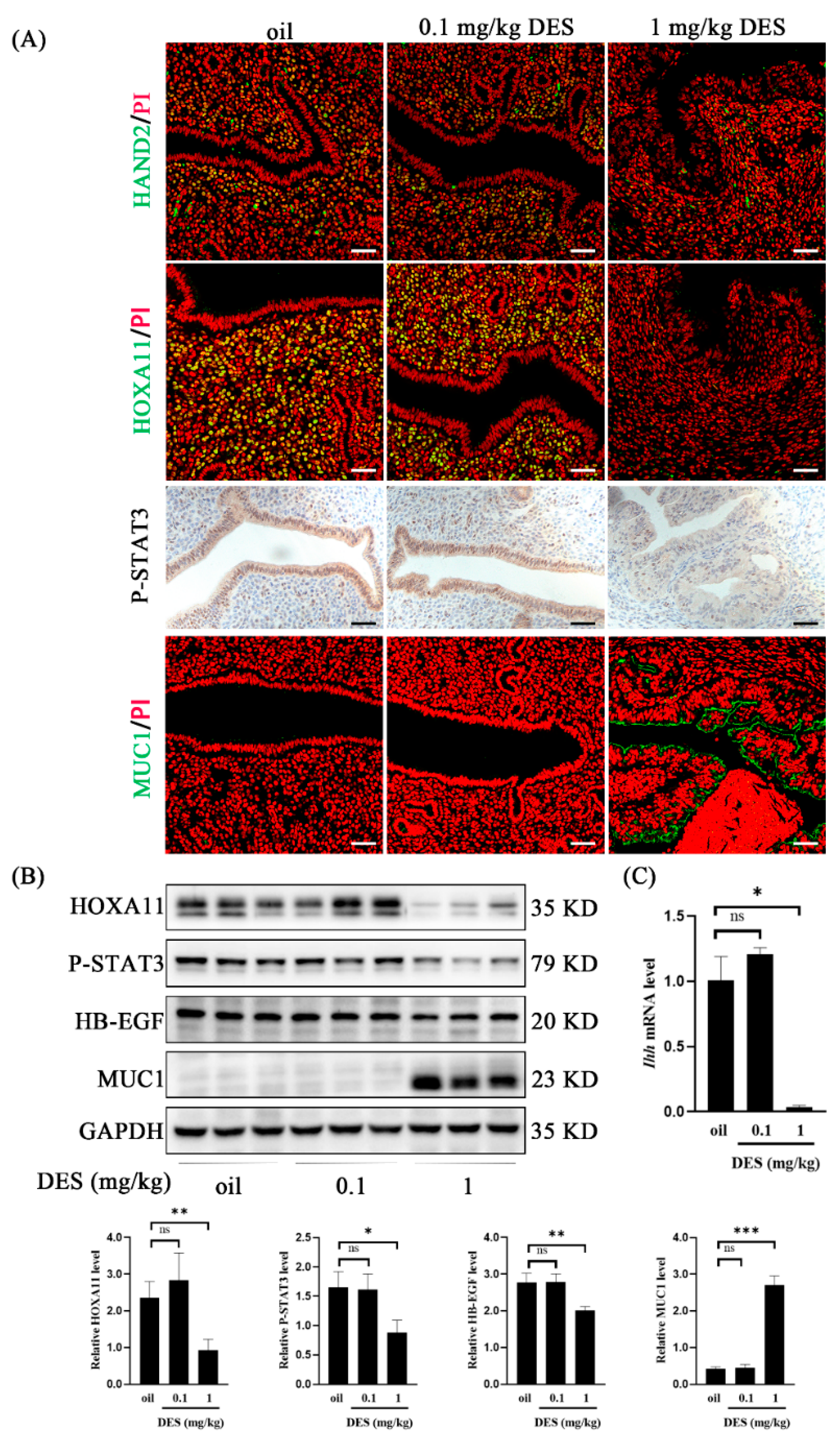
2.4. DES Causes Abnormal Endometrial Receptivity
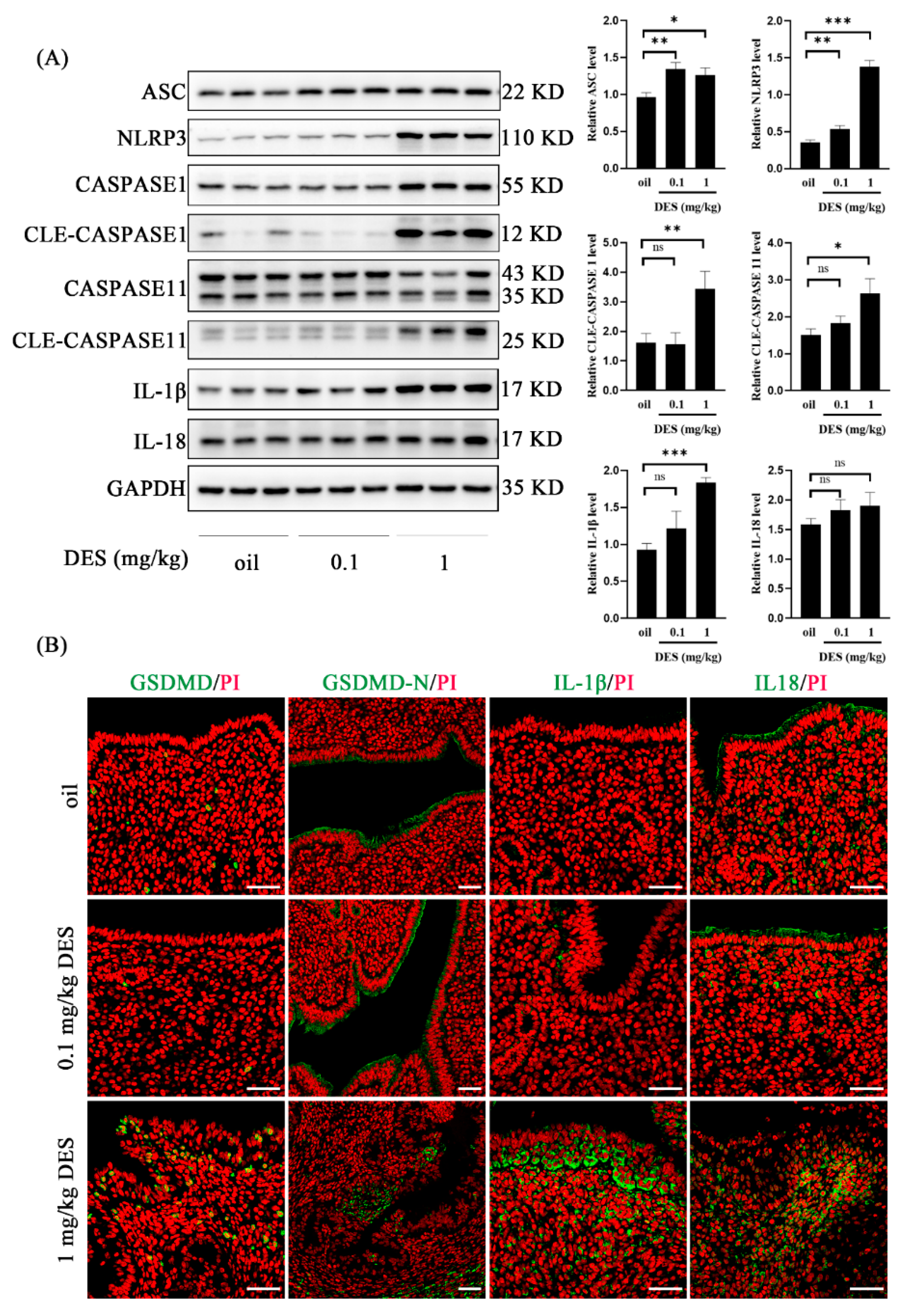
2.5. DES Leads to the Activation of the Pyroptosis Pathway
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Treatment Methods
4.2. Immunofluorescence
4.3. Immunohistochemistry
4.4. Isolation and Treatment of Mouse Stromal Cells
4.5. Western Blot
4.6. Real-Time RT-PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giusti, R.M.; Iwamoto, K.; Hatch, E.E. Diethylstilbestrol revisited: A review of the long-term health effects. Ann. Intern. Med. 1995, 122, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.M.; Waring, R.H. Diethylstilboestrol—A long-term legacy. Maturitas 2012, 72, 108–112. [Google Scholar] [CrossRef]
- Al Jishi, T.; Sergi, C. Current perspective of diethylstilbestrol (DES) exposure in mothers and offspring. Reprod. Toxicol. 2017, 71, 71–77. [Google Scholar] [CrossRef]
- Can, A.; Semiz, O. Diethylstilbestrol (DES)-induced cell cycle delay and meiotic spindle disruption in mouse oocytes during in-vitro maturation. Mol. Hum. Reprod. 2000, 6, 154–162. [Google Scholar] [CrossRef]
- Horiguchi, H.; Oguma, E.; Sakamoto, T.; Murata, K.; Kayama, F. Suppression of erythropoietin induction by diethylstilbestrol in rats. Arch. Toxicol. 2014, 88, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.H.B.; Zhang, M.; de Haan, L.H.J.; van Ravenzwaay, B.; Louisse, J.; Rietjens, I. The in vivo developmental toxicity of diethylstilbestrol (DES) in rat evaluated by an alternative testing strategy. Arch. Toxicol. 2019, 93, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- Nanjappa, M.K.; Medrano, T.I.; Mesa, A.M.; Ortega, M.T.; Caldo, P.D.; Mao, J.; Kinkade, J.A.; Levin, E.R.; Rosenfeld, C.S.; Cooke, P.S. Mice lacking membrane estrogen receptor 1 are protected from reproductive pathologies resulting from developmental estrogen exposure†. Biol. Reprod. 2019, 101, 392–404. [Google Scholar] [CrossRef]
- Li, S.; Hansman, R.; Newbold, R.; Davis, B.; McLachlan, J.A.; Barrett, J.C. Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol. Carcinog. 2003, 38, 78–84. [Google Scholar] [CrossRef]
- Adedeji, O.B.; Durhan, E.J.; Garcia-Reyero, N.; Kahl, M.D.; Jensen, K.M.; Lalone, C.A.; Makynen, E.A.; Perkins, E.J.; Thomas, L.; Villeneuve, D.L.; et al. Short-term study investigating the estrogenic potency of diethylstilbesterol in the fathead minnow (Pimephales promelas). Environ. Sci. Technol. 2012, 46, 7826–7835. [Google Scholar] [CrossRef]
