Acridine-Based Chalcone 1C and ABC Transporters
Abstract
1. Introduction
2. Results
2.1. Viability Assays
2.2. Effect of 1C on ABCB1
2.2.1. Chalcone 1C and ABCB1 Protein Expression
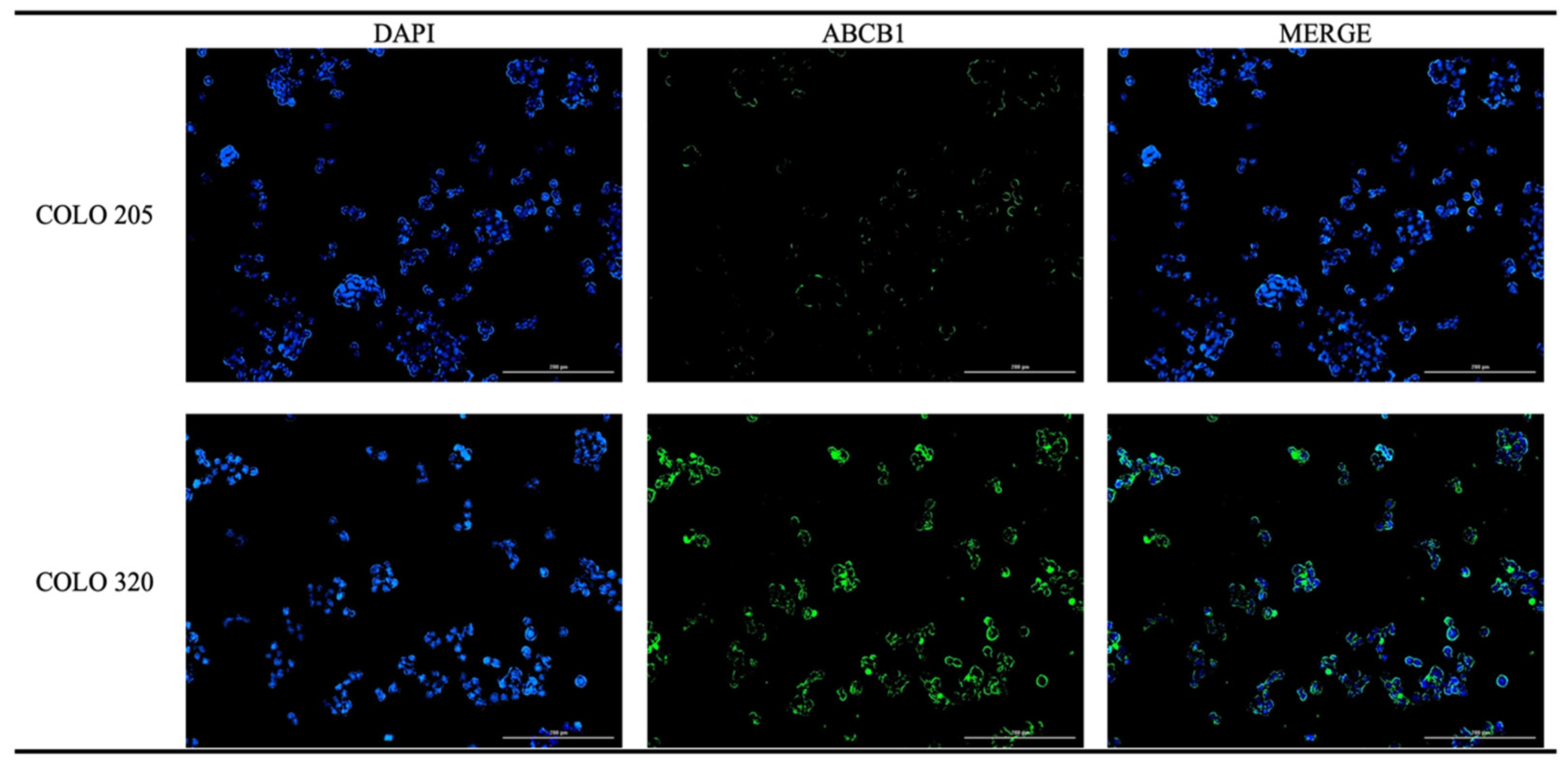
2.2.2. Immunofluorescence Analysis of ABCB1
2.2.3. Chalcone 1C and ABCB1 Gene Expression
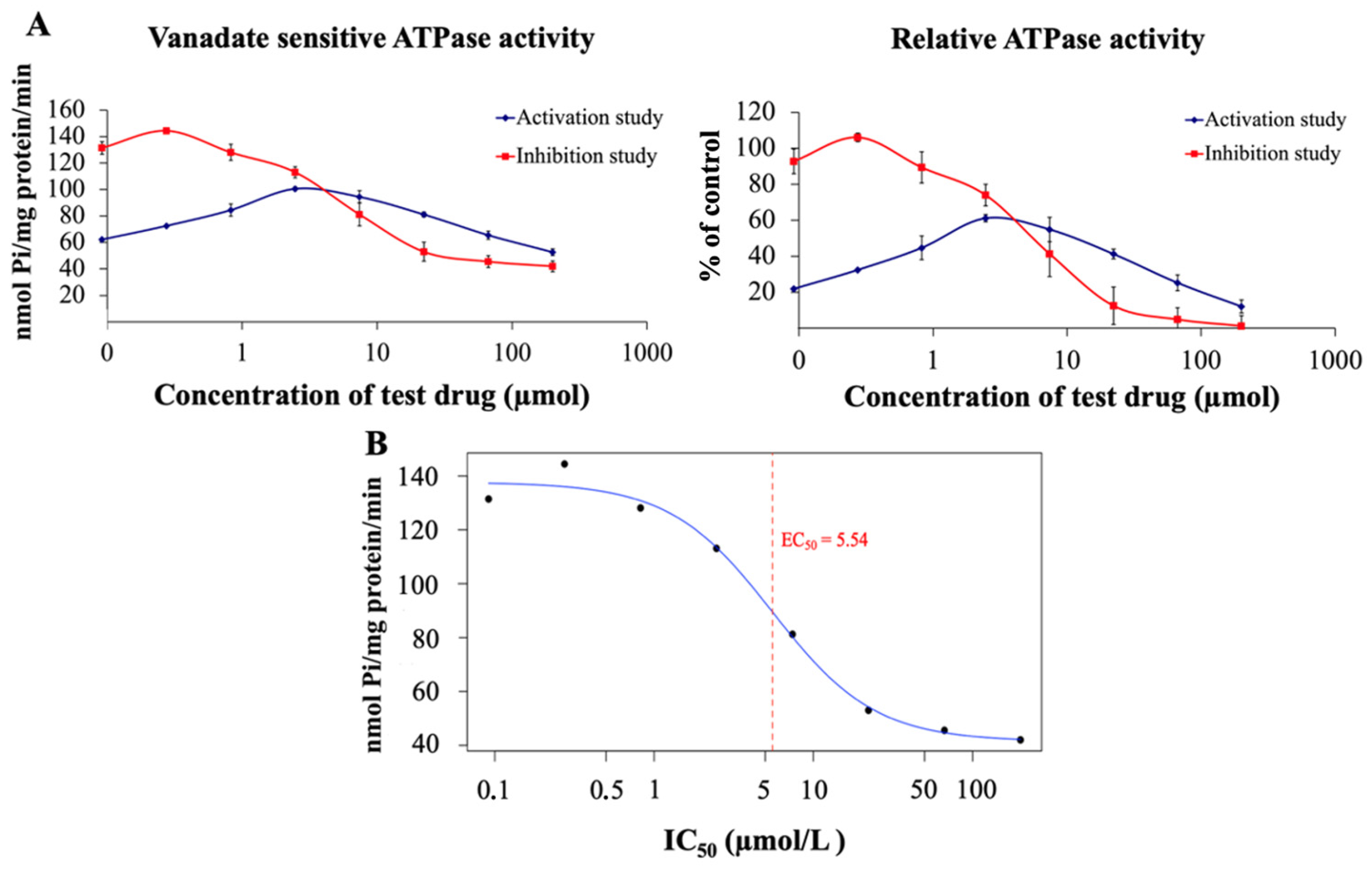
2.2.4. 1C and ATPase Activity Assay
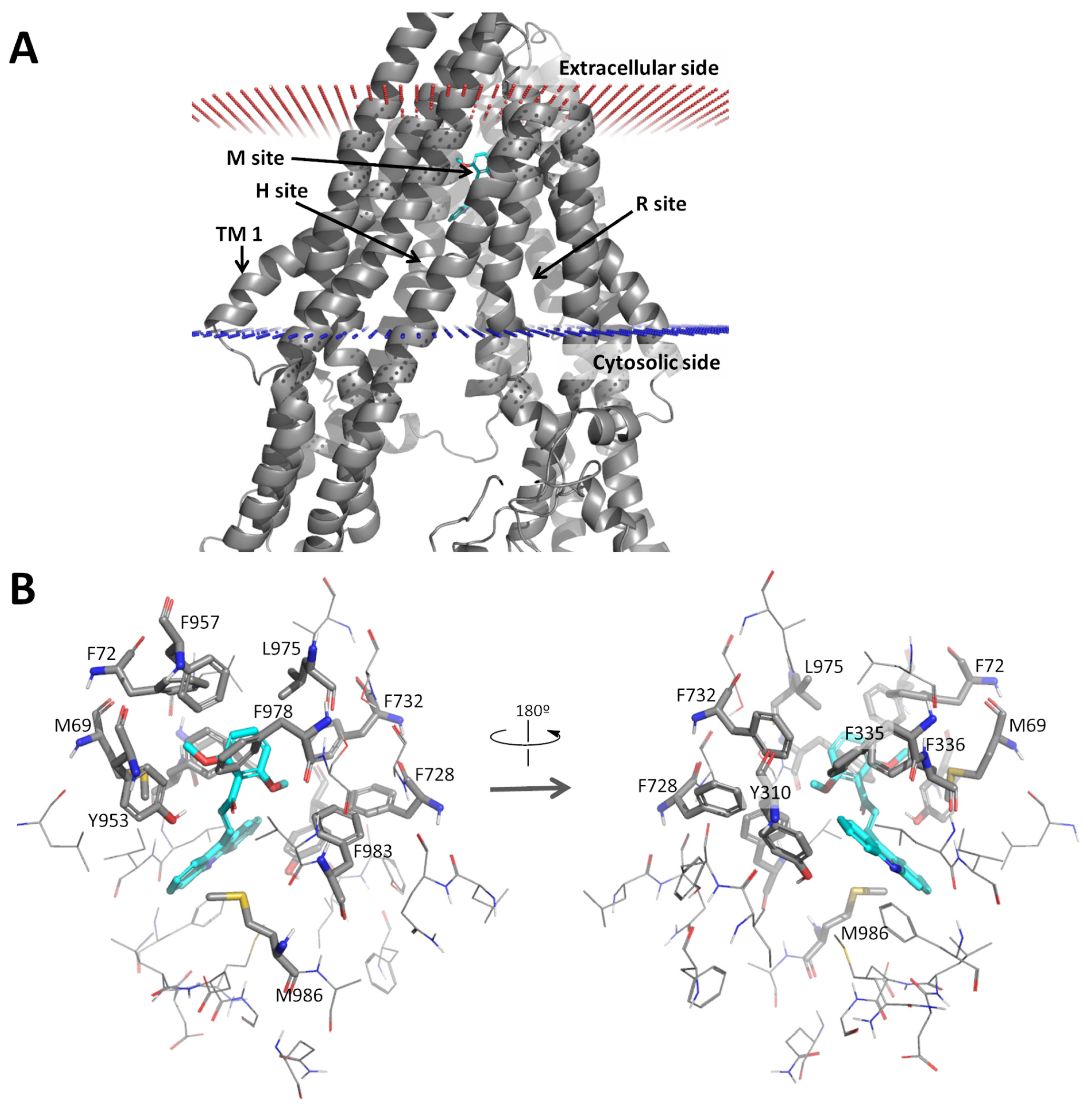
2.2.5. Docking Analysis of 1C
2.3. Effect of 1C on ABCC1 and ABCG2
2.3.1. Chalcone 1C and the Protein Expression of ABCC1 and ABCG2
2.3.2. Chalcone 1C and the Gene Expression of ABCC1 and ABCG2
2.3.3. Effect of 1C on Galectin-1 Expression
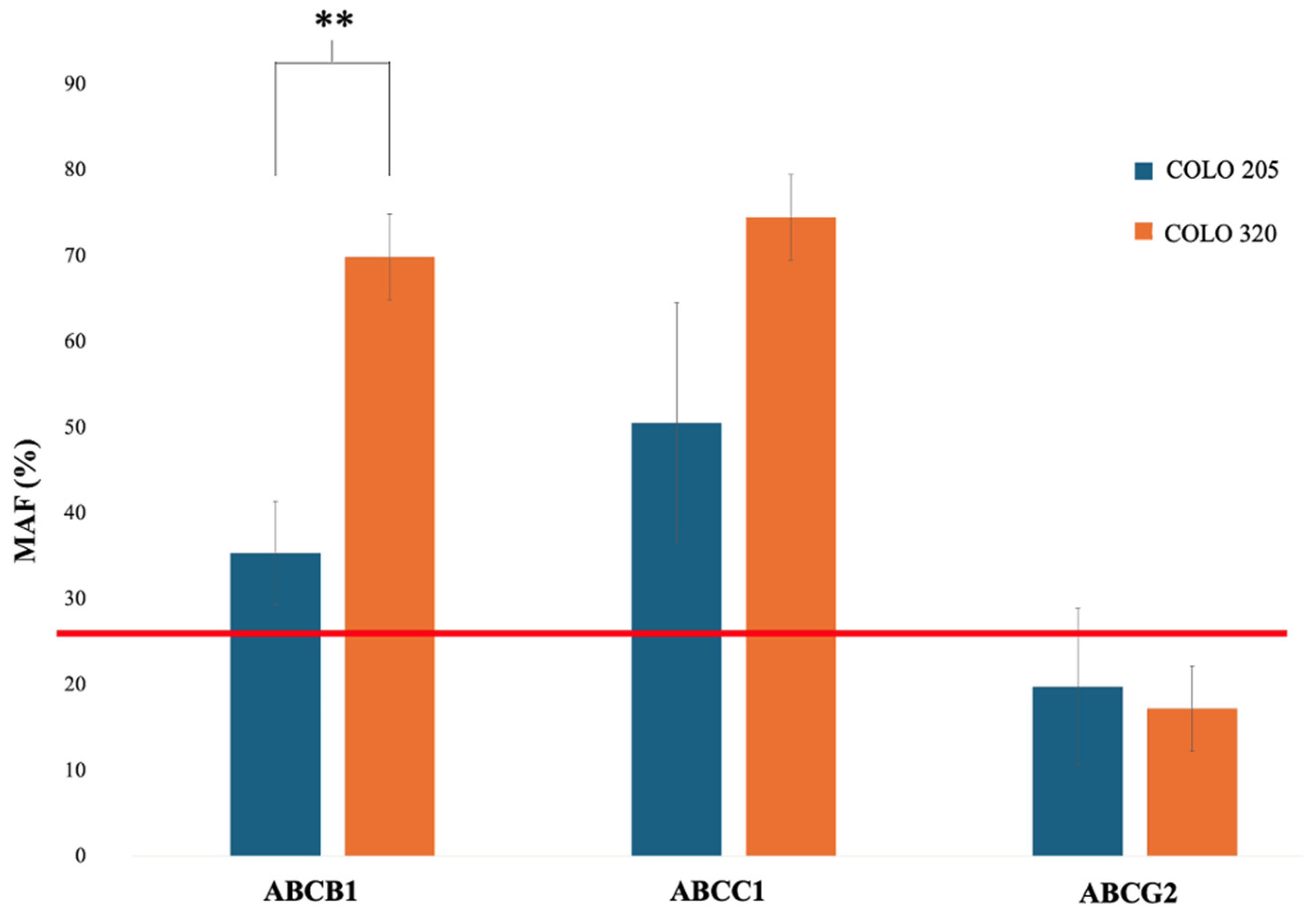
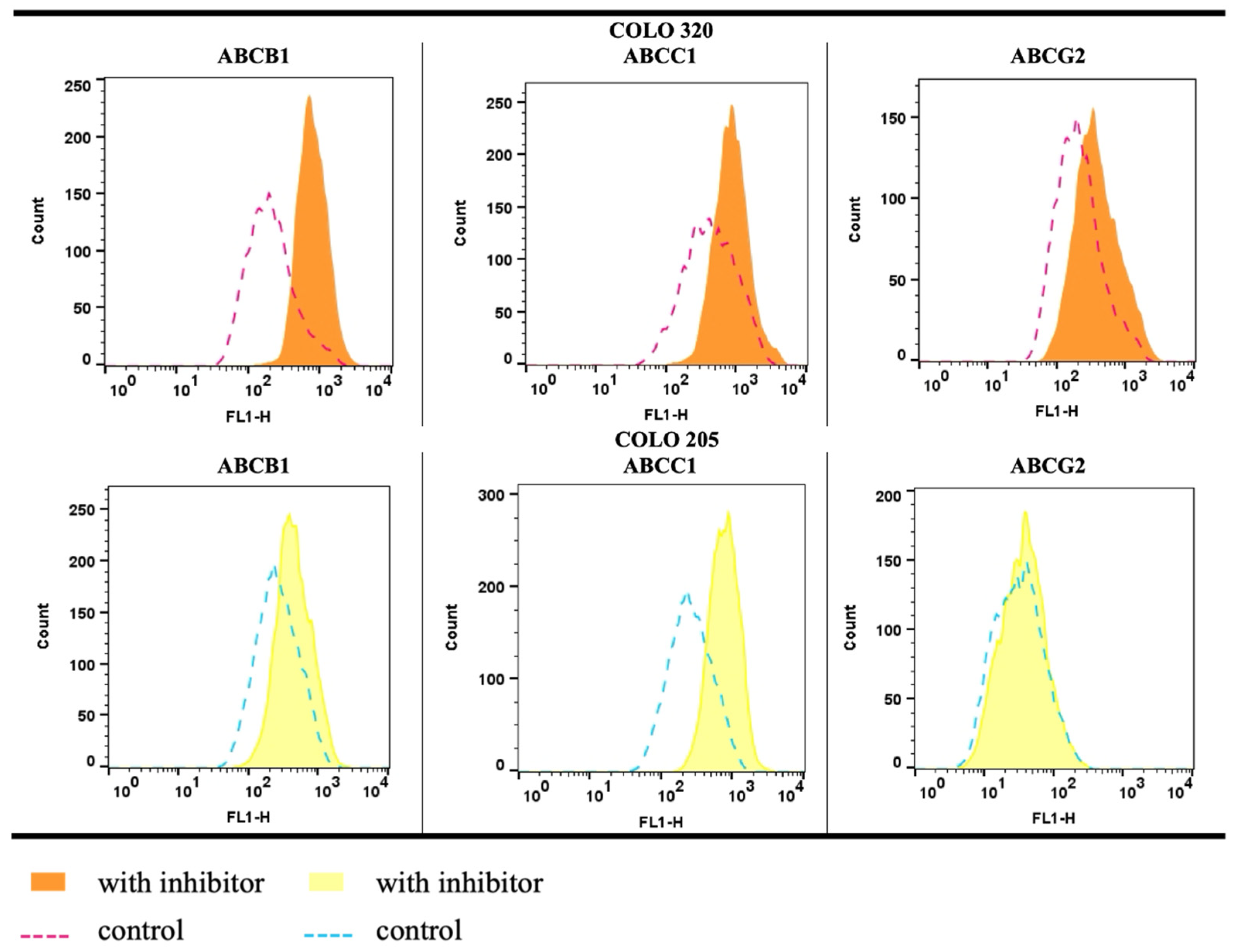
2.4. Functional Efflux Assay in COLO 205 and COLO 320
3. Discussion
Limitations and Future Directions
4. Materials and Methods
4.1. Test Compound
4.2. Cell Culture
4.3. Molecular Docking
4.4. Viability Assays
4.5. Immunofluorescence
4.6. ATPase Activity Analysis
4.7. Functional Assessment of ABC Proteins
4.8. Quantitative Reverse Transcription PCR (qRT-PCR)
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A549/T | Human paclitaxel resistant lung adenocarcinoma cell line |
| ABC | ATP-binding cassette |
| ABCB1 | ATP-binding cassette subfamily b member 1 (P-glycoprotein) |
| ABCC1 | ATP-binding cassette subfamily c member 1 (multidrug resistance-associated protein 1) |
| ABCG2 | ATP-binding cassette subfamily g member 2 (breast cancer resistance protein) |
| ATPase | Adenosine triphosphatase |
| BJ-5ta | Human fibroblast cell line immortalized |
| BT-20 | Human breast carcinoma cell line |
| Caco-2 | Human colorectal adenocarcinoma cell line |
| COLO 205 | Human colorectal adenocarcinoma cell line |
| COLO 320 | Human multidrug resistant colorectal adenocarcinoma cell line |
| DMSO | Dimethyl sulfoxide |
| EC50 | Half-maximal effective concentration |
| FHC | Fetal human colon cell line |
| GAL | Galectin |
| HCT-15 | Human colorectal adenocarcinoma cell line |
| HT-29 | Human colorectal adenocarcinoma cell line |
| IC50 | Half-maximal inhibitory concentration |
| LoVo | Human colorectal adenocarcinoma cell line |
| MAF | Multidrug resistance activity factor |
| MCF-7 | Michigan cancer foundation-7 breast cancer cell line |
| MRP1 | Multidrug resistance-associated protein 1 (ABCC1) |
| MTT | Methylthiazoltetrazolium assay |
| NCI-H460/MX20 | Human mitoxantrone resistant large cell lung carcinoma cell line |
| NBD | Nucleotide-binding domain |
| PBS | Phosphate-buffered saline |
| PVDF | Polyvinylidene fluoride |
| RPMI | Roswell Park memorial institute medium |
| SDS | Sodium dodecyl sulfate |
| SI | Selectivity index |
| SRB | Sulforhodamine B assay |
| TCA | Trichloroacetic acid |
| TMD | Transmembrane domain |
| qRT-PCR | Quantitative reverse transcription polymerase chain reaction |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gautam, V.; Sandhu, A.; Rawat, K.; Sharma, A.; Saha, L. Current and Emerging Therapeutic Approaches for Colorectal Cancer: A Comprehensive Review. World J. Gastrointest. Surg. 2023, 15, 495–519. [Google Scholar] [CrossRef]
- Fadlallah, H.; El Masri, J.; Fakhereddine, H.; Youssef, J.; Chemaly, C.; Doughan, S.; Abou-Kheir, W. Colorectal Cancer: Recent Advances in Management and Treatment. World J. Clin. Oncol. 2024, 15, 1136–1156. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Ye, X.-C.; Wang, R.; Ling, X.; McManus, M.; Fan, F.; Boulbes, D.; Ellis, L.M. Intracrine VEGF Signaling Mediates the Activity of Prosurvival Pathways in Human Colorectal Cancer Cells. Cancer Res. 2016, 76, 3014–3024. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Fan, F.; Wang, R.; Ye, X.; Xia, L.; Boulbes, D.; Ellis, L.M. Intracrine VEGF Signalling Mediates Colorectal Cancer Cell Migration and Invasion. Br. J. Cancer 2017, 117, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, T.; Zhang, L.; Zhu, P.; Deng, M.; Huang, C.; Hu, T.; Jiang, L.; Li, J. Mammalian Drug Efflux Transporters of the ATP Binding Cassette (ABC) Family in Multidrug Resistance: A Review of the Past Decade. Cancer Lett. 2016, 370, 153–164. [Google Scholar] [CrossRef]
