Ethylene Signaling Modulates Dehydrin Expression in Arabidopsis thaliana Under Prolonged Dehydration
Abstract
1. Introduction
2. Results
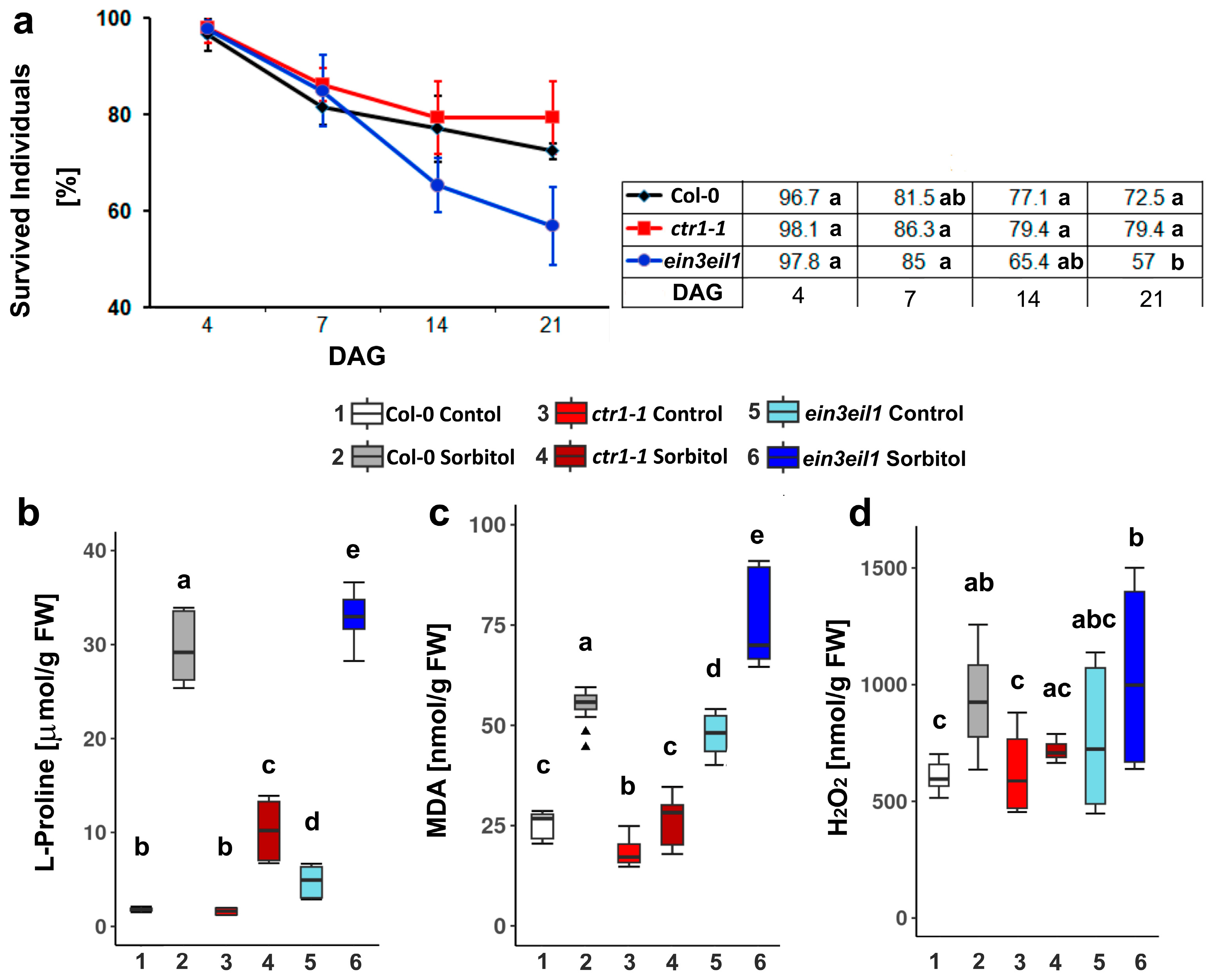
2.1. Survival and Accumulation of Stress Markers in the Wild-Type Col-0 and the Ethylene Signaling Mutants Subjected to Prolonged Dehydration
2.1.1. Survival Rate
2.1.2. L-Proline, MDA and H2O2 Content Measured in Col-0, ctr1-1, and ein3eil1 After 21 Days of Growth on Sorbitol-Containing Medium
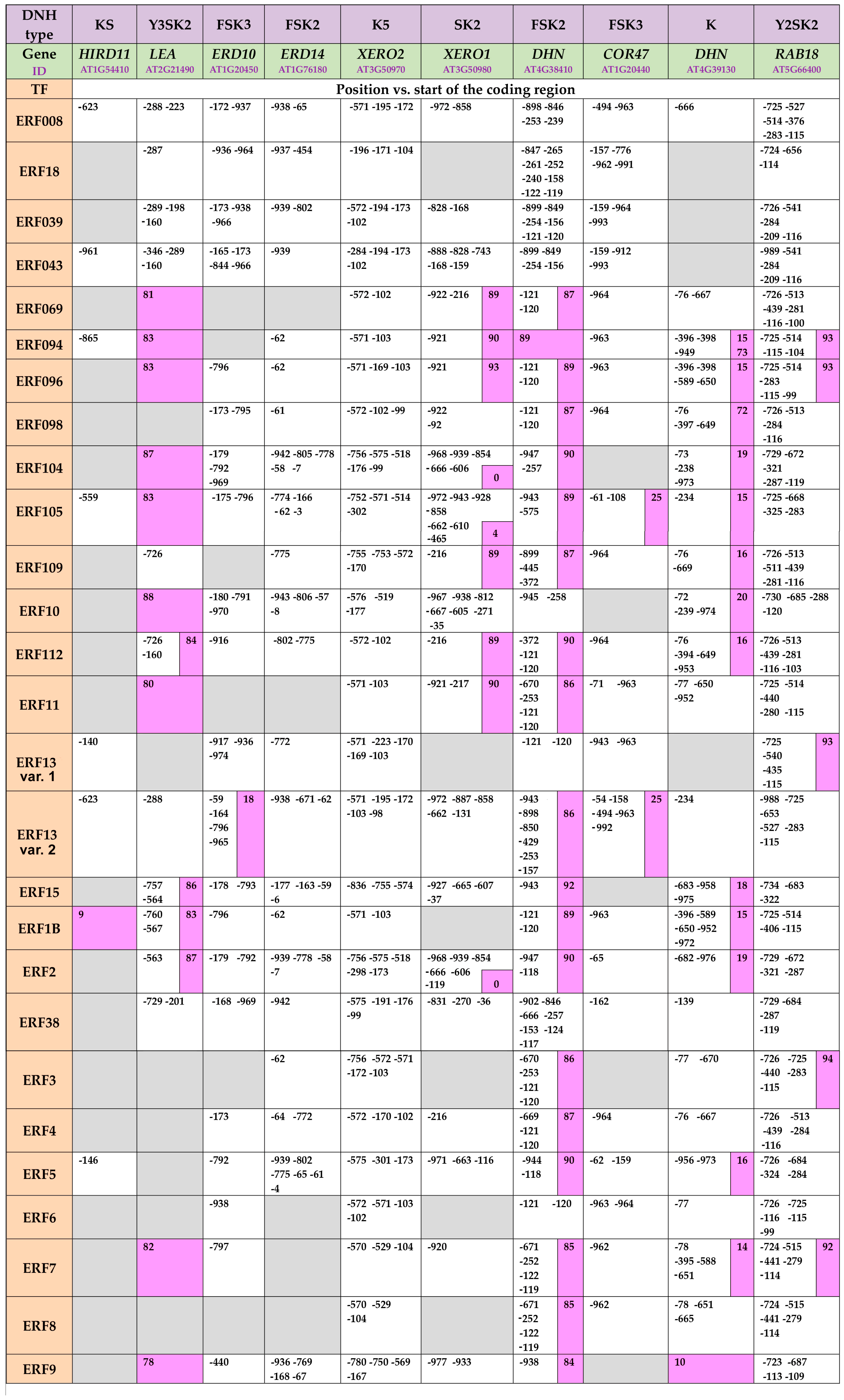
2.2. In Silico Analyses of Promoter Regions of the Dehydrin-Coding A. thaliana Genes
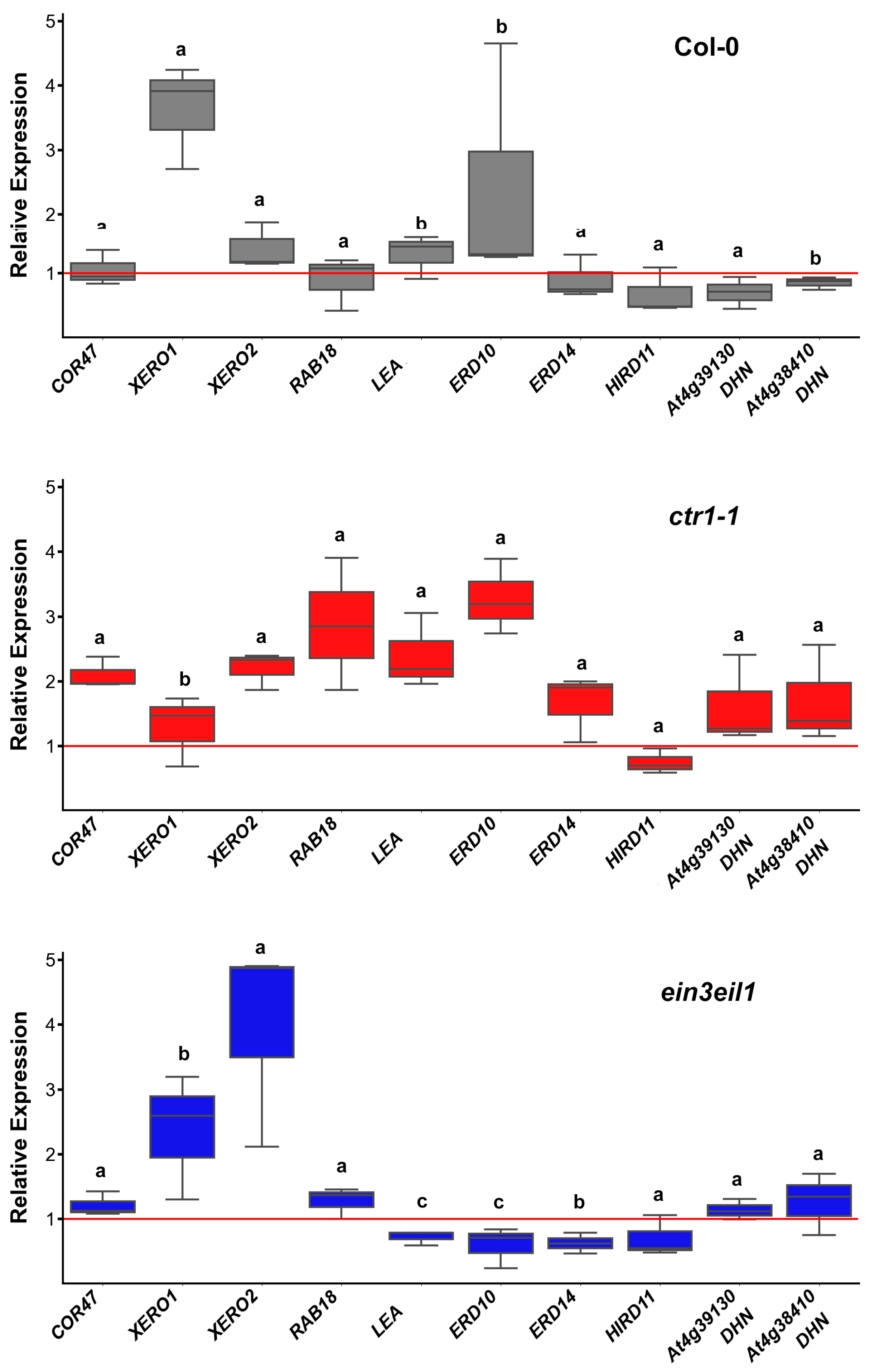
2.3. Transcript Profiling of Dehydrin-Coding Genes in Organs of A. thaliana Wild-Type (Col-0) and Ethylene Mutants (ctr1-1 and ein3eil1) Under Prolonged Dehydration
2.3.1. Dehydrin Transcript Profiles in Leaves
2.3.2. Dehydrin Transcript Profiles in Roots
2.4. Immunodetection of Dehydrin Types in Leaves and Roots of A. thaliana Wild Type (Col-0) and Ethylene Mutants (ctr1-1 and ein3eil1) Under Prolonged Dehydration
2.4.1. Dehydrin Immunosignals in Leaves
2.4.2. DHN Immunosignals in Roots
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Survival Test
4.3. Measurements of MDA, H2O2, and Free Proline Contents
4.4. RT-qPCR Analysis of A. thaliana Dehydrin Genes
4.5. Immunodetection of Dehydrins
4.6. Statistical Analysis
5. Conclusions
- ethylene signaling fine-tuned the expression of specific dehydrin genes;
- ethylene signaling was negatively correlated with the accumulation of Y-segment-containing dehydrins in Arabidopsis.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-Dependent and ABA-Independent Signaling in Response to Osmotic Stress in Plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Urano, K.; Maruyama, K.; Ogata, Y.; Morishita, Y.; Takeda, M.; Sakurai, N.; Suzuki, H.; Saito, K.; Shibata, D.; Kobayashi, M.; et al. Characterization of the ABA-Regulated Global Responses to Dehydration in Arabidopsis by Metabolomics. Plant J. 2009, 57, 1065–1078. [Google Scholar] [CrossRef]