- Fu, B.; Gao, H.; Fang, C.; Cheng, G.; Wang, H.; Wang, Y.; Hao, H.; Wang, X.; Huang, L.; Peng, D. Development of a monoclonal antibody-based indirect competitive enzyme-linked immunosorbent assay for screening of diethylstilbestrol in animal-derived foods. Heliyon 2024, 10, e39769. [Google Scholar] [CrossRef]
- Ding, Z.M.; Hua, L.P.; Ahmad, M.J.; Safdar, M.; Chen, F.; Wang, Y.S.; Zhang, S.X.; Miao, Y.L.; Xiong, J.J.; Huo, L.J. Diethylstilbestrol exposure disrupts mouse oocyte meiotic maturation in vitro through affecting spindle assembly and chromosome alignment. Chemosphere 2020, 249, 126182. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ji, Y.; Shao, Y.; Jiang, X.; Zhang, H. Novel molecularly imprinted polymer prepared by nanoattapulgite as matrix for selective solid-phase extraction of diethylstilbestrol. J. Chromatogr. A 2009, 1216, 7546–7552. [Google Scholar] [CrossRef]
- Marselos, M.; Tomatis, L. Diethylstilboestrol: II, pharmacology, toxicology and carcinogenicity in experimental animals. Eur. J. Cancer 1992, 29, 149–155. [Google Scholar] [CrossRef]
- Aschbacher, P.W. Diethylstilbestrol metabolism in food-producing animals. J. Toxicol. Environ. Health Suppl. 1976, 1, 45–59. [Google Scholar] [PubMed]
- Huang, Y.; Zhao, T.; He, J. Preparation of magnetic molecularly imprinted polymers for the rapid detection of diethylstilbestrol in milk samples. J. Sci. Food Agric. 2019, 99, 4452–4459. [Google Scholar] [CrossRef]
- Kugathas, I.; Johansson, H.K.L.; Chan Sock Peng, E.; Toupin, M.; Evrard, B.; Darde, T.A.; Boberg, J.; Draskau, M.K.; Rolland, A.D.; Mazaud-Guittot, S.; et al. Transcriptional profiling of the developing rat ovary following intrauterine exposure to the endocrine disruptors diethylstilbestrol and ketoconazole. Arch. Toxicol. 2023, 97, 849–863. [Google Scholar] [CrossRef] [PubMed]
- vom Saal, F.S.; Timms, B.G.; Montano, M.M.; Palanza, P.; Thayer, K.A.; Nagel, S.C.; Dhar, M.D.; Ganjam, V.K.; Parmigiani, S.; Welshons, W.V. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc. Natl. Acad. Sci. USA 1997, 94, 2056–2061. [Google Scholar] [CrossRef]
- Ivell, R.; Heng, K.; Nicholson, H.; Anand-Ivell, R. Brief maternal exposure of rats to the xenobiotics dibutyl phthalate or diethylstilbestrol alters adult-type Leydig cell development in male offspring. Asian J. Androl. 2013, 15, 261–268. [Google Scholar] [CrossRef]
