Antioxidant Peptide Production Using Keratin from Feather Waste: Effect of Extraction and Thiol Blocking Method
Abstract
:1. Introduction
2. Results
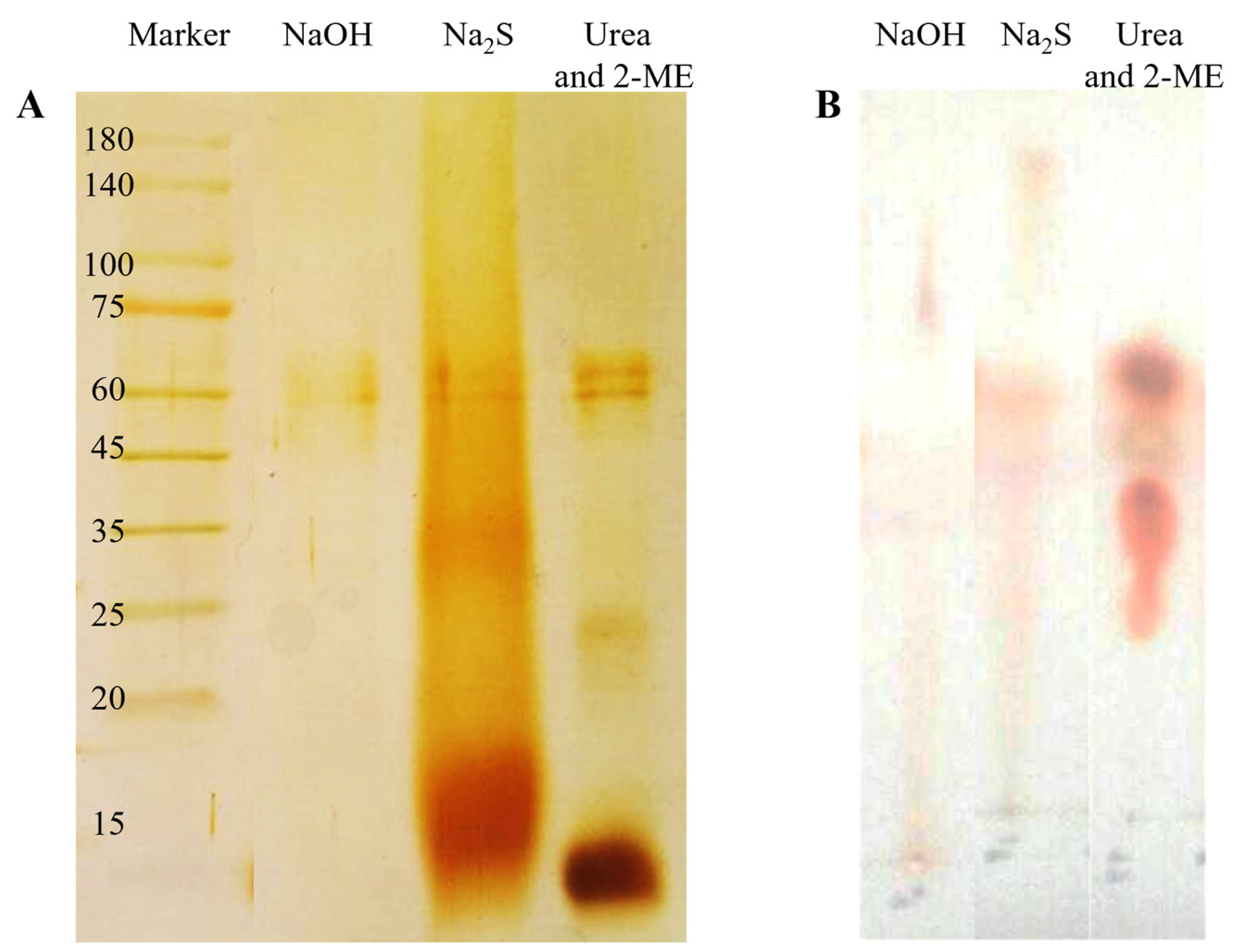
2.1. Feather Dissolution
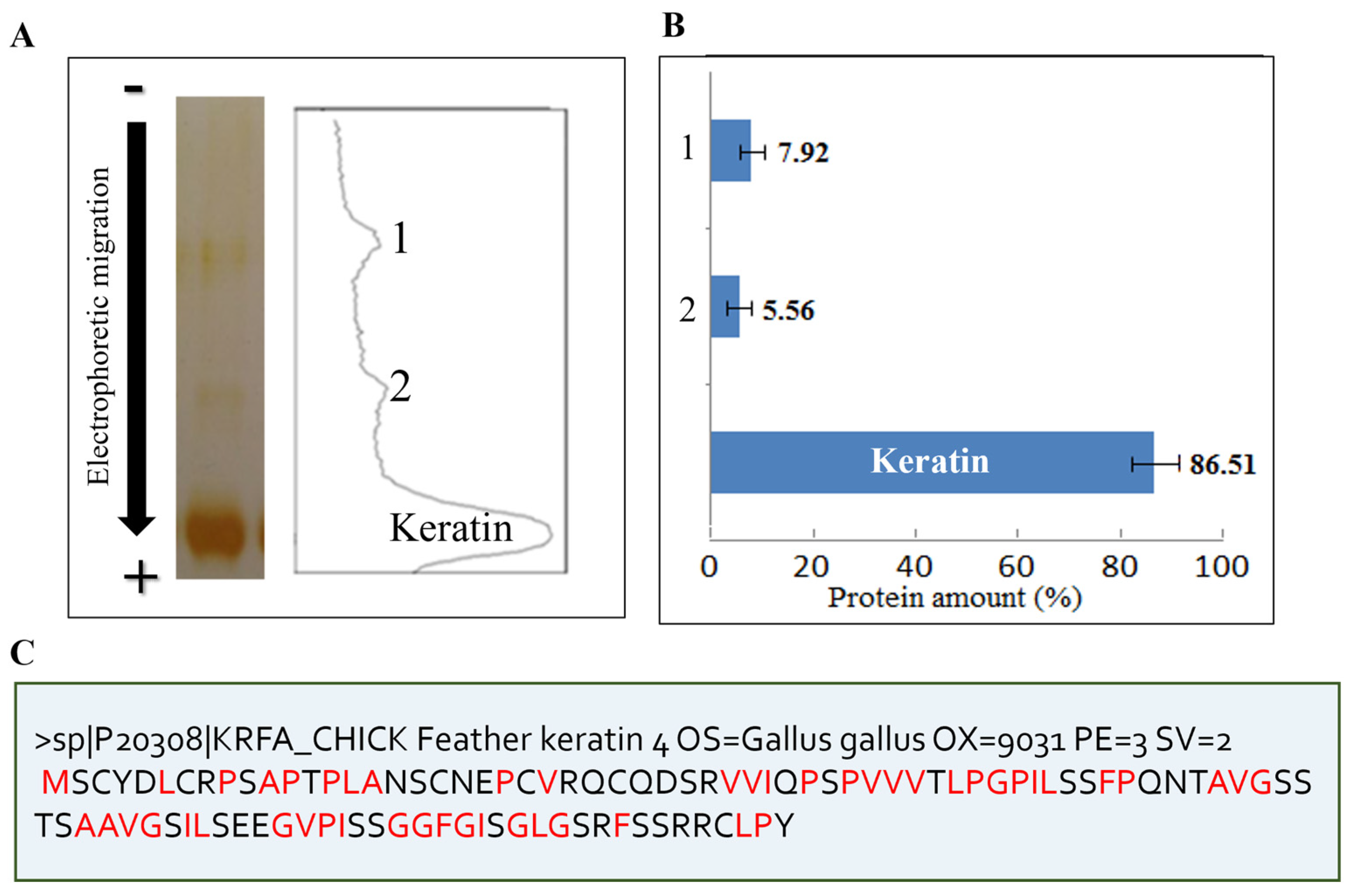
2.2. Characterization of Three Dialysates
2.3. Biochemical Characterization of Feather Keratin
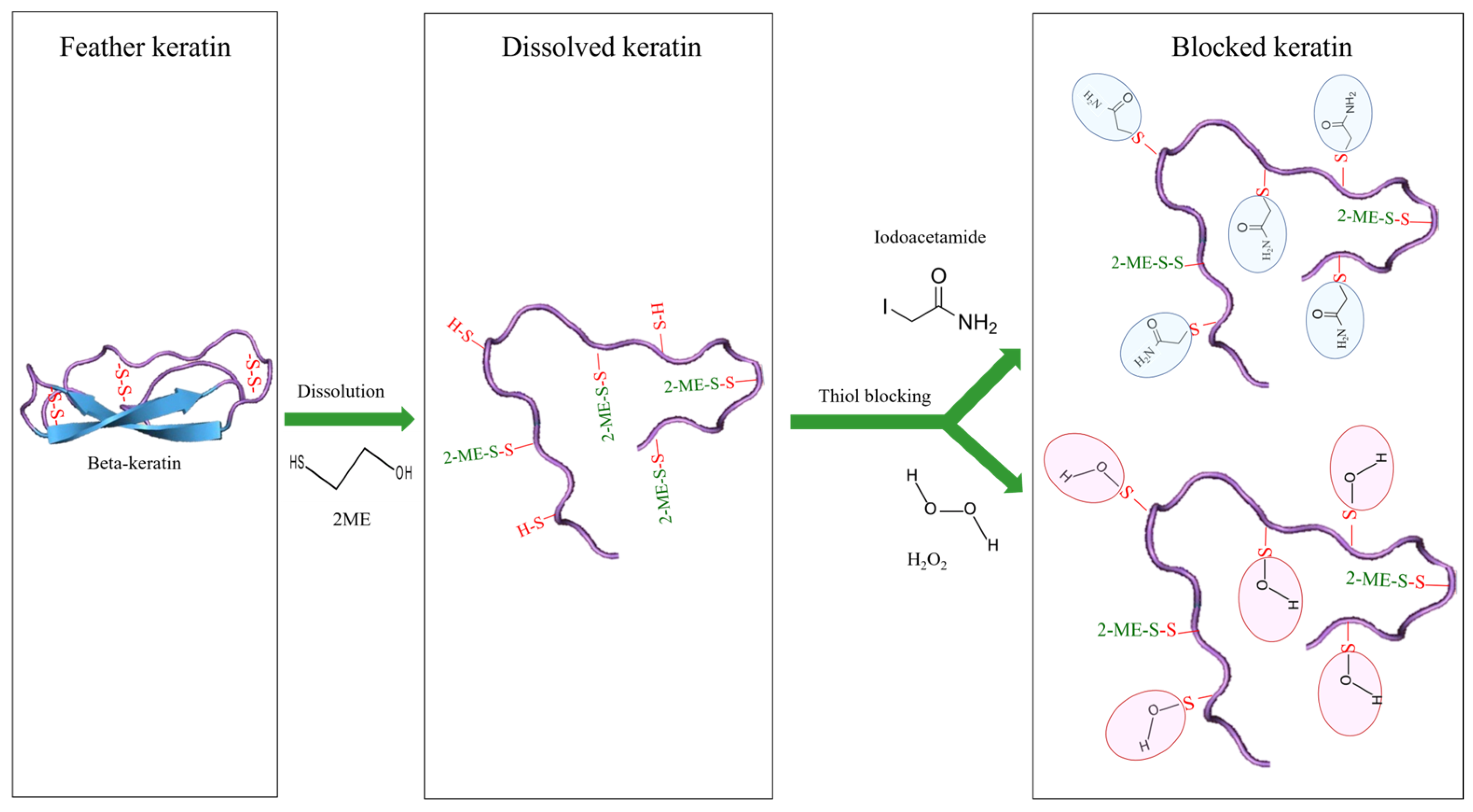
2.4. Effect of Blocking Agents on Antioxidant Activity
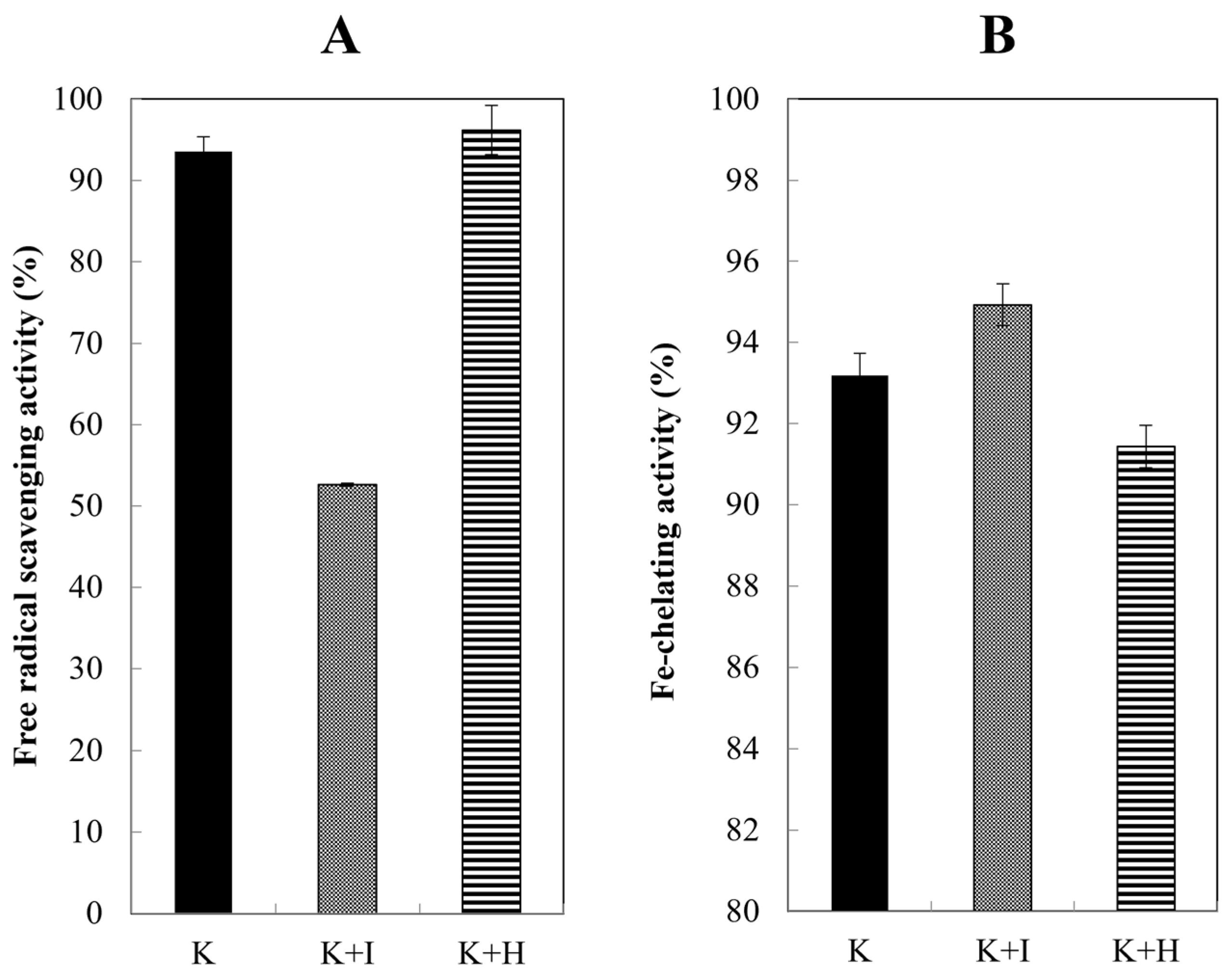
2.5. Radical Scavenging Activity
2.6. Fe-Chelating Activity
2.7. Kinetics Studies
2.7.1. Kinetics of Free Radical Scavenging
2.7.2. Kinetics of Fe-Chelating
2.8. Comparison of Rate of Inactivation
3. Discussion
4. Materials and Methods
4.1. Dissolution of Feathers
4.1.1. Dissolution Using Sodium Hydroxide
4.1.2. Dissolution Using Sodium Sulfide
4.1.3. Dissolution Using Urea and 2-Mercaptoethanol
4.2. Protein Characterization
4.2.1. Tricine-SDS-PAGE Analysis
4.2.2. Thin-Layer Chromatography (TLC)
4.3. Protein Quantification
4.4. Blocking of Free Thiol Groups with Iodoacetamide
4.4.1. Blocking of Free Thiol Groups with H2O2
4.4.2. Measurement of Free Radical Scavenging Activity
Control absorption (methanol) = Ac
Sample absorption = As
4.5. Measurement of Fe-Chelating Activity
Ac = absorbance control (EDTA)
As = absorbance of keratin sample
4.6. Kinetics of Antioxidant Activity
4.7. Bioinformatic Analysis
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alzaydi, A.; Barbhuiya, R.I.; Routray, W.; Elsayed, A.; Singh, A. Bioactive peptides: Synthesis, applications, and associated challenges. Food Bioeng. 2023, 2, 273–290. [Google Scholar] [CrossRef]