- Ha, S.; Vankova, R.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S. Cytokinins: Metabolism and Function in Plant Adaptation to Environmental Stresses. Trends Plant Sci. 2012, 17, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Urano, K.; Yoshiwara, K.; Morishita, Y.; Sakurai, N.; Suzuki, H.; Kojima, M.; Sakakibara, H.; Shibata, D.; Saito, K.; et al. Integrated Analysis of the Effects of Cold and Dehydration on Rice Metabolites, Phytohormones, and Gene Transcripts. Plant Physiol. 2014, 164, 1759–1771. [Google Scholar] [CrossRef]
- Skirycz, A.; De Bodt, S.; Obata, T.; De Clercq, I.; Claeys, H.; De Rycke, R.; Andriankaja, M.; Van Aken, O.; Van Breusegem, F.; Fernie, A.R.; et al. Developmental Stage Specificity and the Role of Mitochondrial Metabolism in the Response of Arabidopsis Leaves to Prolonged Mild Osmotic Stress. Plant Physiol. 2010, 152, 226–244. [Google Scholar] [CrossRef]
- Naing, A.H.; Campol, J.R.; Kang, H.; Xu, J.; Chung, M.Y.; Kim, C.K. Role of Ethylene Biosynthesis Genes in the Regulation of Salt Stress and Drought Stress Tolerance in Petunia. Front. Plant Sci. 2022, 13, 844449. [Google Scholar] [CrossRef] [PubMed]
- Narayana, I.; Lalonde, S.; Saini, H.S. Water-Stress-Induced Ethylene Production in Wheat: A Fact or Artifact? Plant Physiol. 1991, 96, 406–410. [Google Scholar] [CrossRef]
- Arraes, F.B.; Beneventi, M.A.; Lisei de Sa, M.E.; Paixao, J.F.; Albuquerque, E.V.; Marin, S.R.; Purgatto, E.; Nepomuceno, A.L.; Grossi-de-Sa, M.F. Implications of Ethylene Biosynthesis and Signaling in Soybean Drought Stress Tolerance. BMC Plant Biol. 2015, 15, 213. [Google Scholar] [CrossRef]
- Sarapat, S.; Songwattana, P.; Longtonglang, A.; Umnajkitikorn, K.; Girdthai, T.; Tittabutr, P.; Boonkerd, N.; Teaumroong, N. Effects of Increased 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Activity in Bradyrhizobium sp. SUTN9-2 on Mung Bean Symbiosis under Water Deficit Conditions. Microbes Environ. 2020, 35, ME20024. [Google Scholar] [CrossRef]
- Ge, X.M.; Cai, H.L.; Lei, X.; Zhou, X.; Yue, M.; He, J.M. Heterotrimeric G Protein Mediates Ethylene-Induced Stomatal Closure via Hydrogen Peroxide Synthesis in Arabidopsis. Plant J. 2015, 82, 138–150. [Google Scholar] [CrossRef]