- Newbold, R.R. Prenatal exposure to diethylstilbestrol (DES). Fertil. Steril. 2008, 89 (Suppl. S2), e55–e56. [Google Scholar] [CrossRef]
- Varayoud, J.; Ramos, J.G.; Bosquiazzo, V.L.; Lower, M.; Muñoz-de-Toro, M.; Luque, E.H. Neonatal exposure to bisphenol A alters rat uterine implantation-associated gene expression and reduces the number of implantation sites. Endocrinology 2011, 152, 1101–1111. [Google Scholar] [CrossRef]
- Jin, Z.Y.; Liu, C.K.; Hong, Y.Q.; Liang, Y.X.; Liu, L.; Yang, Z.M. BHPF exposure impairs mouse and human decidualization. Environ. Pollut. 2022, 304, 119222. [Google Scholar] [CrossRef] [PubMed]
- Sehring, J.; Beltsos, A.; Jeelani, R. Human implantation: The complex interplay between endometrial receptivity, inflammation, and the microbiome. Placenta 2022, 117, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, T.; Guo, X.; Wong, C.K.; Chen, X.; Chan, Y.L.; Wang, C.C.; Laird, S.; Li, T.C. Successful implantation is associated with a transient increase in serum pro-inflammatory cytokine profile followed by a switch to anti-inflammatory cytokine profile prior to confirmation of pregnancy. Fertil. Steril. 2021, 115, 1044–1053. [Google Scholar] [CrossRef]
- Mor, G.; Aldo, P.; Alvero, A.B. The unique immunological and microbial aspects of pregnancy. Nat. Rev. Immunol. 2017, 17, 469–482. [Google Scholar] [CrossRef]
- Dekel, N.; Gnainsky, Y.; Granot, I.; Mor, G. Inflammation and implantation. Am. J. Reprod. Immunol. 2010, 63, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Bianco, J.J.; McPherson, S.J.; Wang, H.; Prins, G.S.; Risbridger, G.P. Transient neonatal estrogen exposure to estrogen-deficient mice (aromatase knockout) reduces prostate weight and induces inflammation in late life. Am. J. Pathol. 2006, 168, 1869–1878. [Google Scholar] [CrossRef]
- Yang, Q.; Ali, M.; Treviño, L.S.; Mas, A.; Ismail, N.; Al-Hendy, A. Epigenetic Modulation of Inflammatory Pathways in Myometrial Stem Cells and Risk of Uterine Fibroids. Int. J. Mol. Sci. 2023, 24, 11641. [Google Scholar] [CrossRef]
- Rao, Z.; Zhu, Y.; Yang, P.; Chen, Z.; Xia, Y.; Qiao, C.; Liu, W.; Deng, H.; Li, J.; Ning, P.; et al. Pyroptosis in inflammatory diseases and cancer. Theranostics 2022, 12, 4310–4329. [Google Scholar] [CrossRef]