- Marques, A.V.L.; Ruginsk, B.E.; Prado, L.d.O.; de Lima, D.E.; Daniel, I.W.; Moure, V.R.; Valdameri, G. The Association of ABC Proteins with Multidrug Resistance in Cancer. Biochim. Biophys. Acta Mol. Cell Res. 2025, 1872, 119878. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yu, A.-M. ABC Transporters in Multidrug Resistance and Pharmacokinetics, and Strategies for Drug Development. Curr. Pharm. Des. 2014, 20, 793. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Ashique, S.; Bhowmick, M.; Pal, R.; Khatoon, H.; Kumar, P.; Sharma, H.; Garg, A.; Kumar, S.; Das, U. Multi Drug Resistance in Colorectal Cancer- Approaches to Overcome, Advancements and Future Success. Adv. Cancer Biol. Metastasis 2024, 10, 100114. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Z.; Peng, C.; You, J.; Shen, J.; Han, S.; Chen, J. Dietary Compound Isoliquiritigenin Targets GRP78 to Chemosensitize Breast Cancer Stem Cells via β-Catenin/ABCG2 Signaling. Carcinogenesis 2014, 35, 2544–2554. [Google Scholar] [CrossRef] [PubMed]
- Skinner, K.T.; Palkar, A.M.; Hong, A.L. Genetics of ABCB1 in Cancer. Cancers 2023, 15, 4236. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, F.; Zhou, H.; Chen, Y.; Du, J.; Zhang, X.; Song, D.; Zhao, H. MDIG Promotes Cisplatin Resistance of Lung Adenocarcinoma by Regulating ABC Transporter Expression via Activation of the WNT/Β-catenin Signaling Pathway. Oncol. Lett. 2019, 18, 4294–4307. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Pata, J.; Chaptal, V.; Boumendjel, A.; Falson, P.; Prasad, R. Structure, Function, and Inhibition of Catalytically Asymmetric ABC Transporters: Lessons from the PDR Subfamily. Drug Resist. Updates 2023, 71, 100992. [Google Scholar] [CrossRef]
- Tian, Y.; Han, X.; Tian, D. The Biological Regulation of ABCE1. IUBMB Life 2012, 64, 795–800. [Google Scholar] [CrossRef]
- Gerovac, M.; Tampé, R. Control of MRNA Translation by Versatile ATP-Driven Machines. Trends Biochem. Sci. 2019, 44, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Aller, S.G.; Beis, K.; Carpenter, E.P.; Chang, G.; Chen, L.; Dassa, E.; Dean, M.; Duong Van Hoa, F.; Ekiert, D.; et al. Structural and Functional Diversity Calls for a New Classification of ABC Transporters. FEBS Lett. 2020, 594, 3767–3775. [Google Scholar] [CrossRef]
- Wu, C.-P.; Hung, C.-Y.; Hsieh, Y.-J.; Murakami, M.; Huang, Y.-H.; Su, T.-Y.; Hung, T.-H.; Yu, J.-S.; Wu, C.-P.; Hung, C.-Y.; et al. ABCB1 and ABCG2 Overexpression Mediates Resistance to the Phosphatidylinositol 3-Kinase Inhibitor HS-173 in Cancer Cell Lines. Cells 2023, 12, 1056. [Google Scholar] [CrossRef]
- Fan, W.; Shao, K.; Luo, M. Structural View of Cryo-Electron Microscopy-Determined ATP-Binding Cassette Transporters in Human Multidrug Resistance. Biomolecules 2024, 14, 231. [Google Scholar] [CrossRef]
- Kiełbowski, K.; Król, M.; Bakinowska, E.; Pawlik, A. The Role of ABCB1, ABCG2, and SLC Transporters in Pharmacokinetic Parameters of Selected Drugs and Their Involvement in Drug–Drug Interactions. Membranes 2024, 14, 223. [Google Scholar] [CrossRef]
- Wu, C.-P.; Hsiao, S.-H.; Wu, Y.-S. Perspectives on Drug Repurposing to Overcome Cancer Multidrug Resistance Mediated by ABCB1 and ABCG2. Drug Resist. Updates 2023, 71, 101011. [Google Scholar] [CrossRef] [PubMed]
- Szebényi, K.; Füredi, A.; Bajtai, E.; Sama, S.N.; Csiszar, A.; Gombos, B.; Szabó, P.; Grusch, M.; Szakács, G. Effective Targeting of Breast Cancer by the Inhibition of P-Glycoprotein Mediated Removal of Toxic Lipid Peroxidation Byproducts from Drug Tolerant Persister Cells. Drug Resist. Updates 2023, 71, 101007. [Google Scholar] [CrossRef] [PubMed]