- Li, Z.; Huang, R. The Reciprocal Regulation of Abscisic Acid and Ethylene Biosyntheses. Plant Signal Behav. 2011, 6, 1647–1650. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Yu, Y.; Li, S.; Wang, J.; Tang, S.; Huang, R. Abscisic Acid Antagonizes Ethylene Production through the ABI4-Mediated Transcriptional Repression of ACS4 and ACS8 in Arabidopsis. Mol. Plant 2016, 9, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Ethylene Inhibits Abscisic Acid-Induced Stomatal Closure in Arabidopsis. Plant Physiol. 2005, 138, 2337–2343. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, L.; Ma, W.; Peng, Z.; Shi, J.; Pan, F.; Gao, Y.; Sui, X.; Rengel, Z.; Chen, Q.; et al. Ethylene Inhibits ABA-Induced Stomatal Closure via Regulating NtMYB184-Mediated Flavonol Biosynthesis in Tobacco. J. Exp. Bot. 2023, 74, 6735–6748. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Cytokinin and Auxin Inhibit Abscisic Acid-Induced Stomatal Closure by Enhancing Ethylene Production in Arabidopsis. J. Exp. Bot. 2006, 57, 2259–2266. [Google Scholar] [CrossRef]
- Skirycz, A.; Vandenbroucke, K.; Clauw, P.; Maleux, K.; De Meyer, B.; Dhondt, S.; Pucci, A.; Gonzalez, N.; Hoeberichts, F.; Tognetti, V.B.; et al. Survival and Growth of Arabidopsis Plants Given Limited Water are not Equal. Nat. Biotechnol. 2011, 29, 212–214. [Google Scholar] [CrossRef]
- Vaseva, I.I.; Qudeimat, E.; Potuschak, T.; Du, Y.; Genschik, P.; Vandenbussche, F.; Van Der Straeten, D. The Plant Hormone Ethylene Restricts Arabidopsis Growth via the Epidermis. Proc. Nat. Acad. Sci. USA 2018, 115, E4130–E4139. [Google Scholar] [CrossRef]
- Scharwies, J.D.; Clarke, T.; Zheng, Z.; Dinneny, A.; Birkeland, S.; Veltman, M.A.; Sturrock, C.J.; Banda, J.; Torres-Martínez, H.H.; Viana, W.G.; et al. Moisture-Responsive Root-Branching Pathways Identified in Diverse Maize Breeding Germplasm. Science 2025, 387, 666–673. [Google Scholar] [CrossRef]
- Peng, J.; Li, Z.; Wen, X.; Li, W.; Shi, H.; Yang, L.; Zhu, H.; Guo, H. Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in Arabidopsis. PLoS Genet. 2014, 10, e1004664. [Google Scholar] [CrossRef]
