Impact of Tire-Derived Microplastics on Microbiological Activity of Aerobic Granular Sludge
Abstract
1. Introduction
2. Results and Discussion
2.1. Impact of TWP on Nitrogen Compound Transformations in GSBRs
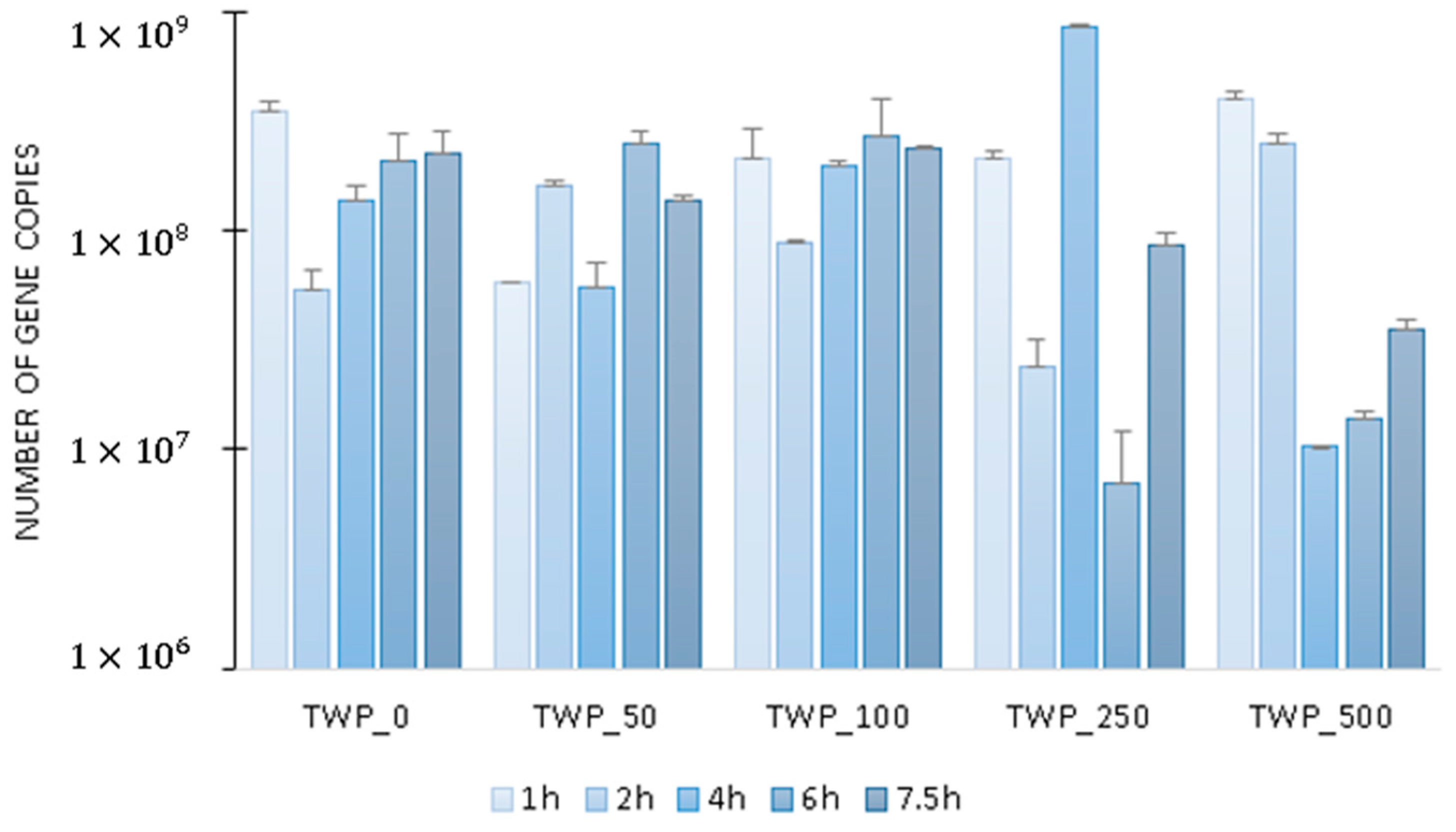
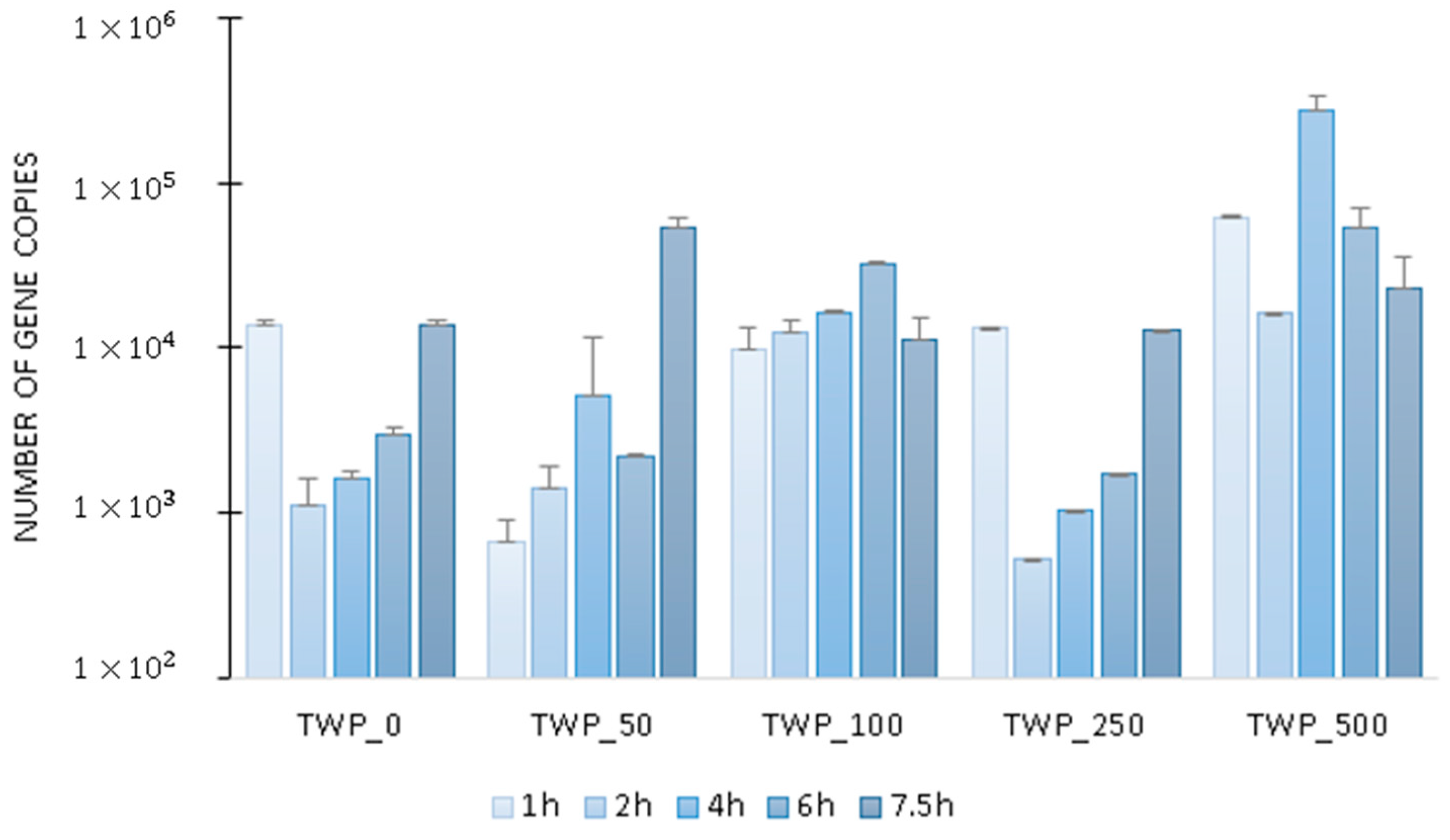
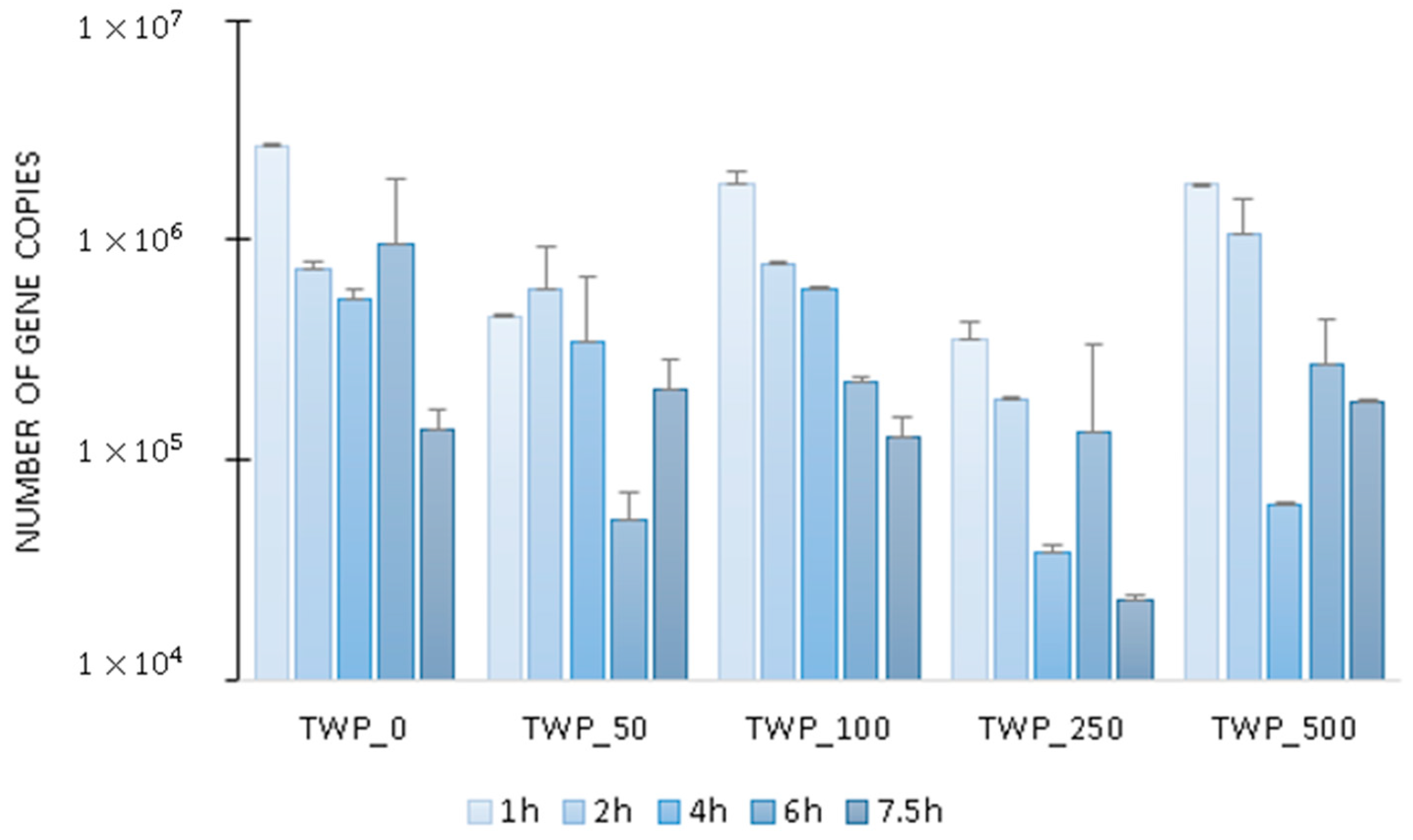
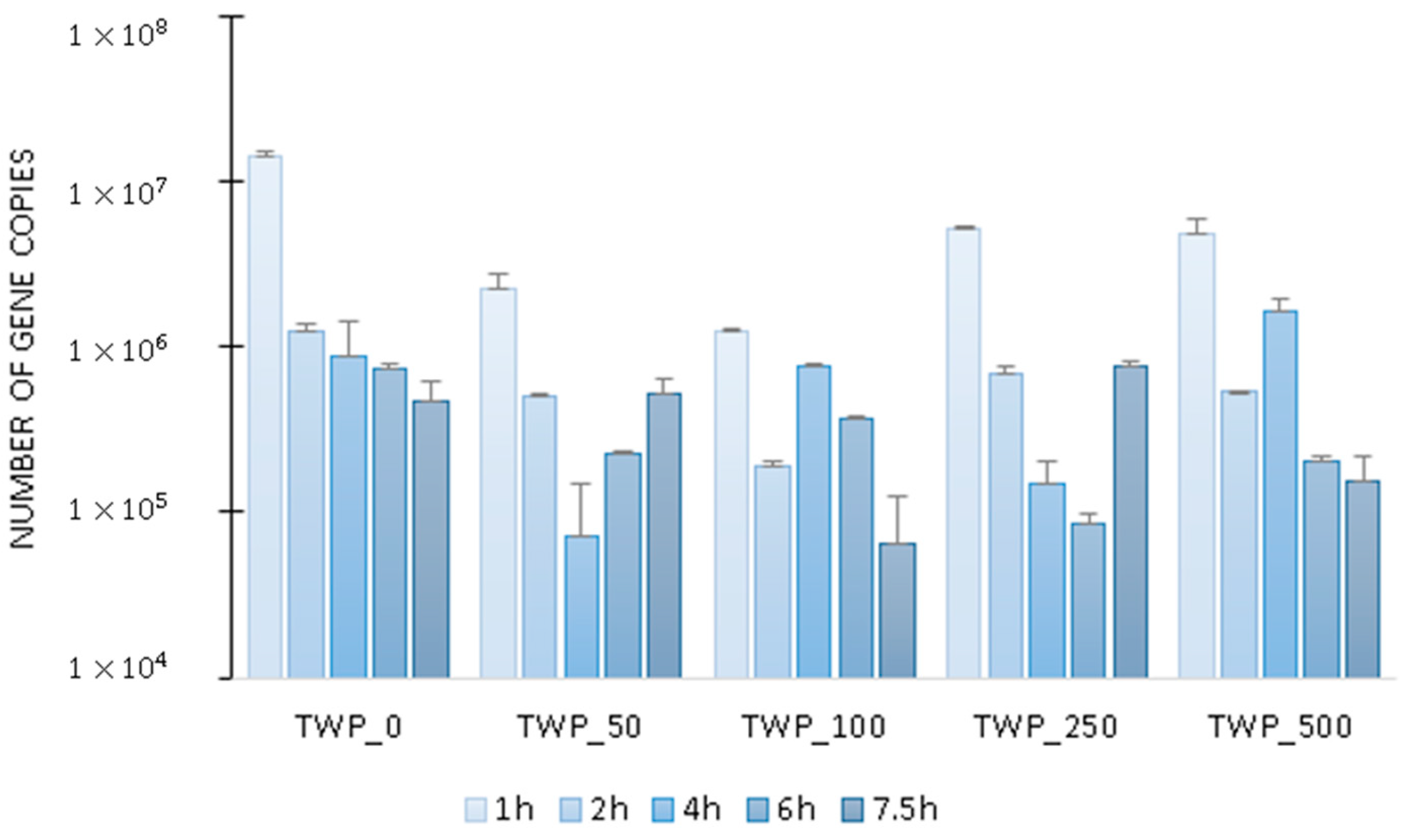
2.2. Microbial Activity
3. Materials and Methods
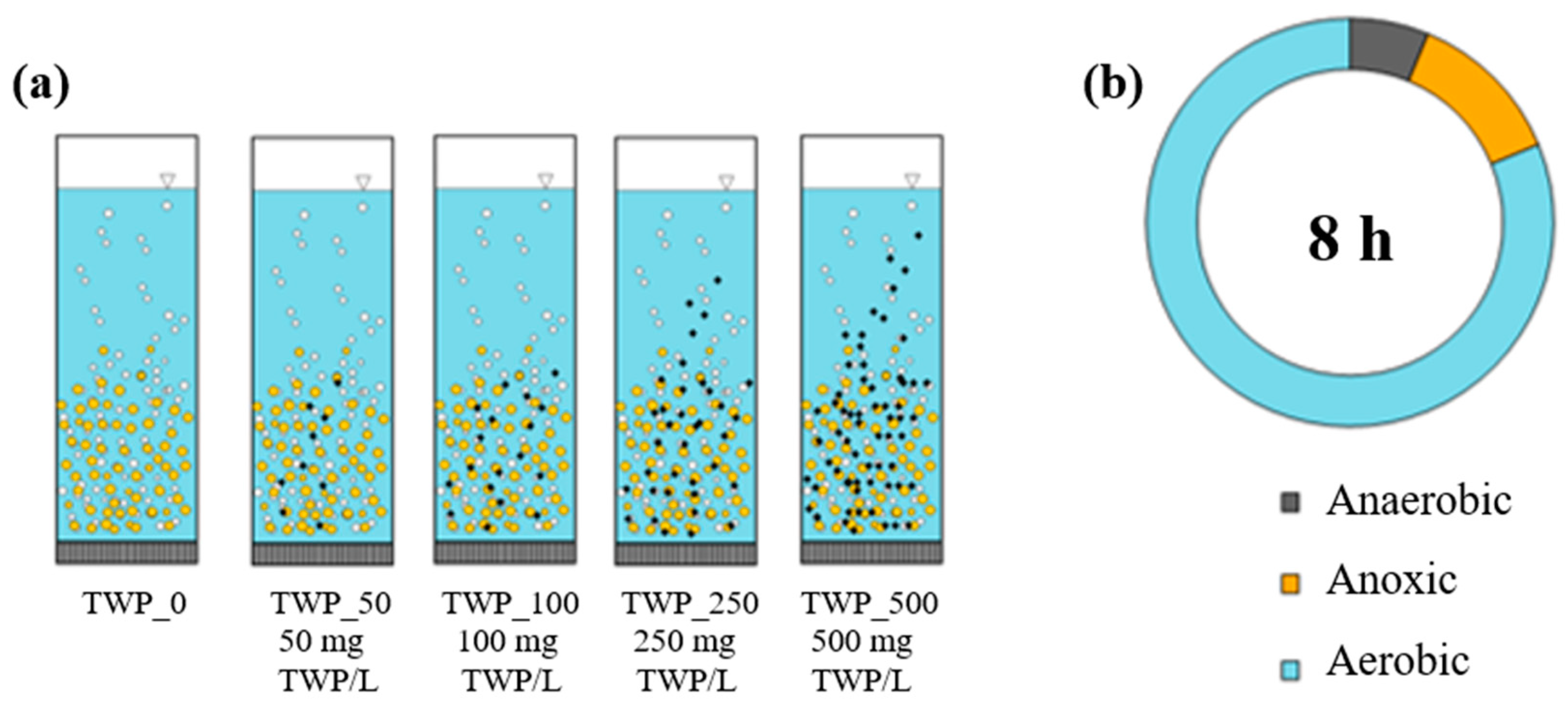
3.1. Organization of the Experiment
3.2. Technological Analyses
3.3. Molecular Analyses
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene | Primer Set | Thermal Profile | Reference |
|---|---|---|---|
| 16S rRNA | 519F/907R | 94 °C/15 s 50 °C/45 s 60 °C/45 s | Lane (1991) [55] |
| amoA | amoA1F/amoA2R | 94 °C/15 s 50 °C/45 s 60 °C/45 s | Rotthauwe et al. (1997) [56] |
| nirK | F1aCu/R3Cu | 94 °C/15 s 55 °C/1 min 60 °C/1 min | Throbäck et al. (2004) [57] |
| nosZ | NosZ-F/NosZ1622R | 94 °C/15 s 60 °C/2.5 min | Throbäck et al. (2004) [57] |
| TWP Concentration mg/L | N-NH4 mg/(L·h) | N-Nox mg/(L·h) |
|---|---|---|
| 0 | 3.9 | 2.0 |
| 50 | 4.1 | 2.3 |
| 100 | 3.7 | 1.4 |
| 250 | 4.5 | 2.5 |
| 500 | 4.5 | 3.2 |
| Dose | Time | amoA | nirK | nosZ | 16S rRNA | |
|---|---|---|---|---|---|---|
| Dose | 1.000000 | 0.000000 | 0.489366 | −0.095148 | −0.077729 | −0.039697 |
| Time | 0.000000 | 1.000000 | 0.047333 | −0.609123 | −0.472511 | −0.121096 |
| amoA | 0.489366 | 0.047333 | 1.000000 | −0.104749 | 0.042104 | −0.179776 |
| nirK | −0.095148 | −0.609123 | −0.104749 | 1.000000 | 0.739879 | 0.221573 |
| nosZ | −0.077729 | −0.472511 | 0.042104 | 0.739879 | 1.000000 | 0.220938 |
| 16S rRNA | −0.039697 | −0.121096 | −0.179776 | 0.221573 | 0.220938 | 1.000000 |
References
- Plastics Europe. Plastics Europe Plastics—The Fast Facts 2024; Plastics Europe: Brussels, Belgium, 2024. [Google Scholar]
