O-Desmethyltramadol Enhanced Anti-Cancer Efficacy over Tramadol Through Non-μ-Opioid Receptor and Differential Cellular Contexts of Human Breast Cancer Cells
Abstract
1. Introduction
2. Results
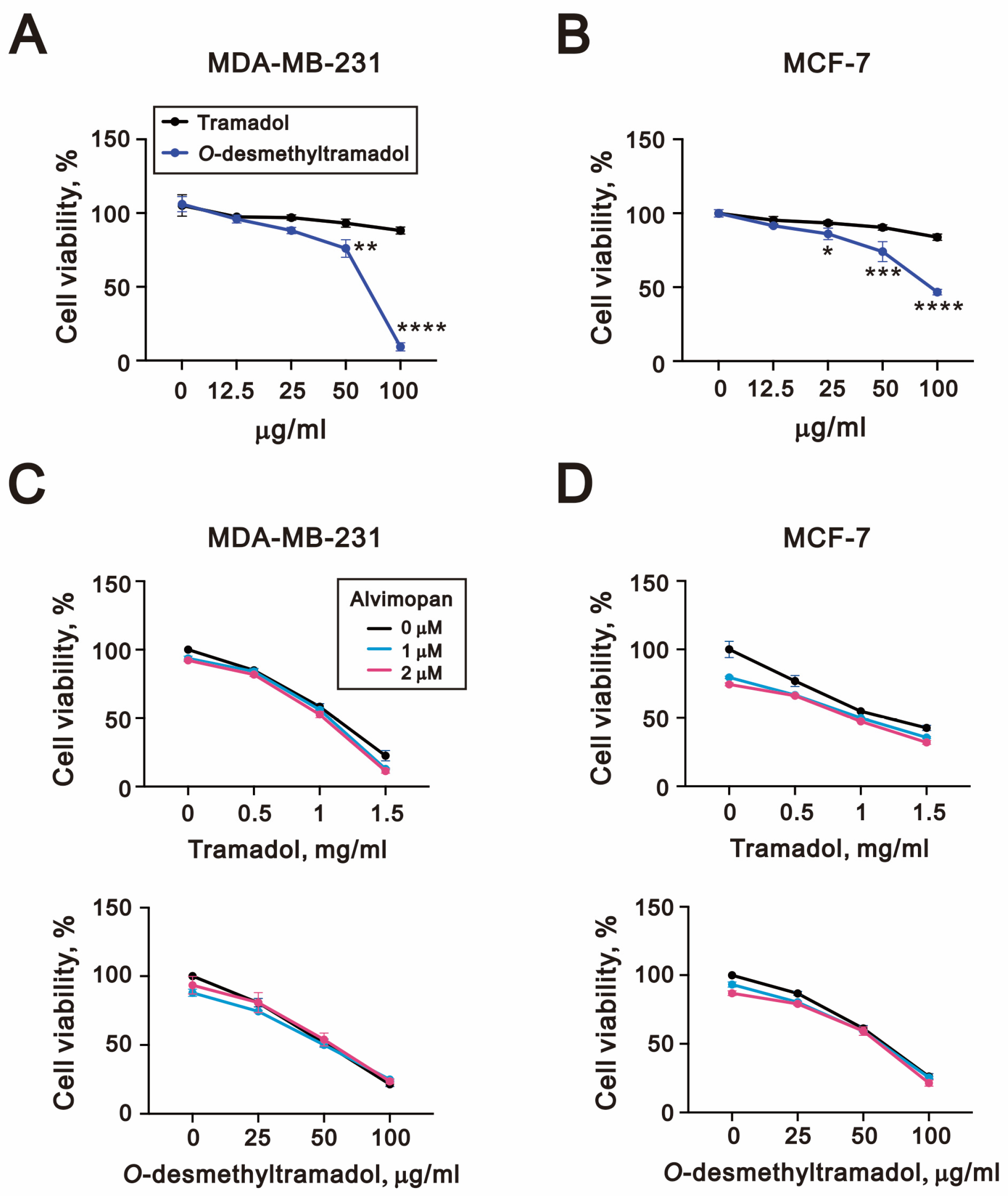
2.1. O-Desmethyltramadol Had a Better Effect than Tramadol in Inhibiting the Cell Viability of Breast Cancer Cells
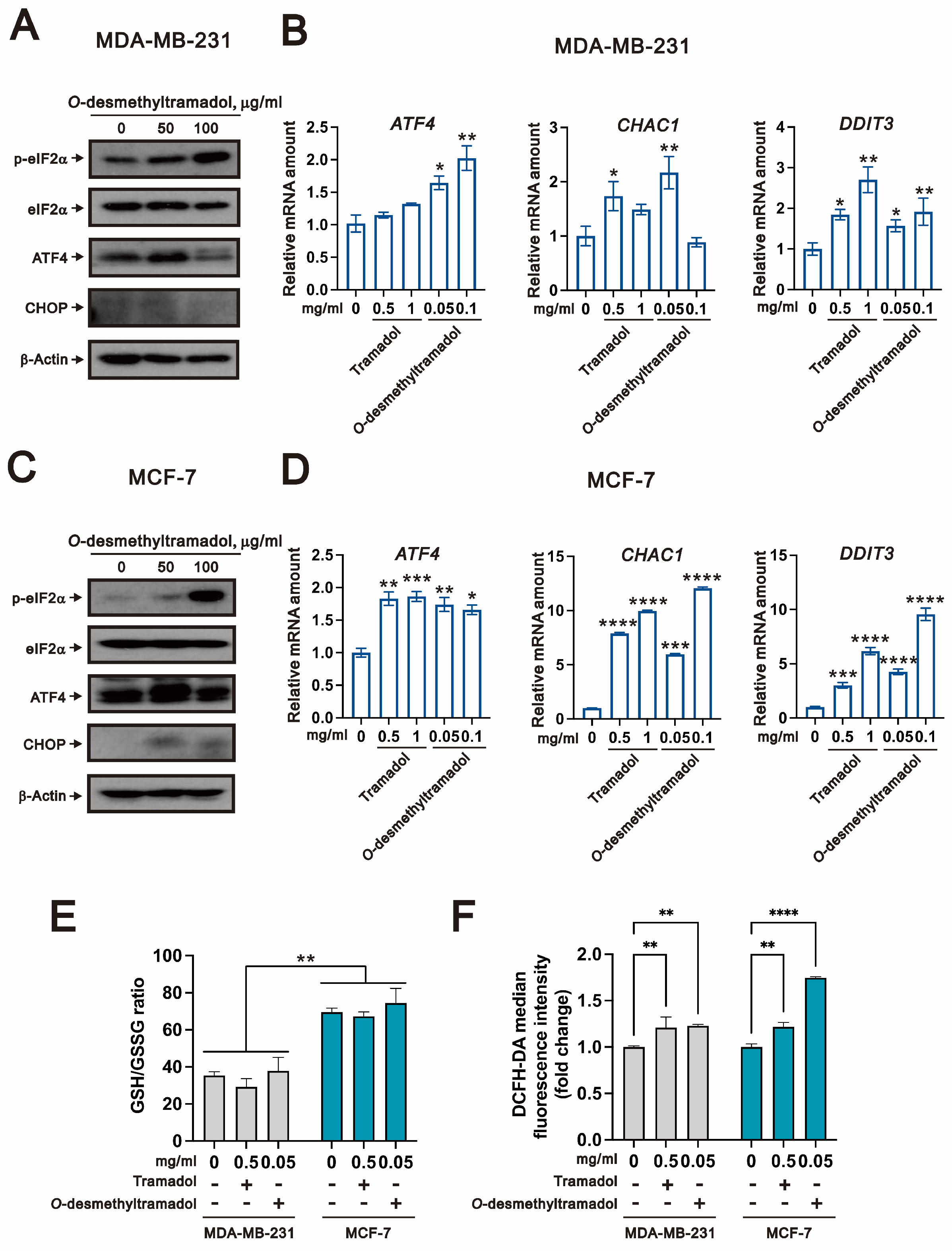
2.2. O-Desmethyltramadol Triggered ER Stress in Breast Cancer Cells
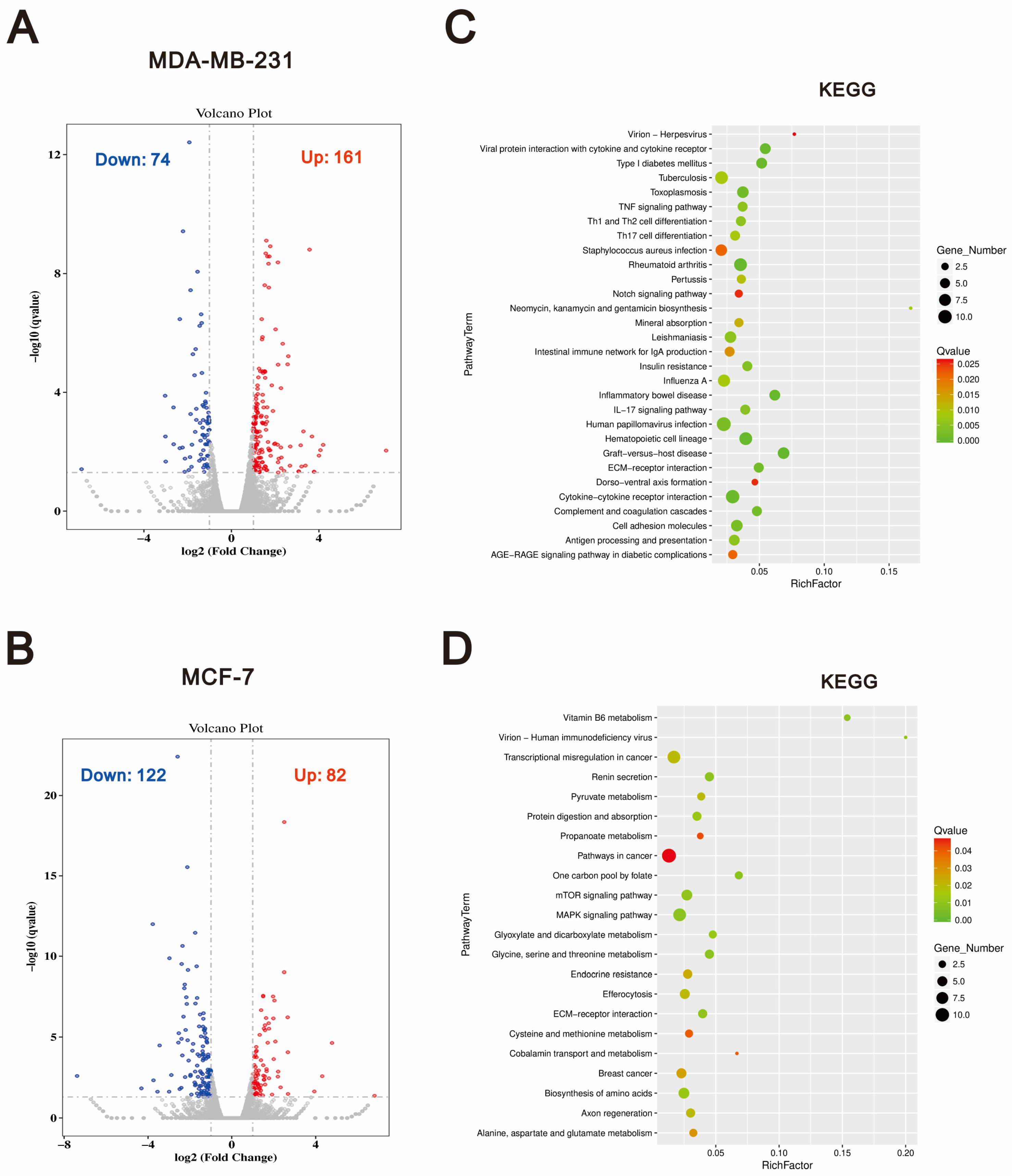
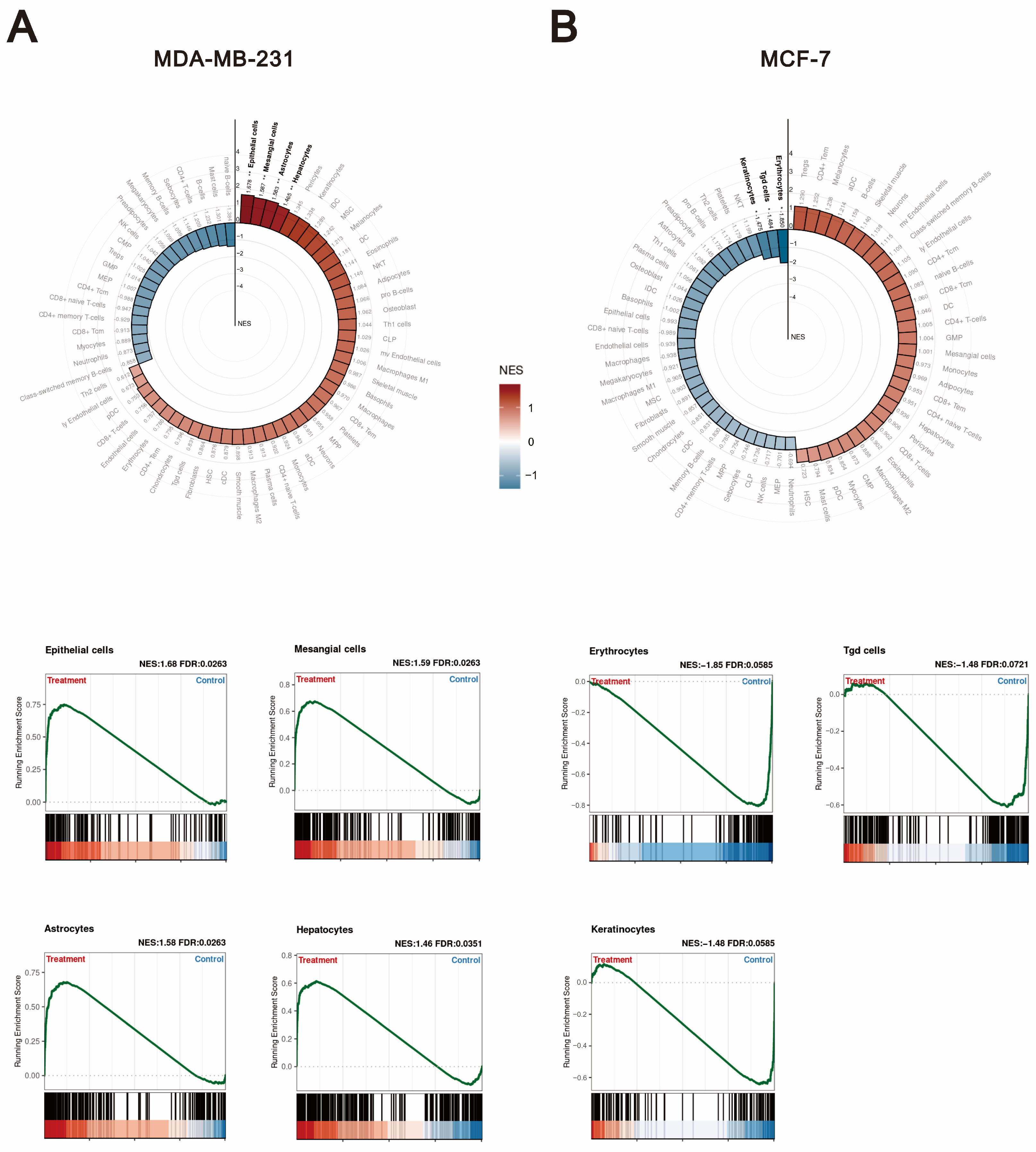
2.3. Compared to Tramadol, O-Desmethyltramadol Exerts Distinct Effects on Different Types of Breast Cancer Cells by Modulating Various Functional Pathways
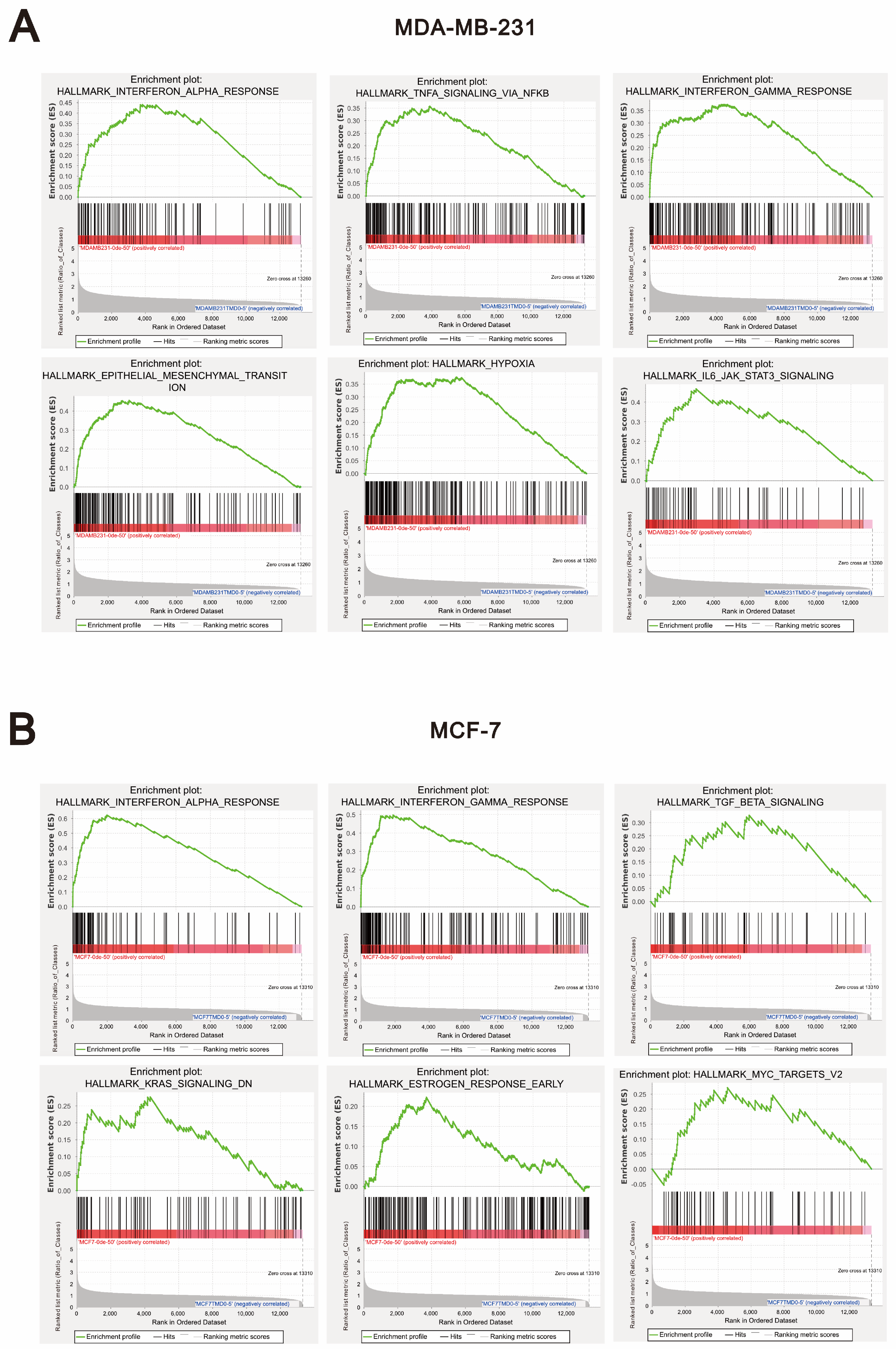
2.4. The Differential Effects of O-Desmethyltramadol and Tramadol in MDA-MB-231 and MCF-7 Cells: Identifying Cell Type-Specific Responses
2.5. Shared Differentially Expressed Genes Regulated by O-Desmethyltramadol Compared to Tramadol in Breast Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Viability and Cytotoxicity Analysis
4.3. Western Blot
4.4. RNA Isolation, Quantitative Polymerase Chain Reaction (qPCR), and RNA-seq
4.5. Differential Expression Analysis and Enrichment Analysis
4.6. GSH/GSSG Measurement
4.7. ROS Measurement
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, L.J.; Perry, C.M. Tramadol: A review of its use in perioperative pain. Drugs 2000, 60, 139–176. [Google Scholar] [CrossRef] [PubMed]
- Miotto, K.; Cho, A.K.; Khalil, M.A.; Blanco, K.; Sasaki, J.D.; Rawson, R. Trends in Tramadol: Pharmacology, Metabolism, and Misuse. Anesth. Analg. 2017, 124, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Subedi, M.; Bajaj, S.; Kumar, M.S.; Yc, M. An overview of tramadol and its usage in pain management and future perspective. Biomed. Pharmacother. 2019, 111, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Grond, S.; Sablotzki, A. Clinical pharmacology of tramadol. Clin. Pharmacokinet. 2004, 43, 879–923. [Google Scholar] [CrossRef]