- Muriithi, W.; Macharia, L.W.; Heming, C.P.; Echevarria, J.L.; Nyachieo, A.; Filho, P.N.; Neto, V.M. ABC Transporters and the Hallmarks of Cancer: Roles in Cancer Aggressiveness beyond Multidrug Resistance. Cancer Biol. Med. 2020, 17, 253. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.P.; Li, Y.C.; Murakami, M.; Hsiao, S.H.; Lee, Y.C.; Huang, Y.H.; Chang, Y.T.; Hung, T.H.; Wu, Y.S.; Ambudkar, S.V. Furmonertinib, a Third-Generation EGFR Tyrosine Kinase Inhibitor, Overcomes Multidrug Resistance through Inhibiting ABCB1 and ABCG2 in Cancer Cells. Int. J. Mol. Sci. 2023, 24, 13972. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Hoti, S. Development of Fourth Generation ABC Inhibitors from Natural Products: A Novel Approach to Overcome Cancer Multidrug Resistance. Anti-Cancer Agents Med. Chem. 2015, 15, 605–615. [Google Scholar] [CrossRef]
- Duan, C.; Yu, M.; Xu, J.; Li, B.-Y.; Zhao, Y.; Kankala, R.K. Overcoming Cancer Multi-Drug Resistance (MDR): Reasons, Mechanisms, Nanotherapeutic Solutions, and Challenges. Biomed. Pharmacother. 2023, 162, 114643. [Google Scholar] [CrossRef]
- Leite, F.F.; de Sousa, N.F.; de Oliveira, B.H.M.; Duarte, G.D.; Ferreira, M.D.L.; Scotti, M.T.; Filho, J.M.B.; Rodrigues, L.C.; de Moura, R.O.; Mendonça-Junior, F.J.B.; et al. Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies. Molecules 2023, 28, 4009. [Google Scholar] [CrossRef]
- Sheikh, K.A.; Gupta, A.; Umar, M.; Ali, R.; Shaquiquzzaman, M.; Akhter, M.; Khan, M.A.; Kaleem, M.; Ambast, P.K.; Charan, S.; et al. Advances in Chalcone Derivatives: Unravelling Their Anticancer Potential through Structure-Activity Studies. J. Mol. Struct. 2024, 1299, 137154. [Google Scholar] [CrossRef]
- Takac, P.; Kello, M.; Vilkova, M.; Vaskova, J.; Michalkova, R.; Mojzisova, G.; Mojzis, J. Antiproliferative Effect of Acridine Chalcone Is Mediated by Induction of Oxidative Stress. Biomolecules 2020, 10, 345. [Google Scholar] [CrossRef]
- Rossi, M.; Pellegrino, C.; Rydzyk, M.M.; Farruggia, G.; de Biase, D.; Cetrullo, S.; D’Adamo, S.; Bisi, A.; Blasi, P.; Malucelli, E.; et al. Chalcones Induce Apoptosis, Autophagy and Reduce Spreading in Osteosarcoma 3D Models. Biomed. Pharmacother. 2024, 179, 117284. [Google Scholar] [CrossRef]
- Bułakowska, A.; Sławiński, J.; Hering, A.; Gucwa, M.; Ochocka, J.R.; Hałasa, R.; Balewski, Ł.; Stefanowicz-Hajduk, J. New Chalcone Derivatives Containing 2,4-Dichlorobenzenesulfonamide Moiety with Anticancer and Antioxidant Properties. Int. J. Mol. Sci. 2023, 25, 274. [Google Scholar] [CrossRef] [PubMed]
- Kudličková, Z.; Michalková, R.; Salayová, A.; Ksiažek, M.; Vilková, M.; Bekešová, S.; Mojžiš, J. Design, Synthesis, and Evaluation of Novel Indole Hybrid Chalcones and Their Antiproliferative and Antioxidant Activity. Molecules 2023, 28, 6583. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N. Anticancer Activity of Natural and Synthetic Chalcones. Int. J. Mol. Sci. 2021, 22, 11306. [Google Scholar] [CrossRef] [PubMed]
- Michalkova, R.; Mirossay, L.; Kello, M.; Mojzisova, G.; Baloghova, J.; Podracka, A.; Mojzis, J. Anticancer Potential of Natural Chalcones: In Vitro and In Vivo Evidence. Int. J. Mol. Sci. 2023, 24, 10354. [Google Scholar] [CrossRef]
- Lindamulage, I.K.; Vu, H.-Y.; Karthikeyan, C.; Knockleby, J.; Lee, Y.-F.; Trivedi, P.; Lee, H. Novel Quinolone Chalcones Targeting Colchicine-Binding Pocket Kill Multidrug-Resistant Cancer Cells by Inhibiting Tubulin Activity and MRP1 Function. Sci. Rep. 2017, 7, 10298. [Google Scholar] [CrossRef]
- Silbermann, K.; Shah, C.P.; Sahu, N.U.; Juvale, K.; Stefan, S.M.; Kharkar, P.S.; Wiese, M. Novel Chalcone and Flavone Derivatives as Selective and Dual Inhibitors of the Transport Proteins ABCB1 and ABCG2. Eur. J. Med. Chem. 2019, 164, 193–213. [Google Scholar] [CrossRef]
- Peña-Solórzano, D.; Scholler, M.; Bernhardt, G.; Buschauer, A.; König, B.; Ochoa-Puentes, C. Tariquidar-Related Chalcones and Ketones as ABCG2 Modulators. ACS Med. Chem. Lett. 2018, 9, 854–859. [Google Scholar] [CrossRef]
- Kaczor, A.; Szemerédi, N.; Kucwaj-Brysz, K.; Dąbrowska, M.; Starek, M.; Latacz, G.; Spengler, G.; Handzlik, J. Computer-Aided Search for 5-Arylideneimidazolone Anticancer Agents Able To Overcome ABCB1-Based Multidrug Resistance. ChemMedChem 2021, 16, 2386–2401. [Google Scholar] [CrossRef] [PubMed]