- Wei, Y.; Lan, B.; Zheng, T.; Yang, L.; Zhang, X.; Cheng, L.; Tuerhongjiang, G.; Yuan, Z.; Wu, Y. GSDME-mediated pyroptosis promotes the progression and associated inflammation of atherosclerosis. Nat. Commun. 2023, 14, 929. [Google Scholar] [CrossRef]
- Sborgi, L.; Rühl, S.; Mulvihill, E.; Pipercevic, J.; Heilig, R.; Stahlberg, H.; Farady, C.J.; Müller, D.J.; Broz, P.; Hiller, S. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016, 35, 1766–1778. [Google Scholar] [CrossRef]
- Xue, Y.; Enosi Tuipulotu, D.; Tan, W.H.; Kay, C.; Man, S.M. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019, 40, 1035–1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, S.; Zhang, Z.; Wu, H.; Lieberman, J. Channelling inflammation: Gasdermins in physiology and disease. Nat. Rev. Drug Discov. 2021, 20, 384–405. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wan, J.; Tan, G. The mechanisms of NLRP3 inflammasome/pyroptosis activation and their role in diabetic retinopathy. Front. Immunol. 2023, 14, 1151185. [Google Scholar] [CrossRef]
- Coll, R.C.; Schroder, K.; Pelegrín, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, H.; Tong, Z.; Cai, J. Nanoparticle-mediated cell pyroptosis: A new therapeutic strategy for inflammatory diseases and cancer. J. Nanobiotechnol. 2024, 22, 504. [Google Scholar] [CrossRef]
- Yang, Z.S.; Pan, H.Y.; Shi, W.W.; Chen, S.T.; Wang, Y.; Li, M.Y.; Zhang, H.Y.; Yang, C.; Liu, A.X.; Yang, Z.M. Regulation and Function of Laminin A5 during Mouse and Human Decidualization. Int. J. Mol. Sci. 2021, 23, 199. [Google Scholar] [CrossRef] [PubMed]
- Finn, C.A.; Martin, L. The role of the oestrogen secreted before oestrus in the preparation of the uterus for implantation in the mouse. J. Endocrinol. 1970, 47, 431–438. [Google Scholar] [CrossRef]
- Miller, I.; Min, M.; Yang, C.; Tian, C.; Gookin, S.; Carter, D.; Spencer, S.L. Ki67 is a Graded Rather than a Binary Marker of Proliferation versus Quiescence. Cell Rep. 2018, 24, 1105–1112.e5. [Google Scholar] [CrossRef]
- Gusterson, B.A.; Ross, D.T.; Heath, V.J.; Stein, T. Basal cytokeratins and their relationship to the cellular origin and functional classification of breast cancer. Breast Cancer Res. 2005, 7, 143–148. [Google Scholar] [CrossRef]
- Kurita, T.; Cunha, G.R. Roles of p63 in differentiation of Müllerian duct epithelial cells. Ann. N. Y. Acad. Sci. 2001, 948, 9–12. [Google Scholar] [CrossRef]