- Raffa, R.B.; Friderichs, E.; Reimann, W.; Shank, R.P.; Codd, E.E.; Vaught, J.L. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J. Pharmacol. Exp. Ther. 1992, 260, 275–285. [Google Scholar] [CrossRef]
- Lambert, D.G. Opioids and opioid receptors; understanding pharmacological mechanisms as a key to therapeutic advances and mitigation of the misuse crisis. BJA Open 2023, 6, 100141. [Google Scholar] [CrossRef]
- Reeves, K.C.; Shah, N.; Muñoz, B.; Atwood, B.K. Opioid Receptor-Mediated Regulation of Neurotransmission in the Brain. Front. Mol. Neurosci. 2022, 15, 919773. [Google Scholar]
- Zhang, H.; Sun, M.; Zhou, D.; Gorur, A.; Sun, Z.; Zeng, W.; Cata, J.P.; Chen, W.; Miao, C. Increased mu-opioid receptor expression is associated with reduced disease-free and overall survival in laryngeal squamous cell carcinoma. Br. J. Anaesth. 2020, 125, 722–729. [Google Scholar] [CrossRef]
- Weingaertner, I.R.; Koutnik, S.; Ammer, H. Chronic morphine treatment attenuates cell growth of human BT474 breast cancer cells by rearrangement of the ErbB signalling network. PLoS ONE 2013, 8, e53510. [Google Scholar] [CrossRef]
- Maneckjee, R.; Biswas, R.; Vonderhaar, B.K. Binding of opioids to human MCF-7 breast cancer cells and their effects on growth. Cancer Res. 1990, 50, 2234–2238. [Google Scholar]
- Montagna, G.; Gupta, H.V.; Hannum, M.; Tan, K.S.; Lee, J.; Scarpa, J.R.; Plitas, G.; Irie, T.; McCormick, P.J.; Fischer, G.W.; et al. Intraoperative opioids are associated with improved recurrence-free survival in triple-negative breast cancer. Br. J. Anaesth. 2021, 126, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Tong, J.H.; Zhou, Z.Q.; Duan, M.L.; Xu, J.G.; Zeng, H.J.; Wang, S.H. Tramadol inhibits proliferation, migration and invasion via α2-adrenoceptor signaling in breast cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 157–165. [Google Scholar]