- Edelson, M.; Håbesland, D.; Traldi, R. Uncertainties in Global Estimates of Plastic Waste Highlight the Need for Monitoring Frameworks. Mar. Pollut. Bull. 2021, 171, 112720. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and Characteristics of Microplastics in Market Bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Plastics and Microplastics: A Threat to Environment. Environ. Technol. Innov. 2019, 14, 100352. [Google Scholar] [CrossRef]
- An, L.; Liu, Q.; Deng, Y.; Wu, W.; Gao, Y.; Ling, W. Sources of Microplastic in the Environment. In Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; Volume 95, pp. 143–159. [Google Scholar]
- Baensch-Baltruschat, B.; Kocher, B.; Kochleus, C.; Stock, F.; Reifferscheid, G. Tyre and Road Wear Particles—A Calculation of Generation, Transport and Release to Water and Soil with Special Regard to German Roads. Sci. Total Environ. 2021, 752, 141939. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef]
- Jan Kole, P.; Löhr, A.J.; Van Belleghem, F.G.A.J.; Ragas, A.M.J. Wear and Tear of Tyres: A Stealthy Source of Microplastics in the Environment. Int. J. Environ. Res. Public Health 2017, 14, 1265. [Google Scholar] [CrossRef]
- Mierzyńska, K.; Pol, W.; Martyniuk, M.; Zieliński, P. Traffic Intensity as a Factor Influencing Microplastic and Tire Wear Particle Pollution in Snow Accumulated on Urban Roads. Water 2024, 16, 2907. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Recycling Waste Tires into Ground Tire Rubber (Gtr)/Rubber Compounds: A Review. J. Compos. Sci. 2020, 4, 103. [Google Scholar] [CrossRef]
- Bond, C.; Li, H.; Rate, A.W. Land Use Pattern Affects Microplastic Concentrations in Stormwater Drains in Urban Catchments in Perth, Western Australia. Land 2022, 11, 1815. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in Wastewater Treatment Plants: Detection, Occurrence and Removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Otieno, J.O.; Cydzik-Kwiatkowska, A.; Jachimowicz, P. Enhancing Biogas Production Amidst Microplastic Contamination in Wastewater Treatment Systems: A Strategic Review. Energies 2024, 17, 2555. [Google Scholar] [CrossRef]