- Gazdova, M.; Michalkova, R.; Kello, M.; Vilkova, M.; Kudlickova, Z.; Baloghova, J.; Mirossay, L.; Mojzis, J. Chalcone-Acridine Hybrid Suppresses Melanoma Cell Progression via G2/M Cell Cycle Arrest, DNA Damage, Apoptosis, and Modulation of MAP Kinases Activity. Int. J. Mol. Sci. 2022, 23, 12266. [Google Scholar] [CrossRef]
- Takac, P.; Kello, M.; Pilatova, M.B.; Kudlickova, Z.; Vilkova, M.; Slepcikova, P.; Petik, P.; Mojzis, J. New Chalcone Derivative Exhibits Antiproliferative Potential by Inducing G2/M Cell Cycle Arrest, Mitochondrial-Mediated Apoptosis and Modulation of MAPK Signalling Pathway. Chem. Biol. Interact. 2018, 292, 37–49. [Google Scholar] [CrossRef]
- Čižmáriková, M.; Takáč, P.; Spengler, G.; Kincses, A.; Nové, M.; Vilková, M.; Mojžiš, J. New Chalcone Derivative Inhibits ABCB1 in Multidrug Resistant T-Cell Lymphoma and Colon Adenocarcinoma Cells. Anticancer Res. 2019, 39, 6499–6505. [Google Scholar] [CrossRef] [PubMed]
- Bharathiraja, P.; Yadav, P.; Sajid, A.; Ambudkar, S.V.; Prasad, N.R. Natural Medicinal Compounds Target Signal Transduction Pathways to Overcome ABC Drug Efflux Transporter-Mediated Multidrug Resistance in Cancer. Drug Resist. Updates 2023, 71, 101004. [Google Scholar] [CrossRef]
- Dong, J.; Qin, Z.; Zhang, W.-D.; Cheng, G.; Yehuda, A.G.; Ashby, C.R.; Chen, Z.-S.; Cheng, X.-D.; Qin, J.-J. Medicinal Chemistry Strategies to Discover P-Glycoprotein Inhibitors: An Update. Drug Resist. Updates 2020, 49, 100681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tan, Y.; Li, T.; Tan, D.; Fu, B.; Yang, M.; Chen, Y.; Cao, M.; Xuan, C.; Du, Q.; et al. Intercellular Adhesion Molecule-1 Suppresses TMZ Chemosensitivity in Acquired TMZ-Resistant Gliomas by Increasing Assembly of ABCB1 on the Membrane. Drug Resist. Updates 2024, 76, 101112. [Google Scholar] [CrossRef]
- Ganesan, M.; Kanimozhi, G.; Pradhapsingh, B.; Khan, H.A.; Alhomida, A.S.; Ekhzaimy, A.; Brindha, G.; Prasad, N.R. Phytochemicals Reverse P-Glycoprotein Mediated Multidrug Resistance via Signal Transduction Pathways. Biomed. Pharmacother. 2021, 139, 111632. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, M.P.; Rigalli, J.P.; Ceré, L.I.; Semeniuk, M.; Catania, V.A.; Ruiz, M.L. ABC Transporters: Regulation and Association with Multidrug Resistance in Hepatocellular Carcinoma and Colorectal Carcinoma. Curr. Med. Chem. 2019, 26, 1224–1250. [Google Scholar] [CrossRef]
- Harazono, Y.; Kho, D.H.; Balan, V.; Nakajima, K.; Hogan, V.; Raz, A. Extracellular Galectin-3 Programs Multidrug Resistance through Na+/K+-ATPase and P-Glycoprotein Signaling. Oncotarget 2015, 6, 19592–19604. [Google Scholar] [CrossRef]
- Luo, W.; Song, L.; Chen, X.-L.; Zeng, X.-F.; Wu, J.-Z.; Zhu, C.-R.; Huang, T.; Tan, X.-P.; Lin, X.-M.; Yang, Q.; et al. Identification of Galectin-1 as a Novel Mediator for Chemoresistance in Chronic Myeloid Leukemia Cells. Oncotarget 2016, 7, 26709–26723. [Google Scholar] [CrossRef]
- Carabias, P.; Espelt, M.V.; Bacigalupo, M.L.; Rojas, P.; Sarrias, L.; Rubin, A.; Saffioti, N.A.; Elola, M.T.; Rossi, J.P.; Wolfenstein-Todel, C.; et al. Galectin-1 Confers Resistance to Doxorubicin in Hepatocellular Carcinoma Cells through Modulation of P-Glycoprotein Expression. Cell Death Dis. 2022, 13, 79. [Google Scholar] [CrossRef]
- Wang, F.; Lv, P.; Gu, Y.; Li, L.; Ge, X.; Guo, G. Galectin-1 Knockdown Improves Drug Sensitivity of Breast Cancer by Reducing P-Glycoprotein Expression through Inhibiting the Raf-1/AP-1 Signaling Pathway. Oncotarget 2017, 8, 25097–25106. [Google Scholar] [CrossRef]
- Sethi, A.; Sasikala, K.; Jakkula, P.; Gadde, D.; Sanam, S.; Qureshi, I.A.; Talla, V.; Alvala, M. Design, Synthesis and Computational Studies Involving Indole-Coumarin Hybrids as Galectin-1 Inhibitors. Chem. Pap. 2021, 75, 2791–2805. [Google Scholar] [CrossRef]
- Luís, C.; Costa, R.; Rodrigues, I.; Castela, Â.; Coelho, P.; Guerreiro, S.; Gomes, J.; Reis, C.; Soares, R. Xanthohumol and 8-Prenylnaringenin Reduce Type 2 Diabetes–Associated Oxidative Stress by Downregulating Galectin-3. Porto Biomed. J. 2019, 4, e23. [Google Scholar] [CrossRef]