- Suen, A.A.; Jefferson, W.N.; Wood, C.E.; Padilla-Banks, E.; Bae-Jump, V.L.; Williams, C.J. SIX1 Oncoprotein as a Biomarker in a Model of Hormonal Carcinogenesis and in Human Endometrial Cancer. Mol. Cancer Res. 2016, 14, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Suen, A.A.; Jefferson, W.N.; Wood, C.E.; Williams, C.J. SIX1 Regulates Aberrant Endometrial Epithelial Cell Differentiation and Cancer Latency Following Developmental Estrogenic Chemical Exposure. Mol. Cancer Res. 2019, 17, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lin, H.; Kong, S.; Wang, S.; Wang, H.; Wang, H.; Armant, D.R. Physiological and molecular determinants of embryo implantation. Mol. Aspects Med. 2013, 34, 939–980. [Google Scholar] [CrossRef]
- Chen, S.T.; Shi, W.W.; Ran, F.; Liu, C.K.; Luo, H.N.; Wu, L.J.; Wu, Y.; Zhang, T.T.; Yang, Z.M. The activation of cGAS-STING pathway causes abnormal uterine receptivity in aged mice. Aging Cell 2024, 23, e14303. [Google Scholar] [CrossRef] [PubMed]
- Hantak, A.M.; Bagchi, I.C.; Bagchi, M.K. Role of uterine stromal-epithelial crosstalk in embryo implantation. Int. J. Dev. Biol. 2014, 58, 139–146. [Google Scholar] [CrossRef]
- Matsumoto, H.; Daikoku, T.; Wang, H.; Sato, E.; Dey, S.K. Differential expression of ezrin/radixin/moesin (ERM) and ERM-associated adhesion molecules in the blastocyst and uterus suggests their functions during implantation. Biol. Reprod. 2004, 70, 729–736. [Google Scholar] [CrossRef]
- Rackow, B.W.; Jorgensen, E.; Taylor, H.S. Endometrial polyps affect uterine receptivity. Fertil. Steril. 2011, 95, 2690–2692. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Gendler, S.J.; Carson, D.D. Expression of human MUC1 during early pregnancy in the human MUC1 transgenic mouse model. Biol. Reprod. 2009, 81, 1182–1188. [Google Scholar] [CrossRef]
- Li, M.Y.; Wu, Y.; Tang, H.L.; Wang, Y.; Li, B.; He, Y.Y.; Yan, G.J.; Yang, Z.M. Embryo-Derived Cathepsin B Promotes Implantation and Decidualization by Activating Pyroptosis. Adv. Sci. 2024, 11, e2402299. [Google Scholar] [CrossRef]