- Jachimowicz, P.; Jo, Y.J.; Cydzik-Kwiatkowska, A. Polyethylene Microplastics Increase Extracellular Polymeric Substances Production in Aerobic Granular Sludge. Sci. Total Environ. 2022, 851, 158208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y. Effects of Microplastics on Wastewater and Sewage Sludge Treatment and Their Removal: A Review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Mosquera-Corral, A.; De Kreuk, M.K.; Heijnen, J.J.; Van Loosdrecht, M.C.M. Effects of Oxygen Concentration on N-Removal in an Aerobic Granular Sludge Reactor. Water Res. 2005, 39, 2676–2686. [Google Scholar] [CrossRef]
- Winkler, M.K.H.; Bassin, J.P.; Kleerebezem, R.; van der Lans, R.G.J.M.; van Loosdrecht, M.C.M. Temperature and Salt Effects on Settling Velocity in Granular Sludge Technology. Water Res. 2012, 46, 5445–5451. [Google Scholar] [CrossRef]
- Li, W.; Shan, X.Y.; Wang, Z.Y.; Lin, X.Y.; Li, C.X.; Cai, C.Y.; Abbas, G.; Zhang, M.; Shen, L.D.; Hu, Z.Q.; et al. Effect of Self-Alkalization on Nitrite Accumulation in a High-Rate Denitrification System: Performance, Microflora and Enzymatic Activities. Water Res. 2016, 88, 758–765. [Google Scholar] [CrossRef]
- Liu, C.; Shen, Y.; Li, Y.; Huang, F.; Wang, S.; Li, J. Aerobic Granular Sludge for Complex Heavy Metal-Containing Wastewater Treatment: Characterization, Performance, and Mechanisms Analysis. Front. Microbiol. 2024, 15, 1356386. [Google Scholar] [CrossRef]
- Perez-Bou, L.; Gonzalez-Martinez, A.; Gonzalez-Lopez, J.; Correa-Galeote, D. Promising Bioprocesses for the Efficient Removal of Antibiotics and Antibiotic-Resistance Genes from Urban and Hospital Wastewaters: Potentialities of Aerobic Granular Systems. Environ. Pollut. 2024, 342, 123115. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Majewsky, M. Cometabolic Degradation of Organic Wastewater Micropollutants by Activated Sludge and Sludge-Inherent Microorganisms. Appl. Microbiol. Biotechnol. 2014, 98, 6583–6597. [Google Scholar] [CrossRef]