- Vilková, M.; Michalková, R.; Kello, M.; Sabolová, D.; Takáč, P.; Kudličková, Z.; Garberová, M.; Tvrdoňová, M.; Béres, T.; Mojžiš, J. Discovery of Novel Acridine-Chalcone Hybrids with Potent DNA Binding and Antiproliferative Activity against MDA-MB-231 and MCF-7 Cells. Med. Chem. Res. 2022, 31, 1323–1338. [Google Scholar] [CrossRef]
- Salanci, Š.; Vilková, M.; Martinez, L.; Mirossay, L.; Michalková, R.; Mojžiš, J. The Induction of G2/M Phase Cell Cycle Arrest and Apoptosis by the Chalcone Derivative 1C in Sensitive and Resistant Ovarian Cancer Cells Is Associated with ROS Generation. Int. J. Mol. Sci. 2024, 25, 7541. [Google Scholar] [CrossRef] [PubMed]
- Michalkova, R.; Mirossay, L.; Gazdova, M.; Kello, M.; Mojzis, J. Molecular Mechanisms of Antiproliferative Effects of Natural Chalcones. Cancers 2021, 13, 2730. [Google Scholar] [CrossRef]
- Sakagami, H.; Masuda, Y.; Tomomura, M.; Yokose, S.; Uesawa, Y.; Ikezoe, N.; Asahara, D.; Takao, K.; Kanamoto, T.; Terakubo, S.; et al. Quantitative Structure-Cytotoxicity Relationship of Chalcones. Anticancer Res. 2017, 37, 1091–1098. [Google Scholar] [CrossRef]
- Michalkova, R.; Kello, M.; Kudlickova, Z.; Gazdova, M.; Mirossay, L.; Mojzisova, G.; Mojzis, J. Programmed Cell Death Alterations Mediated by Synthetic Indole Chalcone Resulted in Cell Cycle Arrest, DNA Damage, Apoptosis and Signaling Pathway Modulations in Breast Cancer Model. Pharmaceutics 2022, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Callejas, G.; Piñeros-Avila, M.; Celis, C.A.; Torrenegra, R.; Espinosa-Benitez, A.; Pestana-Nobles, R.; Yosa-Reyes, J. Natural 2′,4-Dihydroxy-4′,6′-Dimethoxy Chalcone Isolated from Chromolaena Tacotana Inhibits Breast Cancer Cell Growth through Autophagy and Mitochondrial Apoptosis. Plants 2024, 13, 570. [Google Scholar] [CrossRef]
- Mendez-Callejas, G.; Piñeros-Avila, M.; Yosa-Reyes, J.; Pestana-Nobles, R.; Torrenegra, R.; Camargo-Ubate, M.F.; Bello-Castro, A.E.; Celis, C.A. A Novel Tri-Hydroxy-Methylated Chalcone Isolated from Chromolaena Tacotana with Anti-Cancer Potential Targeting Pro-Survival Proteins. Int. J. Mol. Sci. 2023, 24, 15185. [Google Scholar] [CrossRef]
- Kashmiry, A.A.; Ibrahim, N.S.; Mohamed, M.F.; Abdelhamid, I.A. Novel α-Cyano-Indolyl Chalcones as Anti-Cancer Candidates, Induce G1/S Cell Cycle Arrest and Sequentially Activate Caspases-7, 8, and 9 in Breast Carcinoma. Polycycl. Aromat. Compd. 2024, 1–20. [Google Scholar] [CrossRef]
- Ragheb, M.A.; Abdelrashid, H.E.; Elzayat, E.M.; Abdelhamid, I.A.; Soliman, M.H. Novel Cyanochalcones as Potential Anticancer Agents: Apoptosis, Cell Cycle Arrest, DNA Binding, and Molecular Docking Studies. J. Biomol. Struct. Dyn. 2024, 1–19. [Google Scholar] [CrossRef]
- Zeid, M.M.; El-Badry, O.M.; Elmeligie, S.; Hassan, R.A. Design, Synthesis, and Molecular Docking of Novel Miscellaneous Chalcones as P38α Mitogen-Activated Protein Kinase Inhibitors. Chem. Biodivers. 2024, 21, e202400077. [Google Scholar] [CrossRef]
- Xu, S.; Chen, M.; Chen, W.; Hui, J.; Ji, J.; Hu, S.; Zhou, J.; Wang, Y.; Liang, G. Chemopreventive Effect of Chalcone Derivative, L2H17, in Colon Cancer Development. BMC Cancer 2015, 15, 870. [Google Scholar] [CrossRef] [PubMed]
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Rychlicka, M.; Sordon, S.; Filip-Psurska, B.; Milczarek, M.; Wietrzyk, J.; Huszcza, E. Prenylated Flavonoids with Selective Toxicity against Human Cancers. Int. J. Mol. Sci. 2023, 24, 7408. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw-Pierce, E.L.; Pitts, T.M.; Tan, A.-C.; McPhillips, K.; West, M.; Gustafson, D.L.; Halsey, C.; Nguyen, L.; Lee, N.V.; Kan, J.L.C.; et al. Tumor P-Glycoprotein Correlates with Efficacy of PF-3758309 in in Vitro and in Vivo Models of Colorectal Cancer. Front. Pharmacol. 2013, 4, 22. [Google Scholar] [CrossRef]
- The Human Protein Atlas ABCB1. Available online: https://www.proteinatlas.org/ensg00000085563-abcb1/cell+line (accessed on 3 March 2025).