- Fatma, M.; Asgher, M.; Iqbal, N.; Rasheed, F.; Sehar, Z.; Sofo, A.; Khan, N.A. Ethylene Signaling under Stressful Environments: Analyzing Collaborative Knowledge. Plants 2022, 11, 2211. [Google Scholar] [CrossRef]
- Close, T.J. Dehydrins: Emergence of a Biochemical Role of a Family of Plant Dehydration Proteins. Physiol. Plant 1996, 97, 795–803. [Google Scholar] [CrossRef]
- Szlachtowska, Z.; Rurek, M. Plant Dehydrins and Dehydrin-Like Proteins: Characterization and Participation in Abiotic stress Response. Front. Plant Sci. 2023, 14, 1213188. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, Z.; Liu, H.; Zhang, Y.; Bai, Z.; Zhang, L. Effect of K-/S- Segments on Subcellular Localization and Dimerization of Wheat Dehydrin WZY1-2. Plant Signal Behav. 2020, 15, 1827583. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Zhang, T.; Yang, X.; Li, D. Functional Characterization of KS-Type Dehydrin ZmDHN13 and its Related Conserved Domains under Oxidative Stress. Sci. Rep. 2017, 7, 7361. [Google Scholar] [CrossRef] [PubMed]
- Maszkowska, J.; Dębski, J.; Kulik, A.; Kistowski, M.; Bucholc, M.; Lichocka, M.; Klimecka, M.; Sztatelman, O.; Szymańska, K.P.; Dadlez, M.; et al. Phosphoproteomic Analysis Reveals that Dehydrins ERD10 and ERD14 are Phosphorylated by SNF1-Related Protein Kinase 2.10 in Response to Osmotic Stress. Plant Cell Environ. 2019, 42, 931–946. [Google Scholar] [CrossRef]
- Wei, H.; Yang, Y.; Himmel, M.E.; Tucker, M.P.; Ding, S.-Y.; Yang, S.; Arora, R. Identification and Characterization of Five Cold Stress-Related Rhododendron Dehydrin Genes: Spotlight on a FSK-Type Dehydrin With Multiple F-Segments. Front. Bioeng. Biotechnol. 2019, 7, 30. [Google Scholar] [CrossRef]
- Sun, Z.; Li, S.; Chen, W.; Zhang, J.; Zhang, L.; Sun, W.; Wang, Z. Plant Dehydrins: Expression, Regulatory Networks, and Protective Roles in Plants Challenged by Abiotic Stress. Int. J. Mol. Sci. 2021, 22, 12619. [Google Scholar] [CrossRef]