- Quintal-Bojórquez, N.; Segura-Campos, M.R. Bioactive peptides as therapeutic adjuvants for cancer. Nutr. Cancer 2021, 73, 1309–1321. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Fields, K.; Falla, T.J.; Rodan, K.; Bush, L. Bioactive peptides: Signaling the future. J. Cosmet. Dermatol. 2009, 8, 8–13. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Moosavi-Nejad, Z.; Mohammadi, P. A new nanobiotic: Synthesis and characterization of an albumin nanoparticle with intrinsic antibiotic activity. Iran. J. Microbiol. 2023, 15, 697. [Google Scholar] [CrossRef]
- Pakdel, M.; Moosavi-Nejad, Z.; Kermanshahi, R.K.; Hosano, N.; Qamsari, E.M.; Hosano, H. Keratin nanoparticles derived from feather waste for novel antibacterial delivery. Int. J. Biol. Macromol. 2025, 298, 139676. [Google Scholar] [CrossRef]
- Purohit, K.; Reddy, N.; Sunna, A. Exploring the potential of bioactive peptides: From natural sources to therapeutics. Int. J. Mol. Sci. 2024, 25, 1391. [Google Scholar] [CrossRef]
- do Nascimento Alves, R.; dos Santos, J.R.S.; Almeida, F.L.C.; da Silva, F.A.P.; da Silva Araújo, Í.B. Use of Protein By-Products Obtained from Aquatic Organisms as Bioactive Compounds: A Bibliometric Review. Food Rev. Int. 2024, 40, 2662–2681. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A review on bioactive peptides: Physiological functions, bioavailability and safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, antioxidants and international nutrition. Asia Pac. J. Clin. Nutr. 1999, 8, 53–63. [Google Scholar]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Hercberg, S.; Preziosi, P.; Briançon, S.; Galan, P.; Triol, I.; Malvy, D.; Roussel, A.