- Komoto, T.T.; Bernardes, T.M.; Mesquita, T.B.; Bortolotto, L.F.B.; Silva, G.; Bitencourt, T.A.; Baek, S.J.; Marins, M.; Fachin, A.L. Chalcones Repressed the AURKA and MDR Proteins Involved in Metastasis and Multiple Drug Resistance in Breast Cancer Cell Lines. Molecules 2018, 23, 2018. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, L.; Yan, M.; Wu, J.; Liu, Y.; Tian, X.; Jiang, W.; Zhang, L.; Wang, R. Activity and Mechanism of Flavokawain A in Inhibiting P-Glycoprotein Expression in Paclitaxel Resistance of Lung Cancer. Oncol. Lett. 2020, 19, 379–387. [Google Scholar] [CrossRef]
- Cai, C.-Y.; Zhang, W.; Wang, J.-Q.; Lei, Z.-N.; Zhang, Y.-K.; Wang, Y.-J.; Gupta, P.; Tan, C.-P.; Wang, B.; Chen, Z.-S. Biological Evaluation of Non-Basic Chalcone CYB-2 as a Dual ABCG2/ABCB1 Inhibitor. Biochem. Pharmacol. 2020, 175, 113848. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Ablise, M.; Maimaiti, A.; Mutalipu, Z.; Yan, T.; Liu, Z.-Y.; Aihaiti, A. Design, Synthesis, and Ex Vivo Anti-Drug Resistant Cervical Cancer Activity of Novel Molecularly Targeted Chalcone Derivatives. Bioorg. Chem. 2024, 149, 107498. [Google Scholar] [CrossRef]
- Seelig, A.; Li-Blatter, X. P-Glycoprotein (ABCB1)—Weak Dipolar Interactions Provide the Key to Understanding Allocrite Recognition, Binding, and Transport. Cancer Drug Resist. 2022, 6, 1–29. [Google Scholar] [CrossRef]
- Rahman, H.; Ware, M.J.; Sajid, A.; Lusvarghi, S.; Durell, S.R.; Ambudkar, S.V. Residues from Homologous Transmembrane Helices 4 and 10 Are Critical for P-Glycoprotein (ABCB1)-Mediated Drug Transport. Cancers 2023, 15, 3459. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Bahadduri, P.M.; Polli, J.E.; Swaan, P.W.; Ekins, S. Rapid Identification of P-Glycoprotein Substrates and Inhibitors. Drug Metab. Dispos. 2006, 34, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacother. apy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef] [PubMed]
- Nabekura, T.; Hiroi, T.; Kawasaki, T.; Uwai, Y. Effects of Natural Nuclear Factor-Kappa B Inhibitors on Anticancer Drug Efflux Transporter Human P-Glycoprotein. Biomed. Pharmacother. 2015, 70, 140–145. [Google Scholar] [CrossRef]
- Rajagopal, K.; Kalusalingam, A.; Bharathidasan, A.R.; Sivaprakash, A.; Shanmugam, K.; Sundaramoorthy, M.; Byran, G. In Silico Drug Design of Anti-Breast Cancer Agents. Molecules 2023, 28, 4175. [Google Scholar] [CrossRef]
- Montanari, F.; Ecker, G.F. Prediction of Drug–ABC-Transporter Interaction—Recent Advances and Future Challenges. Adv. Drug Deliv. Rev. 2015, 86, 17–26. [Google Scholar] [CrossRef]
- Schäfer, J.; Klösgen, V.J.; Omer, E.A.; Kadioglu, O.; Mbaveng, A.T.; Kuete, V.; Hildebrandt, A.; Efferth, T. In Silico and In Vitro Identification of P-Glycoprotein Inhibitors from a Library of 375 Phytochemicals. Int. J. Mol. Sci. 2023, 24, 10240. [Google Scholar] [CrossRef]
- Wankhede, Y.S.; Khairnar, V.V.; Patil, A.R.; Darekar, A.B. Drug Discovery Tools and In Silico Techniques: A Review. Int. J. Pharm. Sci. Rev. Res. 2024, 84, 63–72. [Google Scholar] [CrossRef]
- Marques, S.M.; Šupolíková, L.; Molčanová, L.; Šmejkal, K.; Bednar, D.; Slaninová, I. Screening of Natural Compounds as P-Glycoprotein Inhibitors against Multidrug Resistance. Biomedicines 2021, 9, 357. [Google Scholar] [CrossRef]
- Ferreira, R.J.; Ferreira, M.-J.U.; dos Santos, D.J.V.A. Molecular Docking Characterizes Substrate-Binding Sites and Efflux Modulation Mechanisms within P-Glycoprotein. J. Chem. Inf. Model. 2013, 53, 1747–1760. [Google Scholar] [CrossRef]
- Zhao, X.; Di, J.; Luo, D.; Vaishnav, Y.; Kamal; Nuralieva, N.; Verma, D.; Verma, P.; Verma, S. Recent Developments of P-Glycoprotein Inhibitors and Its Structure–Activity Relationship (SAR) Studies. Bioorg. Chem. 2024, 143, 106997. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yuan, L.; Hu, C.; Cheng, X.; Qin, J.-J. Strategies to Overcome Cancer Multidrug Resistance (MDR) through Targeting P-Glycoprotein (ABCB1): An Updated Review. Pharmacol. Ther. 2023, 249, 108488. [Google Scholar] [CrossRef]
- Xing, J.; Huang, S.; Heng, Y.; Mei, H.; Pan, X. Computational Insights into Allosteric Conformational Modulation of P-Glycoprotein by Substrate and Inhibitor Binding. Molecules 2020, 25, 6006. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liang, F.; Xia, M.; Cao, W.; Pan, S.; Wu, T.; Xu, X. Structure-Activity Relationship and Mechanism of Flavonoids on the Inhibitory Activity of P-Glycoprotein (P-Gp)-Mediated Transport of Rhodamine123 and Daunorubicin in P-Gp Overexpressed Human Mouth Epidermal Carcinoma (KB/MDR) Cells. Food Chem. Toxicol. 2021, 155, 112381. [Google Scholar] [CrossRef] [PubMed]
- Bonito, C.A.; Ferreira, R.J.; Ferreira, M.-J.U.; Durães, F.; Sousa, E.; Gillet, J.-P.; Cordeiro, M.N.D.S.; dos Santos, D.J.V.A. Probing the Allosteric Modulation of P-Glycoprotein: A Medicinal Chemistry Approach Toward the Identification of Noncompetitive P-Gp Inhibitors. ACS Omega 2023, 8, 11281–11287. [Google Scholar] [CrossRef]
- Sajid, A.; Rahman, H.; Ambudkar, S.V. Advances in the Structure, Mechanism and Targeting of Chemoresistance-Linked ABC Transporters. Nat. Rev. Cancer 2023, 23, 762–779. [Google Scholar] [CrossRef]
- Thomas, C.; Tampé, R. Structural and Mechanistic Principles of ABC Transporters. Annu. Rev. Biochem. 2020, 89, 605–636. [Google Scholar] [CrossRef]
- Liu, M.; Yin, H.; Qian, X.; Dong, J.; Qian, Z.; Miao, J. Xanthohumol, a Prenylated Chalcone from Hops, Inhibits the Viability and Stemness of Doxorubicin-Resistant MCF-7/ADR Cells. Molecules 2016, 22, 36. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Wink, M. Reversal of Multidrug Resistance in Human Colon Cancer and Human Leukemia Cells by Three Plant Extracts and Their Major Secondary Metabolites. Medicines 2018, 5, 123. [Google Scholar] [CrossRef]
- Navarro, P.; Martínez-Bosch, N.; Blidner, A.G.; Rabinovich, G.A. Impact of Galectins in Resistance to Anticancer Therapies. Clin. Cancer Res. 2020, 26, 6086–6101. [Google Scholar] [CrossRef]
- Corral, J.M.; Puerto-Nevado, L.d.; Cedeño, M.; Río-Vilariño, A.; Mahillo-Fernández, I.; Galeano, C.; Baños, N.; García-Foncillas, J.; Dómine, M.; Cebrián, A. Galectin-1, a Novel Promising Target for Outcome Prediction and Treatment in SCLC. Biomed. Pharmacother. 2022, 156, 113987. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.S.; Mohammed, N.B.B.; Dimitroff, C.J. Decoding Strategies to Evade Immunoregulators Galectin-1, -3, and -9 and Their Ligands as Novel Therapeutics in Cancer Immunotherapy. Int. J. Mol. Sci. 2022, 23, 15554. [Google Scholar] [CrossRef]
- Bannoud, N.; Stupirski, J.C.; Cagnoni, A.J.; Hockl, P.F.; Pérez Sáez, J.M.; García, P.A.; Mahmoud, Y.D.; Gambarte Tudela, J.; Scheidegger, M.A.; Marshall, A.; et al. Circulating Galectin-1 Delineates Response to Bevacizumab in Melanoma Patients and Reprograms Endothelial Cell Biology. Proc. Natl. Acad. Sci. USA 2023, 120, 2214350120. [Google Scholar] [CrossRef]
- Carvalho, L.V.d.N.; Assis, R.A.; Montenegro, C.; da Rosa, M.M.; Pereira, M.C.; Pitta, M.G.d.R.; de Melo Rêgo, M.J.B. Galectin Plasmatic Levels Reveal a Cluster Associated with Disease Aggressiveness and Kidney Damage in Multiple Myeloma Patients. Int. J. Mol. Sci. 2024, 25, 13499. [Google Scholar] [CrossRef]
- Herman, K.D.; Holyer, I.; Humphries, D.C.; Adamska, A.; Roper, J.A.; Peterson, K.; Zetterberg, F.R.; Pedersen, A.; MacKinnon, A.C.; Slack, R.J. Galectin-1 Induces the Production of Immune-Suppressive Cytokines in Human and Mouse T Cells. Int. J. Mol. Sci. 2024, 25, 11948. [Google Scholar] [CrossRef] [PubMed]
- Mimura, S.; Morishita, A.; Oura, K.; Takuma, K.; Nakahara, M.; Tadokoro, T.; Fujita, K.; Tani, J.; Kobara, H. Galectins and Liver Diseases. Int. J. Mol. Sci. 2025, 26, 790. [Google Scholar] [CrossRef]
- Wu, K.L.; Chen, H.H.; Pen, C.T.; Yeh, W.L.; Huang, E.Y.; Hsiao, C.C.; Yang, K.D. Circulating Galectin-1 and 90K/Mac-2BP Correlated with the Tumor Stages of Patients with Colorectal Cancer. BioMed Res. Int. 2015, 2015, 306964. [Google Scholar] [CrossRef] [PubMed]