- Dirk, L.M.A.; Abdel, C.G.; Ahmad, I.; Neta, I.C.S.; Pereira, F.E.C.B.; Uneda-Trevisoli, S.H.; Pinheiro, D.G.; Downie, A.B. Late Embryogenesis Abundant Protein-Client Protein Interactions. Plants 2020, 9, 814. [Google Scholar] [CrossRef]
- Richard, S.; Morency, M.J.; Drevet, C.; Jouanin, L.; Séguin, A. Isolation and Characterization of a Dehydrin Gene from White Spruce Induced Upon Wounding, Drought and Cold Stresses. Plant Mol. Biol. 2000, 43, 1–10. [Google Scholar] [CrossRef]
- Mota, A.P.Z.; Oliveira, T.N.; Vinson, C.C.; Williams, T.C.R.; Costa, M.M.C.; Araujo, A.C.G.; Danchin, E.G.J.; Grossi-de-Sá, M.F.; Guimaraes, P.M.; Brasileiro, A.C.M. Contrasting Effects of Wild Arachis Dehydrin Under Abiotic and Biotic Stresses. Front. Plant Sci. 2019, 10, 497. [Google Scholar] [CrossRef]
- Robison, J.D.; Yamasaki, Y.; Randall, S.K. The Ethylene Signaling Pathway Negatively Impacts CBF/DREB-Regulated Cold Response in Soybean (Glycine max). Front. Plant Sci. 2019, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Vaseva, I.; Vissenberg, K.; Van Der Straeten, D. Ethylene in Vegetative Development: A Tale with a Riddle. New Phytol. 2012, 194, 895–909. [Google Scholar] [CrossRef]
- Li, W.; Ma, M.; Feng, Y.; Li, H.; Wang, Y.; Ma, Y.; Li, M.; An, F.; Guo, H. EIN2-Directed Translational Regulation of Ethylene Signaling in Arabidopsis. Cell 2015, 163, 670–683. [Google Scholar] [CrossRef]
- Merchante, C.; Brumos, J.; Yun, J.; Hu, Q.; Spencer, K.R.; Enríquez, P.; Binder, B.M.; Heber, S.; Stepanova, A.N.; Alonso, J.M. Gene-Specific Translation Regulation Mediated by the Hormone-Signaling Molecule EIN2. Cell 2015, 163, 684–697. [Google Scholar] [CrossRef]
- Park, H.L.; Seo, D.H.; Lee, H.Y.; Bakshi, A.; Park, C.; Chien, Y.C.; Kieber, J.J.; Binder, B.M.; Yoon, G.M. Ethylene-Triggered Subcellular Trafficking of CTR1 Enhances the Response to Ethylene Gas. Nat. Commun. 2023, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Vaseva, I.I.; Simova-Stoilova, L.; Kirova, E.; Mishev, K.; Depaepe, T.; Van Der Straeten, D.; Vassileva, V. Ethylene Signaling in Salt-Stressed Arabidopsis thaliana ein2-1 and ctr1-1 Mutants—A dissection of Molecular Mechanisms Involved in Acclimation. Plant Physiol. Biochem. 2021, 167, 999–1010. [Google Scholar] [CrossRef]