-M.; Favier, A. A primary prevention trial using nutritional doses of antioxidant vitamins and minerals in cardiovascular diseases and cancers in a general population: The SU. VI. MAX study—Design, methods, and participant characteristics. Control. Clin. Trials 1998, 19, 336–351. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; De Meyer, G.R.Y.; Herman, A.G.; Kockx, M.M. Reactive oxygen species induce RNA damage in human atherosclerosis. Eur. J. Clin. Investig. 2004, 34, 323–327. [Google Scholar] [CrossRef]
- Gey, K.F. Prospects for the prevention of free radical disease, regarding cancer and cardiovascular disease. Br. Med. Bull. 1993, 49, 679–699. [Google Scholar] [CrossRef]
- Abebe, T. Extraction and Optimization of Natural Protein (Keratin) from Waste Chicken Feather for the Development of Anti-Ageing Cream. Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2017. [Google Scholar]
- Floor, E.; Wetzel, M.G. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J. Neurochem. 1998, 70, 268–275. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Berlett, B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Chem. Res. Toxicol. 1997, 10, 485–494. [Google Scholar] [CrossRef]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef]
- De la Fuente, M. Role of the immune system in aging. Inmunología 2008, 27, 176–191. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Dinesh Kumar, B. Antioxidant activity and functional properties of enzymatic protein hydrolysates from common carp (Cyprinus carpio) roe (egg). J. Food Sci. Technol. 2015, 52, 5817–5825. [Google Scholar] [CrossRef] [PubMed]