- Newbold, R.R.; Padilla-Banks, E.; Jefferson, W.N. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 2006, 147 (Suppl. S6), S11–S17. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, J.; El Zowalaty, A.E.; Li, R.; Dudley, E.A.; Ye, X. Timing and recovery of postweaning exposure to diethylstilbestrol on early pregnancy in CD-1 mice. Reprod. Toxicol. 2014, 49, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, W.R.; Cornwall, G.A.; Carter, M.W.; Bradshaw, W.S. Diethylstilbestrol-induced perinatal lethality in the rat. I. Relationship to reduced maternal weight gain. Biol. Reprod. 1991, 44, 575–582. [Google Scholar] [CrossRef]
- Rogers, R.E.; Chai, S.; Pask, A.J.; Mattiske, D.M. Prenatal exposure to diethylstilbestrol has long-lasting, transgenerational impacts on fertility and reproductive development. Toxicol. Sci. 2023, 195, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Yoshimura, S. Early embryonic losses in mice induced by diethylstilbestrol. Congenit. Anom. 2009, 49, 269–273. [Google Scholar] [CrossRef]
- Li, Y.F.; Canário, A.V.M.; Power, D.M.; Campinho, M.A. Ioxynil and diethylstilbestrol disrupt vascular and heart development in zebrafish. Environ. Int. 2019, 124, 511–520. [Google Scholar] [CrossRef]
- Kurita, T.; Mills, A.A.; Cunha, G.R. Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis. Development 2004, 131, 1639–1649. [Google Scholar] [CrossRef]
- Chu, P.G.; Weiss, L.M. Expression of cytokeratin 5/6 in epithelial neoplasms: An immunohistochemical study of 509 cases. Mod. Pathol. 2002, 15, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Houghton, O.; McCluggage, W.G. The expression and diagnostic utility of p63 in the female genital tract. Adv. Anat. Pathol. 2009, 16, 316–321. [Google Scholar] [CrossRef]
- Timms, B.G.; Howdeshell, K.L.; Barton, L.; Bradley, S.; Richter, C.A.; vom Saal, F.S. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl. Acad. Sci. USA 2005, 102, 7014–7019. [Google Scholar] [CrossRef]
- Padilla-Banks, E.; Jefferson, W.N.; Papas, B.N.; Suen, A.A.; Xu, X.; Carreon, D.V.; Willson, C.J.; Quist, E.M.; Williams, C.J. Developmental estrogen exposure in mice disrupts uterine epithelial cell differentiation and causes adenocarcinoma via Wnt/β-catenin and PI3K/AKT signaling. PLoS Biol. 2023, 21, e3002334. [Google Scholar] [CrossRef]