- Bharadwaj, P.S.; Sanchez, L.; Li, D.; Enyi, D.; Van de Poel, B.; Chang, C. The Plant Hormone Ethylene Promotes Abiotic Stress Tolerance in the Liverwort Marchantia polymorpha. Front. Plant Sci. 2022, 13, 998267. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, M.; Zhu, Z.; Li, S.; Xu, Y.; Zhang, C.; Singer, S.D.; Wang, Y. Identification of the Dehydrin Gene Family from Grapevine Species and Analysis of Their Responsiveness to Various Forms of Abiotic and Biotic Stress. BMC Plant Biol. 2012, 12, 140. [Google Scholar] [CrossRef]
- Voichek, Y.; Hristova, G.; Mollá-Morales, A.; Weigel, D.; Nordborg, M. Widespread Position-Dependent Transcriptional Regulatory Sequences in Plants. Nat. Genet. 2024, 56, 2238–2246. [Google Scholar] [CrossRef]
- Lin, C.H.; Peng, P.H.; Ko, C.Y.; Markhart, A.H.; Lin, T.Y. Characterization of a Novel Y2K-Type Dehydrin VrDhn1 from Vigna radiata. Plant Cell Physiol. 2012, 53, 930–942. [Google Scholar] [CrossRef]
- Hernández-Sánchez, I.E.; Maruri-López, I.; Graether, S.P.; Jiménez-Bremont, J.F. In Vivo Evidence for Homo- and Heterodimeric Interactions of Arabidopsis thaliana Dehydrins AtCOR47, AtERD10, and AtRAB18. Sci. Rep. 2017, 7, 17036. [Google Scholar] [CrossRef] [PubMed]
- Paes de Melo, B.; Carpinetti, P.d.A.; Fraga, O.T.; Rodrigues-Silva, P.L.; Fioresi, V.S.; de Camargos, L.F.; Ferreira, M.F.d.S. Abiotic Stresses in Plants and Their Markers: A Practical View of Plant Stress Responses and Programmed Cell Death Mechanisms. Plants 2022, 11, 1100. [Google Scholar] [CrossRef]
- Martin, R.E.; Marzol, E.; Estevez, J.M.; Muday, G.K. Ethylene Signaling Increases Reactive Oxygen Species Accumulation to Drive Root Hair Initiation in Arabidopsis. Development 2022, 149, dev200487. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, E.J.; Cheng, M.C.; Lin, T.P. Functional Characterization of an Abiotic Stress-Inducible Transcription Factor AtERF53 in Arabidopsis thaliana. Plant Mol. Biol. 2013, 82, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, S.; Tian, H.; Wang, S.; Chen, J.G. The Small Ethylene Response Factor ERF96 is Involved in the Regulation of the Abscisic Acid Response in Arabidopsis. Front. Plant Sci. 2015, 6, 1064. [Google Scholar] [CrossRef]