- Mardani, M.; Badakné, K.; Farmani, J.; Aluko, R.E. Antioxidant peptides: Overview of production, properties, and applications in food systems. Compr. Rev. Food Sci. Food Saf. 2023, 22, 46–106. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Shahidi, F. Antioxidant activity of peptide fractions of capelin protein hydrolysates. Food Chem. 1997, 58, 355–359. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kim, E.-K.; Hwang, J.-W.; Oh, H.-J.; Cheong, S.-H.; Moon, S.-H.; Jeon, B.-T.; Lee, S.M.; Park, P.-J. Purification and characterisation of an antioxidative peptide from enzymatic hydrolysates of duck processing by-products. Food Chem. 2010, 123, 216–220. [Google Scholar] [CrossRef]
- Zhu, Y.; Lao, F.; Pan, X.; Wu, J. Food protein-derived antioxidant peptides: Molecular mechanism, stability and bioavailability. Biomolecules 2022, 12, 1622. [Google Scholar] [CrossRef]
- Kumar, Y.; Yadav, D.N.; Ahmad, T.; Narsaiah, K. Recent trends in the use of natural antioxidants for meat and meat products. Compr. Rev. Food Sci. Food Saf. 2015, 14, 796–812. [Google Scholar] [CrossRef]
- Onifade, A.A.; Al-Sane, N.A.; Al-Musallam, A.A.; Al-Zarban, S. A review: Potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour. Technol. 1998, 66, 1–11. [Google Scholar] [CrossRef]
- Pourjavaheri, F.; Pour, S.O.; Jones, O.A.H.; Smooker, P.M.; Brkljača, R.; Sherkat, F.; Blanch, E.W.; Gupta, A.; Shanks, R.A. Extraction of keratin from waste chicken feathers using sodium sulfide and l-cysteine. Process Biochem. 2019, 82, 205–214. [Google Scholar] [CrossRef]
- Fontoura, R.; Daroit, D.J.; Correa, A.P.F.; Meira, S.M.M.; Mosquera, M.; Brandelli, A. Production of feather hydrolysates with antioxidant, angiotensin-I converting enzyme-and dipeptidyl peptidase-IV-inhibitory activities. New Biotechnol. 2014, 31, 506–513. [Google Scholar] [CrossRef]