- Bodelon, C.; Gierach, G.L.; Hatch, E.E.; Riseberg, E.; Hutchinson, A.; Yeager, M.; Sandler, D.P.; Taylor, J.A.; Hoover, R.N.; Xu, Z.; et al. In utero exposure to diethylstilbestrol and blood DNA methylation in adult women: Results from a meta-analysis of two cohort studies. Environ. Res. 2023, 231 Pt 1, 115990. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Davis, V.L.; Hanson, R.B.; Jefferson, W.N.; McLachlan, J.A.; Bullock, B.C.; Newbold, R.R.; Korach, K.S. Accelerated onset of uterine tumors in transgenic mice with aberrant expression of the estrogen receptor after neonatal exposure to diethylstilbestrol. Mol. Carcinog. 1997, 19, 236–242. [Google Scholar] [CrossRef]
- Padmanabhan, R.; Hendry, I.R.; Knapp, J.R.; Shuai, B.; Hendry, W.J. Altered microRNA expression patterns during the initiation and promotion stages of neonatal diethylstilbestrol-induced dysplasia/neoplasia in the hamster (Mesocricetus auratus) uterus. Cell Biol. Toxicol. 2017, 33, 483–500. [Google Scholar] [CrossRef]
- Nakamura, H.; Kimura, T.; Koyama, S.; Ogita, K.; Tsutsui, T.; Shimoya, K.; Taniguchi, T.; Koyama, M.; Kaneda, Y.; Murata, Y. Mouse model of human infertility: Transient and local inhibition of endometrial STAT-3 activation results in implantation failure. FEBS Lett. 2006, 580, 2717–2722. [Google Scholar] [CrossRef]
- Li, S.Y.; Song, Z.; Song, M.J.; Qin, J.W.; Zhao, M.L.; Yang, Z.M. Impaired receptivity and decidualization in DHEA-induced PCOS mice. Sci. Rep. 2016, 6, 38134. [Google Scholar] [CrossRef]
- Tang, Z.R.; Xu, X.L.; Deng, S.L.; Lian, Z.X.; Yu, K. Oestrogenic Endocrine Disruptors in the Placenta and the Fetus. Int. J. Mol. Sci. 2020, 21, 1519. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, S.P.; Kalimuthu, V.; Chandran Manimegalai, S.; Arulanandu, A.M.; Thiyagarajan, R.; Balamuthu, K. Evaluation of the potential role of diethylstilbestrol on the induction of endometriosis in a rat model—An alternative approach. Biochem. Biophys. Res. Commun. 2022, 617 Pt 2, 18–24. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.T.; He, Y.Y.; Li, B.; Yang, C.; Yang, Z.S.; Yang, Z.M. The regulation and function of acetylated high-mobility group box 1 during implantation and decidualization. Front. Immunol. 2023, 14, 1024706. [Google Scholar] [CrossRef] [PubMed]
- Loveless, R.; Bloomquist, R.; Teng, Y. Pyroptosis at the forefront of anticancer immunity. J. Exp. Clin. Cancer Res. 2021, 40, 264. [Google Scholar] [CrossRef]
- Nadeau-Vallée, M.; Obari, D.; Palacios, J.; Brien, M.; Duval, C.; Chemtob, S.; Girard, S. Sterile inflammation and pregnancy complications: A review. Reproduction 2016, 152, R277–R292. [Google Scholar] [CrossRef]