- Kim, M.H.; Lee, J.R.; Kim, K.J.; Jun, J.H.; Hwang, H.J.; Lee, W.; Nam, S.H.; Oh, J.E.; Yoo, Y.C. Identification for antitumor effects of tramadol in a xenograft mouse model using orthotopic breast cancer cells. Sci. Rep. 2021, 11, 22113. [Google Scholar] [CrossRef]
- Kim, M.H.; Oh, J.E.; Park, S.; Kim, J.H.; Lee, K.Y.; Bai, S.J.; Song, H.; Hwang, H.J.; Kim, D.W.; Yoo, Y.C. Tramadol use is associated with enhanced postoperative outcomes in breast cancer patients: A retrospective clinical study with in vitro confirmation. Br. J. Anaesth. 2019, 123, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Sue, S.H.; Wu, Z.S.; Huang, S.M.; Lee, S.Y.; Wu, Z.F. Antitumorigenic Effect of Tramadol and Synergistic Effect With Doxorubicin in Human Breast Cancer Cells. Front. Oncol. 2022, 12, 811716. [Google Scholar] [CrossRef]
- Chen, M.S.; Wang, S.F.; Hsu, C.Y.; Yin, P.H.; Yeh, T.S.; Lee, H.C.; Tseng, L.M. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2alpha-ATF4 pathway. Oncotarget 2017, 8, 114588–114602. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.C.; Wu, Z.S.; Chen, J.L.; Wu, Z.F.; Lai, H.C.; Huang, Y.H. Mitochondrial Dysfunction Involved in the Cytotoxicity of Tramadol in Human Endometrial Carcinoma Cells. Int. J. Mol. Sci. 2022, 24, 99. [Google Scholar] [CrossRef]
- Chan, W.-H.; Huang, S.-M.; Chiu, Y.-L. Pulmonary Effects of Traumatic Brain Injury in Mice: A Gene Set Enrichment Analysis. Int. J. Mol. Sci. 2024, 25, 3018. [Google Scholar] [CrossRef]
- Danenberg, E.; Bardwell, H.; Zanotelli, V.R.; Provenzano, E.; Chin, S.-F.; Rueda, O.M.; Green, A.; Rakha, E.; Aparicio, S.; Ellis, I.O. Breast tumor microenvironment structures are associated with genomic features and clinical outcome. Nat. Genet. 2022, 54, 660–669. [Google Scholar] [CrossRef]