- Sundaram, M.; Legadevi, R.; Banu, N.A.; Gayathri, V.; Palanisammy, A. A study on anti bacterial activity of keratin nanoparticles from chicken feather waste against Staphylococcus aureus (Bovine Mastitis Bacteria) and its anti oxidant activity. Eur. J. Biotechnol. Biosci. 2015, 3, 1–5. [Google Scholar]
- Nagai, Y.; Nishikawa, T. Alkali solubilization of chicken feather keratin. Agric. Biol. Chem. 1970, 34, 16–22. [Google Scholar] [CrossRef]
- Steiner, R.J.; Kellems, R.O.; Church, D.C. Feather and hair meals for ruminants. IV. Effects of chemical treatments of feathers and processing time on digestibility. J. Anim. Sci. 1983, 57, 495–502. [Google Scholar] [CrossRef]
- Papadopoulos, M.C. Amino acid content and protein solubility of feather meal as affected by different processing conditions. Neth. J. Agric. Sci. 1985, 33, 317–319. [Google Scholar] [CrossRef]
- Kim, W.K.; Lorenz, E.S.; Patterson, P.H. Effect of enzymatic and chemical treatments on feather solubility and digestibility. Poult. Sci. 2002, 81, 95–98. [Google Scholar] [CrossRef]
- Ben Hamad Bouhamed, S.; Krichen, F.; Kechaou, N. Feather protein hydrolysates: A study of physicochemical, functional properties and antioxidant activity. Waste Biomass Valorization 2020, 11, 51–62. [Google Scholar] [CrossRef]
- Sherma, J.; Fried, B. Handbook of Thin-Layer Chromatography; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Karthikeyan, R.; Balaji, S.; Sehgal, P.K. Industrial applications of keratins—A review. J. Sci. Ind. Res. 2007, 66, 710–715. [Google Scholar]
- Schrooyen, P.M.M.; Dijkstra, P.J.; Oberthür, R.C.; Bantjes, A.; Feijen, J. Partially carboxymethylated feather keratins. 1. Properties in aqueous systems. J. Agric. Food Chem. 2000, 48, 4326–4334. [Google Scholar] [CrossRef]