- Cagnoni, A.J.; Giribaldi, M.L.; Blidner, A.G.; Cutine, A.M.; Gatto, S.G.; Morales, R.M.; Salatino, M.; Abba, M.C.; Croci, D.O.; Mariño, K.V.; et al. Galectin-1 Fosters an Immunosuppressive Microenvironment in Colorectal Cancer by Reprogramming CD8+ Regulatory T Cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2102950118. [Google Scholar] [CrossRef]
- Peng, K.-Y.; Jiang, S.-S.; Lee, Y.-W.; Tsai, F.-Y.; Chang, C.-C.; Chen, L.-T.; Yen, B.L. Stromal Galectin-1 Promotes Colorectal Cancer Cancer-Initiating Cell Features and Disease Dissemination Through SOX9 and β-Catenin: Development of Niche-Based Biomarkers. Front. Oncol. 2021, 11, 716055. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Lin, T.-H.; Chang, C.-F.; Lo, Y.-L. Galectin-3 Silencing Inhibits Epirubicin-Induced ATP Binding Cassette Transporters and Activates the Mitochondrial Apoptosis Pathway via β-Catenin/GSK-3β Modulation in Colorectal Carcinoma. PLoS ONE 2013, 8, e82478. [Google Scholar] [CrossRef]
- Dings, R.; Miller, M.; Griffin, R.; Mayo, K. Galectins as Molecular Targets for Therapeutic Intervention. Int. J. Mol. Sci. 2018, 19, 905. [Google Scholar] [CrossRef]
- Goud, N.S.; Bhattacharya, A. Human Galectin-1 in Multiple Cancers: A Privileged Molecular Target in Oncology. Mini-Rev. Med. Chem. 2021, 21, 2169–2186. [Google Scholar] [CrossRef] [PubMed]
- Laderach, D.J.; Compagno, D. Inhibition of Galectins in Cancer: Biological Challenges for Their Clinical Application. Front. Immunol. 2023, 13, 1104625. [Google Scholar] [CrossRef]
- Tarighat, S.S.; Fei, F.; Joo, E.J.; Abdel-Azim, H.; Yang, L.; Geng, H.; Bum-Erdene, K.; Grice, I.D.; von Itzstein, M.; Blanchard, H.; et al. Overcoming Microenvironment-Mediated Chemoprotection through Stromal Galectin-3 Inhibition in Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021, 22, 12167. [Google Scholar] [CrossRef] [PubMed]
- Upreti, M.; Jyoti, A.; Johnson, S.E.; Swindell, E.P.; Napier, D.; Sethi, P.; Chan, R.; Feddock, J.M.; Weiss, H.L.; O’Halloran, T.V.; et al. Radiation-Enhanced Therapeutic Targeting of Galectin-1 Enriched Malignant Stroma in Triple Negative Breast Cancer. Oncotarget 2016, 7, 41559–41574. [Google Scholar] [CrossRef]
- Nambiar, D.K.; Aguilera, T.; Cao, H.; Kwok, S.; Kong, C.; Bloomstein, J.; Wang, Z.; Rangan, V.S.; Jiang, D.; von Eyben, R.; et al. Galectin-1–Driven T Cell Exclusion in the Tumor Endothelium Promotes Immunotherapy Resistance. J. Clin. Investig. 2019, 129, 5553–5567. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas LGALS1. Available online: https://www.proteinatlas.org/ensg00000100097-lgals1/cell+line (accessed on 3 March 2025).
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Rabinovich, G.A.; Bieche, I.; Vidaud, M.; de Gramont, A.; Martinet, M.; Cvitkovic, E.; et al. OTX008, a Selective Small-Molecule Inhibitor of Galectin-1, Downregulates Cancer Cell Proliferation, Invasion and Tumour Angiogenesis. Eur. J. Cancer 2014, 50, 2463–2477. [Google Scholar] [CrossRef]
- Kocibalova, Z.; Guzyova, M.; Borovska, I.; Messingerova, L.; Copakova, L.; Sulova, Z.; Breier, A. Development of Multidrug Resistance in Acute Myeloid Leukemia Is Associated with Alterations of the LPHN1/GAL-9/TIM-3 Signaling Pathway. Cancers 2021, 13, 3629. [Google Scholar] [CrossRef]
- Abudu, O.; Nguyen, D.; Millward, I.; Manning, J.E.; Wahid, M.; Lightfoot, A.; Marcon, F.; Merard, R.; Margielewska-Davies, S.; Roberts, K.; et al. Interplay in Galectin Expression Predicts Patient Outcomes in a Spatially Restricted Manner in PDAC. Biomed. Pharmacother. 2024, 172, 116283. [Google Scholar] [CrossRef]
- EFLUXX-ID® Green Multidrug Resistance Assay Kit. Available online: https://www.enzo.com/product/efluxx-id-green-multidrug-resistance-assay-kit/ (accessed on 3 March 2025).
- Lebedeva, I.V.; Pande, P.; Patton, W.F. Sensitive and Specific Fluorescent Probes for Functional Analysis of the Three Major Types of Mammalian ABC Transporters. PLoS ONE 2011, 6, e22429. [Google Scholar] [CrossRef]
- Krawczenko, A.; Bielawska-Pohl, A.; Wojtowicz, K.; Jura, R.; Paprocka, M.; Wojdat, E.; Kozłowska, U.; Klimczak, A.; Grillon, C.; Kieda, C.; et al. Expression and Activity of Multidrug Resistance Proteins in Mature Endothelial Cells and Their Precursors: A Challenging Correlation. PLoS ONE 2017, 12, e0172371. [Google Scholar] [CrossRef]
- Kosztyu, P.; Dolezel, P.; Vaclavikova, R.; Mlejnek, P. Can the Assessment of ABCB1 Gene Expression Predict Its Function in Vitro? Eur. J. Haematol. 2015, 95, 150–159. [Google Scholar] [CrossRef]
- Shchulkin, A.V.; Abalenikhina, Y.V.; Kosmachevskaya, O.V.; Topunov, A.F.; Yakusheva, E.N. Regulation of P-Glycoprotein during Oxidative Stress. Antioxidants 2024, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.G.; Weiskirchen, R. Advanced In Vitro Models for Preclinical Drug Safety: Recent Progress and Prospects. Curr. Issues Mol. Biol. 2024, 47, 7. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.; Kulin, A.; Jezsó, B.; Kucsma, N.; Sarkadi, B.; Várady, G. Selective Fluorescent Probes for High-Throughput Functional Diagnostics of the Human Multidrug Transporter P-Glycoprotein (ABCB1). Int. J. Mol. Sci. 2022, 23, 10599. [Google Scholar] [CrossRef] [PubMed]
- Hellewell, L.; Bhakta, S. Chalcones, Stilbenes and Ketones Have Anti-Infective Properties via Inhibition of Bacterial Drug-Efflux and Consequential Synergism with Antimicrobial Agents. Access Microbiol. 2020, 2, acmi000105. [Google Scholar] [CrossRef]
- Freitas, T.S.; Xavier, J.C.; Pereira, R.L.S.; Rocha, J.E.; Campina, F.F.; de Araújo Neto, J.B.; Silva, M.M.C.; Barbosa, C.R.S.; Marinho, E.S.; Nogueira, C.E.S.; et al. In Vitro and in Silico Studies of Chalcones Derived from Natural Acetophenone Inhibitors of NorA and MepA Multidrug Efflux Pumps in Staphylococcus Aureus. Microb. Pathog. 2021, 161, 105286. [Google Scholar] [CrossRef]
- Le, M.-T.; Trinh, D.-T.T.; Ngo, T.-D.; Tran-Nguyen, V.-K.; Nguyen, D.-N.; Hoang, T.; Nguyen, H.-M.; Do, T.-G.-S.; Mai, T.T.; Tran, T.-D.; et al. Chalcone Derivatives as Potential Inhibitors of P-Glycoprotein and NorA: An In Silico and In Vitro Study. BioMed Res. Int. 2022, 26, 9982453. [Google Scholar] [CrossRef]
- Silva, J.; Esmeraldo Rocha, J.; da Cunha Xavier, J.; Sampaio de Freitas, T.; Douglas Melo Coutinho, H.; Nogueira Bandeira, P.; Rodrigues de Oliveira, M.; Nunes da Rocha, M.; Machado Marinho, E.; de Kassio Vieira Monteiro, N.; et al. Antibacterial and Antibiotic Modifying Activity of Chalcone (2E)-1-(4′-Aminophenyl)-3-(4-Methoxyphenyl)-Prop-2-En-1-One in Strains of Staphylococcus Aureus Carrying NorA and MepA Efflux Pumps: In Vitro and in Silico Approaches. Microb. Pathog. 2022, 169, 105664. [Google Scholar] [CrossRef]
- Rezende-Júnior, L.M.; Andrade, L.M.d.S.; Leal, A.L.A.B.; Mesquita, A.B.d.S.; Santos, A.L.P.d.A.d.; Neto, J.d.S.L.; Siqueira-Júnior, J.P.; Nogueira, C.E.S.; Kaatz, G.W.; Coutinho, H.D.M.; et al. Chalcones Isolated from Arrabidaea Brachypoda Flowers as Inhibitors of NorA and MepA Multidrug Efflux Pumps of Staphylococcus Aureus. Antibiotics 2020, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, Y.; Xiong, Q.; Zhu, K.; Weng, N.; Zhu, Q. The Role of Artificial Intelligence in the Development of Anticancer Therapeutics from Natural Polyphenols: Current Advances and Future Prospects. Pharmacol. Res. 2024, 208, 107381. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein Structure and Function Prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Bolton, E.E.; Chen, J.; Kim, S.; Han, L.; He, S.; Shi, W.; Simonyan, V.; Sun, Y.; Thiessen, P.A.; Wang, J.; et al. PubChem3D: A New Resource for Scientists. J. Cheminform. 2011, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Jakalian, A.; Bush, B.L.; Jack, D.B.; Bayly, C.I. Fast, Efficient Generation of High-Quality Atomic Charges. AM1-BCC Model: I. Method. J. Comput. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, Efficient Generation of High-quality Atomic Charges. AM1-BCC Model: II. Parameterization and Validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef]
- Case Ross, C.; Walker, D.A.; Darden Junmei Wang, T. Amber 2016 Reference Manual Principal Contributors to the Current Codes. Available online: https://ambermd.org/doc12/Amber16.pdf (accessed on 10 December 2024).