- Tiwari, A.; Trivedi, R.; Lin, S.-Y. Tumor microenvironment: Barrier or opportunity towards effective cancer therapy. J. Biomed. Sci. 2022, 29, 83. [Google Scholar] [CrossRef]
- Savas, P.; Virassamy, B.; Ye, C.; Salim, A.; Mintoff, C.P.; Caramia, F.; Salgado, R.; Byrne, D.J.; Teo, Z.L.; Dushyanthen, S.; et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018, 24, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Keren, L.; Bosse, M.; Marquez, D.; Angoshtari, R.; Jain, S.; Varma, S.; Yang, S.R.; Kurian, A.; Van Valen, D.; West, R.; et al. A Structured Tumor-Immune Microenvironment in Triple Negative Breast Cancer Revealed by Multiplexed Ion Beam Imaging. Cell 2018, 174, 1373–1387. [Google Scholar] [CrossRef] [PubMed]
- Nalio Ramos, R.; Missolo-Koussou, Y.; Gerber-Ferder, Y.; Bromley, C.P.; Bugatti, M.; Núñez, N.G.; Tosello Boari, J.; Richer, W.; Menger, L.; Denizeau, J.; et al. Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Cell 2022, 185, 1189–1207. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, Y.; Hocine, H.R.; Gentric, G.; Pelon, F.; Bernard, C.; Bourachot, B.; Lameiras, S.; Albergante, L.; Bonneau, C.; Guyard, A.; et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020, 10, 1330–1351. [Google Scholar] [CrossRef]
- Ulland, T.K.; Song, W.M.; Huang, S.C.; Ulrich, J.D.; Sergushichev, A.; Beatty, W.L.; Loboda, A.A.; Zhou, Y.; Cairns, N.J.; Kambal, A.; et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 2017, 170, 649–663. [Google Scholar] [CrossRef]
- Costa, A.; Kieffer, Y.; Scholer-Dahirel, A.; Pelon, F.; Bourachot, B.; Cardon, M.; Sirven, P.; Magagna, I.; Fuhrmann, L.; Bernard, C.; et al. Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 2018, 33, 463–479. [Google Scholar] [CrossRef]
- Geurts, V.; Kok, M. Immunotherapy for Metastatic Triple Negative Breast Cancer: Current Paradigm and Future Approaches. Curr. Treat. Options Oncol. 2023, 24, 628–643. [Google Scholar] [CrossRef]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.P.; Hitre, E. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef]