- Mokrejs, P.; Svoboda, P.; Hrncirik, J.; Janacova, D.; Vasek, V. Processing poultry feathers into keratin hydrolysate through alkaline-enzymatic hydrolysis. Waste Manag. Res. 2011, 29, 260–267. [Google Scholar] [CrossRef]
- Pakdel, M.; Moosavi-Nejad, Z.; Kermanshahi, R.K.; Hosano, H. Self-assembled uniform keratin nanoparticles as building blocks for nanofibrils and nanolayers derived from industrial feather waste. J. Clean. Prod. 2022, 335, 130331. [Google Scholar] [CrossRef]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.D.B.; Parry, D.A.D. Molecular packing in the feather keratin filament. J. Struct. Biol. 2008, 162, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.D.B.; Parry, D.A.D. The structural basis of the filament-matrix texture in the avian/reptilian group of hard β-keratins. J. Struct. Biol. 2011, 173, 391–405. [Google Scholar] [CrossRef]
- Alahyaribeik, S.; Ullah, A. Methods of keratin extraction from poultry feathers and their effects on antioxidant activity of extracted keratin. Int. J. Biol. Macromol. 2020, 148, 449–456. [Google Scholar] [CrossRef]
- Fontoura, R.; Daroit, D.J.; Corrêa, A.P.F.; Moresco, K.S.; Santi, L.; Beys-da-Silva, W.O.; Yates III, J.R.; Moreira, J.C.F.; Brandelli, A. Characterization of a novel antioxidant peptide from feather keratin hydrolysates. New Biotechnol. 2019, 49, 71–76. [Google Scholar] [CrossRef]
- Gels, T.; Schagger, H.; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987, 166, 368–379. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Smith, D.J.; Miggio, E.T.; Kenyon, G.L. Simple alkanethiol groups for temporary blocking of sulfhydryl groups of enzymes. Biochemistry 1975, 14, 766–771. [Google Scholar] [CrossRef]