- Wang, K.; Guo, H.; Yin, Y. AP2/ERF Transcription Factors and Their Functions in Arabidopsis Responses to Abiotic Stresses. Environ. Exp. Bot. 2024, 222, 105763. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, J.H.; Lee, M.H.; Yu, J.H.; Kim, S.Y. Isolation and Functional Characterization of CE1 Binding Proteins. BMC Plant Biol. 2010, 10, 277. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, S.J.; Kim, S.Y. AtERF15 is a Positive Regulator of ABA Response. Plant Cell Rep. 2015, 34, 71–81. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Quan, R.; Li, G.; Wang, R.; Huang, R. An AP2 Domain-Containing Gene, ESE1, Targeted by the Ethylene Signaling Component EIN3 is Important for the Salt Response in Arabidopsis. Plant Physiol. 2011, 157, 854–865. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhang, R.; Huang, R. The Ethylene Response Factor AtERF98 Enhances Tolerance to Salt Through the Transcriptional Activation of Ascorbic Acid Synthesis in Arabidopsis. Plant J. 2012, 71, 273–287. [Google Scholar] [CrossRef]
- Illgen, S.; Zintl, S.; Zuther, E.; Hincha, D.K.; Schmülling, T. Characterisation of the ERF102 to ERF105 Genes of Arabidopsis thaliana and Their Role in the Response to Cold Stress. Plant Mol. Biol. 2020, 103, 303–320. [Google Scholar] [CrossRef]
- Hébert-Haché, A.; Willwerth, J.J.; Kemp, B.; Inglis, D.L. Correlation between Dehydrin-Like Proteins and Cold Hardiness of Grapevine. Can. J. Plant Sci. 2023, 103, 494–506. [Google Scholar] [CrossRef]
- Claeys, H.; Van Landeghem, S.; Dubois, M.; Maleux, K.; Inzé, D. What Is Stress? Dose-Response Effects in Commonly Used in Vitro Stress Assays. Plant Physiol. 2014, 165, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Kramer, G.; Norman, H.; Krizek, D.; Mirecki, R. Influence of UV-B Radiation on Polyamines, Lipid Peroxidation and Membrane Lipids in Cucumber. Phytochemistry 1991, 30, 2101–2108. [Google Scholar] [CrossRef]