- Abtahi, S.M.; Petermann, M.; Juppeau Flambard, A.; Beaufort, S.; Terrisse, F.; Trotouin, T.; Joannis Cassan, C.; Albasi, C. Micropollutants Removal in Tertiary Moving Bed Biofilm Reactors (MBBRs): Contribution of the Biofilm and Suspended Biomass. Sci. Total Environ. 2018, 643, 1464–1480. [Google Scholar] [CrossRef]
- Majone, M.; Massanisso, P.; Carucci, A.; Lindrea, K.; Tandoi, V. Influence of Storage on Kinetic Selection to Control Aerobic Filamentous Bulking. Water Sci. Technol. 1996, 34, 223–232. [Google Scholar] [CrossRef]
- Li, L.; Song, K.; Yeerken, S.; Geng, S.; Liu, D.; Dai, Z.; Xie, F.; Zhou, X.; Wang, Q. Effect Evaluation of Microplastics on Activated Sludge Nitrification and Denitrification. Sci. Total Environ. 2020, 707, 135953. [Google Scholar] [CrossRef] [PubMed]
- Brahney, J.; Mahowald, N.; Prank, M.; Cornwell, G.; Klimont, Z.; Matsui, H.; Prather, K.A. Constraining the Atmospheric Limb of the Plastic Cycle. Proc. Natl. Acad. Sci. USA 2021, 118, e2020719118. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Su, H.; Xiu, X.; Liu, C.; Hao, W. Tire Wear Particles in Different Water Environments: Occurrence, Behavior, and Biological Effects—A Review and Perspectives. Environ. Sci. Pollut. Res. 2023, 30, 90574–90594. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.X.; Lu, X.; Liu, B.; Li, Y.; Long, C.; Li, A. Abundance and Diversity of Bacterial Nitrifiers and Denitrifiers and Their Functional Genes in Tannery Wastewater Treatment Plants Revealed by High-Throughput Sequencing. PLoS ONE 2014, 9, e113603. [Google Scholar] [CrossRef]
- Arias, A.H.; Alfonso, M.B.; Girones, L.; Piccolo, M.C.; Marcovecchio, J.E. Synthetic Microfibers and Tyre Wear Particles Pollution in Aquatic Systems: Relevance and Mitigation Strategies. Environ. Pollut. 2022, 295, 118607. [Google Scholar] [CrossRef]