- Onkar, S.S.; Carleton, N.M.; Lucas, P.C.; Bruno, T.C.; Lee, A.V.; Vignali, D.A.A.; Oesterreich, S. The Great Immune Escape: Understanding the Divergent Immune Response in Breast Cancer Subtypes. Cancer Discov. 2023, 13, 23–40. [Google Scholar] [CrossRef]
- Danish, H.H.; Goyal, S.; Taunk, N.K.; Wu, H.; Moran, M.S.; Haffty, B.G. Interferon-induced protein with tetratricopeptide repeats 1 (IFIT1) as a prognostic marker for local control in T1-2 N0 breast cancer treated with breast-conserving surgery and radiation therapy (BCS + RT). Breast J. 2013, 19, 231–239. [Google Scholar] [CrossRef]
- Zhou, W.; Yeerkenbieke, G.; Zhang, Y.; Zhou, M.; Li, J. Guanylate binding protein 4 shapes an inflamed tumor microenvironment and identifies immuno-hot tumors. J. Cancer Res. Clin. Oncol. 2024, 150, 90. [Google Scholar] [CrossRef]
- Mei, J.; Fu, Z.; Cai, Y.; Song, C.; Zhou, J.; Zhu, Y.; Mao, W.; Xu, J.; Yin, Y. SECTM1 is upregulated in immuno-hot tumors and predicts immunotherapeutic efficacy in multiple cancers. iScience 2023, 26, 106027. [Google Scholar] [CrossRef]
- Huang, J.; Liu, F.; Liu, D.; Tang, S.; Shen, D. Exploring SLC16A1 as an Oncogenic Regulator and Therapeutic Target in Cholangiocarcinoma. J. Cancer 2024, 15, 3794–3808. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, G.; Pai, T.; Hardiman, T.; Avery-Kiejda, K.; Scott, R.J.; Spencer, J.; Pinder, S.E.; Grigoriadis, A. Molecular patterns of cancer colonisation in lymph nodes of breast cancer patients. Breast Cancer Res. 2018, 20, 143. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Li, A.X.; Sanders, A.J.; Ye, L.; Frewer, K.; Hargest, R.; Jiang, W.G. NUPR1 and its potential role in cancer and pathological conditions (Review). Int. J. Oncol. 2021, 58, 21. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; He, X.; Zheng, Y.; Feng, W.; Xia, X.; Yu, X.; Lin, Z. Down-regulation of asparagine synthetase induces cell cycle arrest and inhibits cell proliferation of breast cancer. Chem. Biol. Drug Des. 2014, 84, 578–584. [Google Scholar] [CrossRef]
- Qin, C.; Yang, X.; Zhan, Z. High Expression of Asparagine Synthetase Is Associated with Poor Prognosis of Breast Cancer in Chinese Population. Cancer Biother. Radiopharm. 2020, 35, 581–585. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef]
- Yu, X.; Li, W.; Sun, S.; Li, J. DDIT3 is associated with breast cancer prognosis and immune microenvironment: An integrative bioinformatic and immunohistochemical analysis. J. Cancer 2024, 15, 3873–3889. [Google Scholar] [CrossRef]
- Malonne, H.; Sonet, B.; Streel, B.; Lebrun, S.; De Niet, S.; Sereno, A.; Vanderbist, F. Pharmacokinetic evaluation of a new oral sustained release dosage form of tramadol. Br. J. Clin. Pharmacol. 2004, 57, 270–278. [Google Scholar] [CrossRef][Green Version]
- Albrecht, W. Which concentrations are optimal for in vitro testing? Excli J. 2020, 19, 1172–1173. [Google Scholar] [PubMed]
- Aran, D.; Hu, Z.; Butte, A.J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017, 18, 220. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]

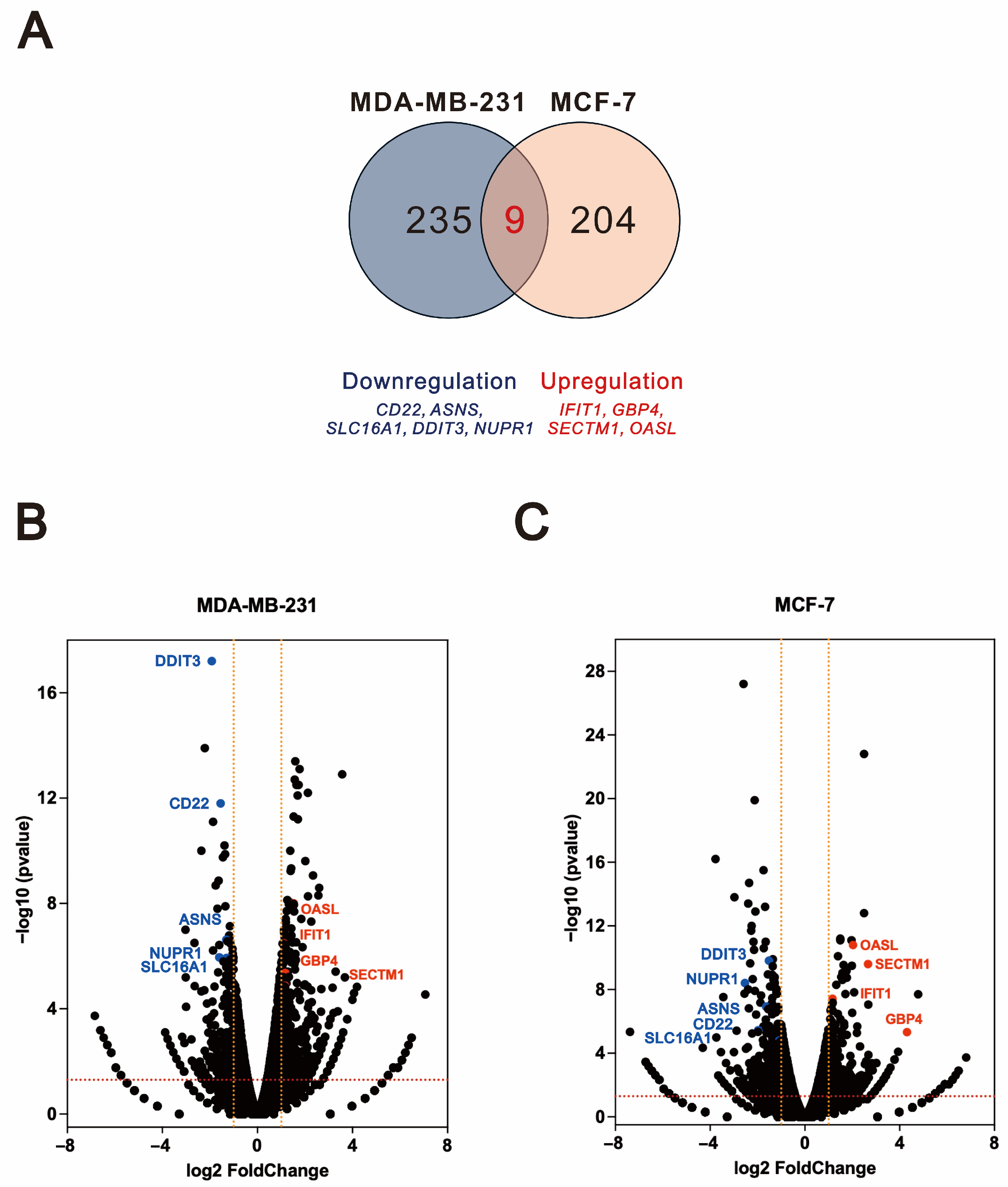
| MDA-MB-231. control vs. tramadol differentially expressed genes | SPX, CLCA3P, ENSG00000283265, ENSG00000272468, PELI2, HEY1, ENSG00000232053, OLAH, SLC16A12, PSMD10P2, LINC00632, LINC02392, CHRM3-AS2, PLA2G4C, PINLYP, ENSG00000253227, NGF, ATP6V0D2, MMP3, NMRAL2P, SLC16A1, TNFSF15, LNCOG,SUN3, NCF2, XIRP2, NUPR1, ENSG00000236453, DDIT3, LAMA1, MAL2, BCAN, LINC01293, ENSG00000237596, LURAP1L-AS1, LINC03002, FLI1,MXD1, LINC01291, GAPLINC, TM4SF19, MMP1, ENSG00000286975, PTPRR, DCLK1, B3GALT5, TM4SF19-AS1, DLGAP1-AS2, AK5, ENSG00000285108, TOX, PTPN22, CREBRF, RCAN1, IL24, HDAC9, RASGRP3, ENSG00000266401, ENSG00000227706, TMEM236, MPP4, DNAJB9, CXCL2, LAMP3, TRIB3, ENSG00000272405, VNN1, RGMB-AS1, PARM1, SEC24A, DOCK9, ENSG00000260604, IGFN1, SP140, PPARGC1A, HERPUD1, LRATD2, PPP1R15A, HKDC1, FGF1, EVI2A, SEC24D, GFPT1, ENSG00000287023, SESN2, BCAN-AS1, COBLL1, OSBP2, ZNF114, GEM, AADACP1, AGRN, FAM20C, COL7A1, LAMA5, ADAMTS15, PRKAR1B, SNRPA, MARCKSL1, C1R, BCAM, UCP2, FCGBP, SREBF1, SCNN1A, ID1, SIGIRR, IGFBP6, VWA1, SHROOM1, ABCA1, SAA1, MXD3, PDE7B, CEMIP, BCL3, U2AF1, TNFSF10, TYMSOS, PFN1P1, CEBPD, SAA2, ENSG00000281181, AQP3, LMNTD2-AS1, TFAP2A-AS1, SOCS1, ST7-AS1, C1QL1, ALG1L5P, ZFP91-CNTF |
| MDA-MB-231 control vs. O-desmethyltramadol differentially expressed genes | IFITM10, HSPA6, ENSG00000272825, F12, DHRS2, PELI2, ANGPT1, HSD11B1, DPP4, ITGB2-AS1, PCDH7, CD44-DT, ENSG00000227496, CTSW, SCN9A, TNFSF15, KYNU, SLCO4C1, TMEM158, SHOC1, ENSG00000272405, ENSG00000227706, SLITRK4, TMEM45A |
| MDA-MB-231 tramadol and O-desmethyltramadol shared differentially expressed genes | PELI2, TNFSF15, ENSG00000227706, ENSG00000272405 |
| MCF-7 control vs. tramadol differentially expressed genes | PSG5, TSKU-AS1, LRRC15, NUPR1, SPRY4, BEST1, ETV4, GUSBP13, ENSG00000256433, DUSP6, SLCO4C1, TMEM156, ENSG00000289223, ENSG00000233052, ETV5, HRK, ENSG00000260604, PSG9, RAP1AP, CLGN, DIO2, RAB3IL1, NIBAN1, STARD4, SHC4, BEX2, LPIN1, NOVA1, CD22, CLDN1, GPNMB, RRAGD, RASD1, BCAT1, RUNX2, ACSS2, SCD, SLITRK6, INSIG1-DT, KCNMA1, COL5A2, SCNN1A, GPX8, DDIT3, TNIK, ICAM1, RIPOR3, NEU1, MVK, TGFBI, ULBP1, SLC7A11, FBN1, MAFF, HMGCS1, MSMO1, INSIG1, PFKFB2, NPC2, MMAB, LIF, SIGLEC15, ACSS1, PSAT1, DUSP4, WLS, BHLHE40, PLA2G4C, ERRFI1, LDLR, FADS2, BRI3, ACSL1, QPRT, CAPG, MUC16, MAFB, ST8SIA4, PCDH19, XAF1, LINC01522, NKAIN1, PLEKHN1, ZDHHC22, ARHGEF37, RNF32-AS1, AFAP1L2, OASL, CPA6, COL4A4, OAS2, GRHL3, PKP1, HCK, FGF18, CMPK2, H19, KRT16, LRP4, MX2, RSAD2, PCDH11X, GCOM1, MGAT4EP, MAB21L4, ZBTB47, A2ML1, GABRP, GNG4, KRT6A, SCNN1G, IL1B |