- Garcia, E.J.; Oldoni, T.L.C.; de Alencar, S.M.; Reis, A.; Loguercio, A.D.; Grande, R.H.M. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, H.; Qian, H. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006, 41, 1296–1302. [Google Scholar] [CrossRef]


| Thiol Blocking Agents | Kin of Free Radical Scavenging Activity (%) | Kin of Fe-Chelating Activity (%) |
|---|---|---|
| - | 0.09 | 0.024 |
| Iodoacetamide | 0.37 | 0.0002 |
| H2O2 | 0.19 | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheikh Hosseini, M.; Moosavi-Nejad, Z.; Rezaei Sadrabadi, F.; Hosano, H. Antioxidant Peptide Production Using Keratin from Feather Waste: Effect of Extraction and Thiol Blocking Method. Int. J. Mol. Sci. 2025, 26, 4149. https://doi.org/10.3390/ijms26094149
Sheikh Hosseini M, Moosavi-Nejad Z, Rezaei Sadrabadi F, Hosano H. Antioxidant Peptide Production Using Keratin from Feather Waste: Effect of Extraction and Thiol Blocking Method. International Journal of Molecular Sciences. 2025; 26(9):4149. https://doi.org/10.3390/ijms26094149
Chicago/Turabian StyleSheikh Hosseini, Mehrnaz, Zahra Moosavi-Nejad, Fatemeh Rezaei Sadrabadi, and Hamid Hosano. 2025. "Antioxidant Peptide Production Using Keratin from Feather Waste: Effect of Extraction and Thiol Blocking Method" International Journal of Molecular Sciences 26, no. 9: 4149. https://doi.org/10.3390/ijms26094149
APA StyleSheikh Hosseini, M., Moosavi-Nejad, Z., Rezaei Sadrabadi, F., & Hosano, H. (2025). Antioxidant Peptide Production Using Keratin from Feather Waste: Effect of Extraction and Thiol Blocking Method. International Journal of Molecular Sciences, 26(9), 4149. https://doi.org/10.3390/ijms26094149