- Sarvajith, M.; Kiran Kumar Reddy, G.; Nancharaiah, Y.V. Aerobic Granular Sludge for High-Strength Ammonium Wastewater Treatment: Effect of COD/N Ratios, Long-Term Stability and Nitrogen Removal Pathways. Bioresour. Technol. 2020, 306, 123150. [Google Scholar] [CrossRef]
- Tang, P.; Yu, D.S.; Chen, G.H.; Zhang, P.Y.; Wang, X.X.; Lü, T.T.; Huang, S.; Liu, C.C. Rapid Cultivation of Anaerobic Ammonium Oxidation Granular Sludge and Inhibition Kinetics of Granular Sludge. Huanjing Kexue Environ. Sci. 2019, 40, 4152–4159. [Google Scholar] [CrossRef]
- Tao, C.; Hamouda, M.A. Steady-State Modeling and Evaluation of Partial Nitrification-Anammox (PNA) for Moving Bed Biofilm Reactor and Integrated Fixed-Film Activated Sludge Processes Treating Municipal Wastewater. J. Water Process Eng. 2019, 31, 100854. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, Y.; Zhang, G.; Ruan, R.; Wang, Y.; Wu, X.; Zheng, H.; Zhang, Q.; Cao, L. New Progress of Ammonia Recovery during Ammonia Nitrogen Removal from Various Wastewaters. World J. Microbiol. Biotechnol. 2020, 36, 144. [Google Scholar] [CrossRef] [PubMed]
- Jachimowicz, P.; Mądzielewska, W.; Cydzik-Kwiatkowska, A. Microplastics in Granular Sequencing Batch Reactors: Effects on Pollutant Removal Dynamics and the Microbial Community. J. Hazard. Mater. 2024, 476, 135061. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, J.; Dai, H.; Zhao, Y.; Li, D.; Duan, W.; Guo, Y. Microplastics Affect the Ammonia Oxidation Performance of Aerobic Granular Sludge and Enrich the Intracellular and Extracellular Antibiotic Resistance Genes. J. Hazard. Mater. 2021, 409, 124981. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeong, S.; Long, C.; Chandran, K. Size Dependent Impacts of a Model Microplastic on Nitrification Induced by Interaction with Nitrifying Bacteria. J. Hazard. Mater. 2022, 424, 127363. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Zielińska, M.; Bernat, K.; Bułkowska, K.; Wojnowska-Baryła, I. Insights into Mechanisms of Bisphenol A Biodegradation in Aerobic Granular Sludge. Bioresour. Technol. 2020, 315, 123806. [Google Scholar] [CrossRef]