| MCF-7 control vs. O-desmethyltramadol differentially expressed genes | SPRY4, SLIT1, ENSG00000287315, ENSG00000277159, ENSG00000281181, IFIT2, OTULINL, NCAPG, UBE2C, CENPE, PRR11, DLGAP5, KIF15, HMMR, ATP8A2, SKA1, FAM83D, KIF14, CDKN2C, NEURL1B, APCDD1, CDCA3, IQGAP3, HASPIN, CDC25C, ANKRD35, TGM2, KRT13, H19, ARHGAP33, ANXA8, CCN5, PIF1, PHGDH, TP63, ALDH1L2, KRT6A, IL1B |
| MCF-7 tramadol and O-desmethyltramadol shared differentially expressed genes | SPRY4, H19, KRT6A, IL1B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-S.; Huang, Y.-H.; Huang, S.-M. O-Desmethyltramadol Enhanced Anti-Cancer Efficacy over Tramadol Through Non-μ-Opioid Receptor and Differential Cellular Contexts of Human Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 4139. https://doi.org/10.3390/ijms26094139
Wu Z-S, Huang Y-H, Huang S-M. O-Desmethyltramadol Enhanced Anti-Cancer Efficacy over Tramadol Through Non-μ-Opioid Receptor and Differential Cellular Contexts of Human Breast Cancer Cells. International Journal of Molecular Sciences. 2025; 26(9):4139. https://doi.org/10.3390/ijms26094139
Chicago/Turabian StyleWu, Zih-Syuan, Yi-Hsuan Huang, and Shih-Ming Huang. 2025. "O-Desmethyltramadol Enhanced Anti-Cancer Efficacy over Tramadol Through Non-μ-Opioid Receptor and Differential Cellular Contexts of Human Breast Cancer Cells" International Journal of Molecular Sciences 26, no. 9: 4139. https://doi.org/10.3390/ijms26094139
APA StyleWu, Z.-S., Huang, Y.-H., & Huang, S.-M. (2025). O-Desmethyltramadol Enhanced Anti-Cancer Efficacy over Tramadol Through Non-μ-Opioid Receptor and Differential Cellular Contexts of Human Breast Cancer Cells. International Journal of Molecular Sciences, 26(9), 4139. https://doi.org/10.3390/ijms26094139