- Christiansen, O.B.; Nielsen, H.S.; Kolte, A.M. Inflammation and miscarriage. Semin. Fetal Neonatal Med. 2006, 11, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.W.; Chen, Z.C.; Yang, Z.S.; Yang, Y.; Yan, Y.P.; Liu, Y.F.; Pan, J.M.; Su, R.W.; Yang, Z.M. Blastocyst-induced ATP release from luminal epithelial cells initiates decidualization through the P2Y2 receptor in mice. Sci. Signal 2020, 13, eaba3396. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, Y.K.; Jaiswal, M.K.; Agrawal, V.; Chaturvedi, M.M. Bacterial endotoxin (LPS)-induced DNA damage in preimplanting embryonic and uterine cells inhibits implantation. Fertil. Steril. 2009, 91 (Suppl. S5), 2095–2103. [Google Scholar] [CrossRef]
- Hou, J.; Lei, Z.; Cui, L.; Hou, Y.; Yang, L.; An, R.; Wang, Q.; Li, S.; Zhang, H.; Zhang, L. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol. Environ. Saf. 2021, 212, 112012. [Google Scholar] [CrossRef]
- Wang, R.; Xu, X.; Yang, J.; Chen, W.; Zhao, J.; Wang, M.; Zhang, Y.; Yang, Y.; Huang, W.; Zhang, H. BPDE exposure promotes trophoblast cell pyroptosis and induces miscarriage by up-regulating lnc-HZ14/ZBP1/NLRP3 axis. J. Hazard. Mater. 2023, 455, 131543. [Google Scholar] [CrossRef]
- Chen, S.T.; Ran, F.; Shi, W.W.; Liu, C.K.; Wang, P.C.; Luo, H.N.; Yang, Z.M. Tryptophan in the mouse diet is essential for embryo implantation and decidualization. Front. Endocrinol. 2024, 15, 1356914. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, F.; Chen, S.-T.; Li, M.-Y.; Jin, D.-D.; Yang, Z.-M. Effect of Diethylstilbestrol on Implantation and Decidualization in Mice. Int. J. Mol. Sci. 2025, 26, 4122. https://doi.org/10.3390/ijms26094122
Ran F, Chen S-T, Li M-Y, Jin D-D, Yang Z-M. Effect of Diethylstilbestrol on Implantation and Decidualization in Mice. International Journal of Molecular Sciences. 2025; 26(9):4122. https://doi.org/10.3390/ijms26094122
Chicago/Turabian StyleRan, Feng, Si-Ting Chen, Meng-Yuan Li, Dan-Dan Jin, and Zeng-Ming Yang. 2025. "Effect of Diethylstilbestrol on Implantation and Decidualization in Mice" International Journal of Molecular Sciences 26, no. 9: 4122. https://doi.org/10.3390/ijms26094122
APA StyleRan, F., Chen, S.-T., Li, M.-Y., Jin, D.-D., & Yang, Z.-M. (2025). Effect of Diethylstilbestrol on Implantation and Decidualization in Mice. International Journal of Molecular Sciences, 26(9), 4122. https://doi.org/10.3390/ijms26094122