- Zhao, D.; Cheah, W.Y.; Lai, S.H.; Ng, E.P.; Khoo, K.S.; Show, P.L.; Ling, T.C. Symbiosis of Microalgae and Bacteria Consortium for Heavy Metal Remediation in Wastewater. J. Environ. Chem. Eng. 2023, 11, 109943. [Google Scholar] [CrossRef]
- Jachimowicz, P.; Peng, R.; Hüffer, T.; Hofmann, T.; Cydzik-Kwiatkowska, A. Tire Materials Disturb Transformations of Nitrogen Compounds and Affect the Structure of Biomass in Aerobic Granular Sludge Reactors. J. Hazard. Mater. 2024, 465, 133223. [Google Scholar] [CrossRef]
- Wang, B.; Ni, B.J.; Yuan, Z.; Guo, J. Cometabolic Biodegradation of Cephalexin by Enriched Nitrifying Sludge: Process Characteristics, Gene Expression and Product Biotoxicity. Sci. Total Environ. 2019, 672, 275–282. [Google Scholar] [CrossRef]
- Ni, S.Q.; Sun, N.; Yang, H.; Zhang, J.; Ngo, H.H. Distribution of Extracellular Polymeric Substances in Anammox Granules and Their Important Roles during Anammox Granulation. Biochem. Eng. J. 2015, 101, 126–133. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Rusanowska, P.; Zielińska, M.; Bernat, K.; Wojnowska-Baryła, I. Microbial Structure and Nitrogen Compound Conversions in Aerobic Granular Sludge Reactors with Non-Aeration Phases and Acetate Pulse Feeding. Environ. Sci. Pollut. Res. 2016, 23, 24857–24870. [Google Scholar] [CrossRef]
- Su, X.; Chen, C.; Li, J.; Lu, S.; Xu, G. Effect of Polypropylene Microplastics Concentration on Wastewater Denitrification. Sci. J. Chem. 2022, 10, 53–60. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, G.; Zhang, J.; Chen, Y.; Yu, M.; Lu, L.; Li, H.; Zhu, Y.; Yuan, Y.; Huang, A.; et al. Response of Denitrifying Genes Coding for Nitrite (NirK or NirS) and Nitrous Oxide (NosZ) Reductases to Different Physico-Chemical Parameters during Agricultural Waste Composting. Appl. Microbiol. Biotechnol. 