- Sanner, M. Python: A Programming Language for Software Integration and Development. J. Mol. Graph. Model 1999, 7, 57–61. [Google Scholar] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Re: [PyMOL] PyMOL 2.3 Released|PyMOL Molecular Graphics System. Available online: https://sourceforge.net/p/pymol/mailman/message/36586593/ (accessed on 10 December 2024).
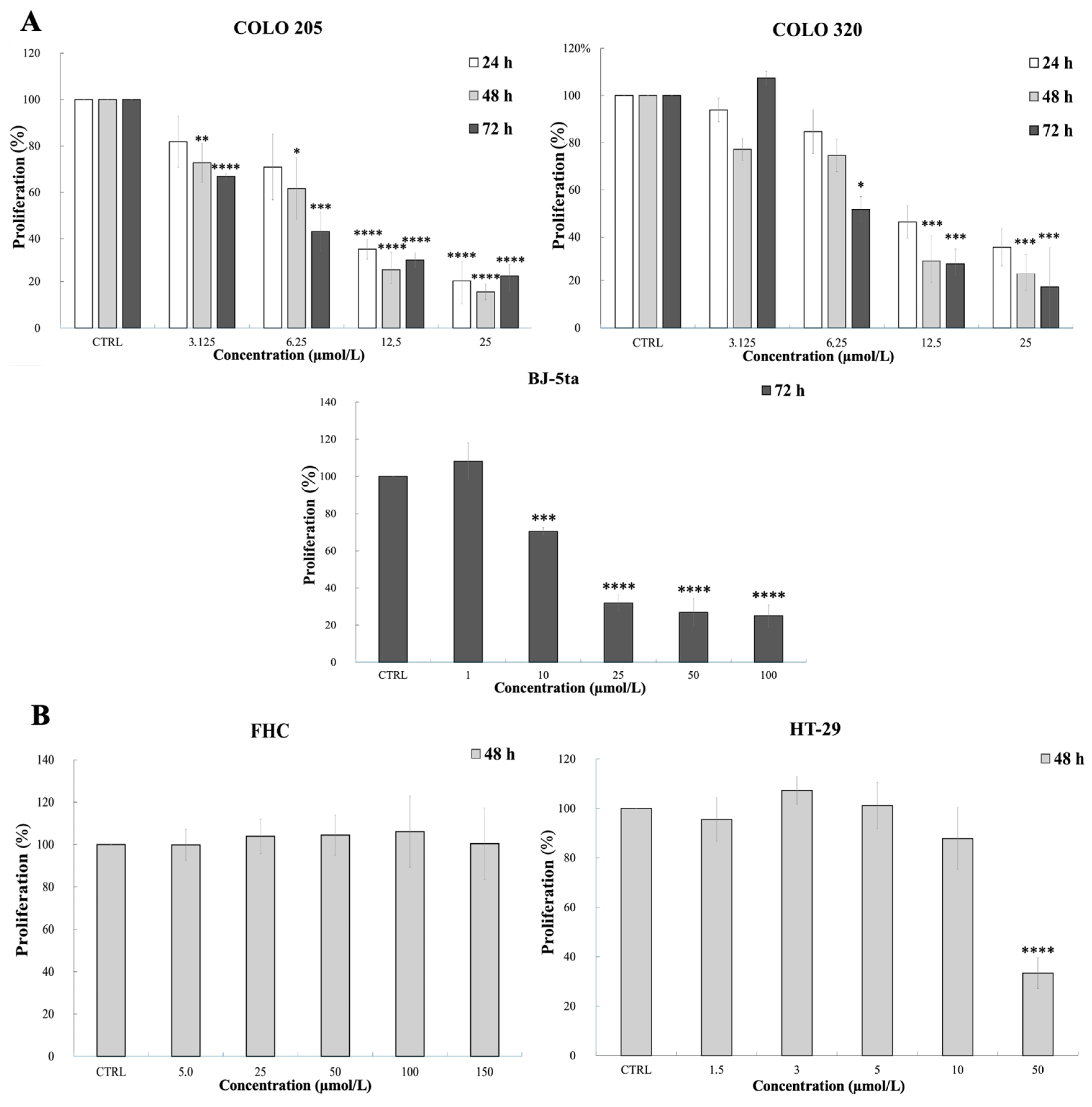

| MTT Assay (72 h) | SRB Assay (48 h) | ||||
|---|---|---|---|---|---|
| BJ-5ta | COLO 205 | COLO 320 | FHC | HT-29 | |
| IC50 (µmol/L) | 18.03 ± 1.23 | 5.34 ± 0.11 | 6.69 ± 0.7 | >150 | 37.89 ± 0.63 |
| p-value | Ref. | <0.0001 | <0.0001 | Ref. | <0.0001 |
| Selectivity index | - | 3.68 ± 0.16 | 2.71 ± 0.17 | - | 3.96 ± 0.07 |
| Gene | Time (hours) | Mean | STDEV | p-Value | p-Value adjusted | RQ |
|---|---|---|---|---|---|---|
| ABCB1 | 24 | 1.17 | 0.05 | 0.002 | 0.013 | 0.44 |
| 48 | 0.68 | 0.05 | 0.308 | 1 | 0.62 | |
| ABCC1 | 24 | −0.21 | 0.12 | 0.308 | 1 | 1.16 |
| 48 | −0.72 | 0.32 | 0.213 | 1 | 1.65 | |
| ABCG2 | 24 | 0.62 | 0.09 | 1 | 1 | 0.65 |
| 48 | −0.26 | 0.02 | 1 | 1 | 1.20 |
| Antibody | MW (kDa) | Origin | Dilution | Catalog No. | Manufacturer |
|---|---|---|---|---|---|
| Anti-ABCB1 | 180 | Rabbit | 1:100 | ab170904 | Abcam, Cambridge, UK |
| Anti-Rabbit IgG Alexa Fluor™ 488 | - | Goat | 1:500 | A11008 | Thermo Fisher Scientific, Waltham, MA, USA |
| Antibody | MW (kDa) | Origin | Dilution | Catalog No. | Manufacturer |
|---|---|---|---|---|---|
| Anti-ABCB1 | 180 | Rabbit | 1:1000 | ab170904 | Abcam, Cambridge, UK |
| Anti-ABCC1 | 170–250 | Rabbit | 1:1000 | ab233383 | Abcam, Cambridge, UK |
| Anti-ABCG2 | 75 | Rabbit | 1:1000 | ab207732 | Abcam, Cambridge, UK |
| Anti-Galectin 1 | 15 | Rabbit | 1:2000 | 11858-1-AP | Proteintech, Manchester, UK |
| β-actin | 45 | Mouse | 1:1000 | 3700S | Cell Signaling, Danvers, MA, USA |
| Anti-Rabbit HRP | - | Goat | 1:1000 | 7074S | Cell Signaling, Danvers, MA, USA |
| Anti-Mouse HRP | - | Horse | 1:1000 | 7076S | Cell Signaling, Danvers, MA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franko, O.; Čižmáriková, M.; Kello, M.; Michalková, R.; Wesołowska, O.; Środa-Pomianek, K.; Marques, S.M.; Bednář, D.; Háziková, V.; Liška, T.J.; et al. Acridine-Based Chalcone 1C and ABC Transporters. Int. J. Mol. Sci. 2025, 26, 4138. https://doi.org/10.3390/ijms26094138
Franko O, Čižmáriková M, Kello M, Michalková R, Wesołowska O, Środa-Pomianek K, Marques SM, Bednář D, Háziková V, Liška TJ, et al. Acridine-Based Chalcone 1C and ABC Transporters. International Journal of Molecular Sciences. 2025; 26(9):4138. https://doi.org/10.3390/ijms26094138
Chicago/Turabian StyleFranko, Ondrej, Martina Čižmáriková, Martin Kello, Radka Michalková, Olga Wesołowska, Kamila Środa-Pomianek, Sérgio M. Marques, David Bednář, Viktória Háziková, Tomáš Ján Liška, and et al. 2025. "Acridine-Based Chalcone 1C and ABC Transporters" International Journal of Molecular Sciences 26, no. 9: 4138. https://doi.org/10.3390/ijms26094138
APA StyleFranko, O., Čižmáriková, M., Kello, M., Michalková, R., Wesołowska, O., Środa-Pomianek, K., Marques, S. M., Bednář, D., Háziková, V., Liška, T. J., & Habalová, V. (2025). Acridine-Based Chalcone 1C and ABC Transporters. International Journal of Molecular Sciences, 26(9), 4138. https://doi.org/10.3390/ijms26094138