2015, 99, 4059–4070. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cheng, H.; Lin, S.; Twagirayezu, G.; Xiao, H.; Gan, C.; Hu, J.; Wang, Y.; Hu, R. Microplastics and Biochar Interactively Affect Nitrous Oxide Emissions from Tobacco Planting Soil. Sci. Total Environ. 2024, 952, 175885. [Google Scholar] [CrossRef]
- Graf, D.R.H.; Jones, C.M.; Hallin, S. Intergenomic Comparisons Highlight Modularity of the Denitrification Pathway and Underpin the Importance of Community Structure for N2O Emissions. PLoS ONE 2014, 9, e114118. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Y.; Hou, L.; Liang, Q.B.; Li, J.C.; Ren, J. Correlation Between Microplastics Pollution and Eutrophication in the Near Shore Waters of Dianchi Lake. Huanjing Kexue/Environ. Sci. 2021, 42, 3166–3175. [Google Scholar] [CrossRef]
- Fu, W.; Song, G.; Wang, Y.; Wang, Q.; Duan, P.; Liu, C.; Zhang, X.; Rao, Z. Advances in Research into and Applications of Heterotrophic Nitrifying and Aerobic Denitrifying Microorganisms. Front. Environ. Sci. 2022, 10, 887093. [Google Scholar] [CrossRef]
- Wigginton, S.K.; Brannon, E.Q.; Kearns, P.J.; Lancellotti, B.V.; Cox, A.; Moseman-Valtierra, S.; Loomis, G.W.; Amador, J.A. Nitrifying and Denitrifying Microbial Communities in Centralized and Decentralized Biological Nitrogen Removing Wastewater Treatment Systems. Water 2020, 12, 1688. [Google Scholar] [CrossRef]
- Coelho, M.A.Z.; Russo, C.; Araújo, O.Q.F. Optimization of a Sequencing Batch Reactor for Biological Nitrogen Removal. Water Res. 2000, 34, 2809–2817. [Google Scholar] [CrossRef]
- OECD. OECD Digital Economy Outlook 2020; OECD Publishing: Paris, France, 2020. [Google Scholar]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Cydzik-Kwiatkowska, A.; Rusanowska, P.; Zielińska, M.; Bernat, K.; Wojnowska-Baryła, I. Structure of Nitrogen-Converting Communities Induced by Hydraulic Retention Time and COD/N Ratio in Constantly Aerated Granular Sludge Reactors Treating Digester Supernatant. Bioresour. Technol. 2014, 154, 162–170. [Google Scholar] [CrossRef]
- Rusanowska, P. Diversity and Abundance of Denitrifying Bacteria in Aerobic Granular Sludge. Ph.D. Thesis, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland, 2015. [Google Scholar]
- Lane, D.J. 16S/23S RRNA Sequencing. Nucleic Acid Techniques in Bacterial Systematics; Wiley: Chichester, UK, 1991. [Google Scholar]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The Ammonia Monooxygenase Structural Gene Amoa as a Functional Marker: Molecular Fine-Scale Analysis of Natural Ammonia-Oxidizing Populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR Primers Targeting NirS, NirK and NosZ Genes for Community Surveys of Denitrifying Bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mądzielewska, W.I.; Jachimowicz, P.; Otieno, J.O.; Cydzik-Kwiatkowska, A. Impact of Tire-Derived Microplastics on Microbiological Activity of Aerobic Granular Sludge. Int. J. Mol. Sci. 2025, 26, 4136. https://doi.org/10.3390/ijms26094136
Mądzielewska WI, Jachimowicz P, Otieno JO, Cydzik-Kwiatkowska A. Impact of Tire-Derived Microplastics on Microbiological Activity of Aerobic Granular Sludge. International Journal of Molecular Sciences. 2025; 26(9):4136. https://doi.org/10.3390/ijms26094136
Chicago/Turabian StyleMądzielewska, Weronika Irena, Piotr Jachimowicz, Job Oliver Otieno, and Agnieszka Cydzik-Kwiatkowska. 2025. "Impact of Tire-Derived Microplastics on Microbiological Activity of Aerobic Granular Sludge" International Journal of Molecular Sciences 26, no. 9: 4136. https://doi.org/10.3390/ijms26094136
APA StyleMądzielewska, W. I., Jachimowicz, P., Otieno, J. O., & Cydzik-Kwiatkowska, A. (2025). Impact of Tire-Derived Microplastics on Microbiological Activity of Aerobic Granular Sludge. International Journal of Molecular Sciences, 26(9), 4136. https://doi.org/10.3390/ijms26094136