- Frew, J.E.; Jones, P.; Scholes, F. Spectrophotometric Determination of Hydrogen Peroxide and Organic Hydroperoxide at Low Concentrations in Aqueous Solution. Anal. Chim. Acta 1983, 155, 139–150. [Google Scholar] [CrossRef]
- Bates, L.; Waldren, R.; Teare, I. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vaseva, I.; Anders, I.; Feller, U. Identification and Expression of Different Dehydrin Subclasses Involved in the Drought Response of Trifolium repens. J. Plant Physiol. 2014, 171, 213–224. [Google Scholar] [CrossRef]
- Hunter, J.D. Matplotlib: A 2D Graphics Environment. Comp. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]


| Gene Name | Locus | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| HIRD11 | At1g54410 | cacgacggagaaggcaaaag | gatgcacggttctcctgtct |
| LEA | At2g21490 | ccggtgttgttagctccact | agatgctcttcaagcgaccc |
| ERD10 | At1g20450 | ggggaaacacttgtttactcaatgg | tggcggaacggactaatttca |
| ERD14 | At1g76180 | gaacaggaggtgccaaaggt | aagaactgtcgcttcggtga |
| XERO1 | At3g50980 | gggtcatcacgactccaaca | ccatgcaacgaccataagcg |
| XERO2 | At3g50970 | caaactgggactaacacggc | ctagtgatgaccaccgggaag |
| RAB18 | At5g66400 | caccacgccgacattttctg | cggttctggtaagacgccat |
| COR47 | At1g20440 | ctacgacggagcttccagtg | cgaatgtcccactcccacat |
| DHN | At4g38410 | cattggtcgacgaaacgcag | cgtttagccaaggtgttgcc |
| DHN | At4g39130 | cgagtttggtaacgccatgc | ggtcttgaagagggtcgtgg |
| EF-1ɑ | At5g60390 | cagatcggcaacggctac | gagaaggtctccaccaccat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaseva, I.I.; Balzhyk, H.; Trailova, M.; Nikolova, T.; Katerova, Z.; Galabova, S.; Todorova, D.; Sergiev, I.; Vassileva, V. Ethylene Signaling Modulates Dehydrin Expression in Arabidopsis thaliana Under Prolonged Dehydration. Int. J. Mol. Sci. 2025, 26, 4148. https://doi.org/10.3390/ijms26094148
Vaseva II, Balzhyk H, Trailova M, Nikolova T, Katerova Z, Galabova S, Todorova D, Sergiev I, Vassileva V. Ethylene Signaling Modulates Dehydrin Expression in Arabidopsis thaliana Under Prolonged Dehydration. International Journal of Molecular Sciences. 2025; 26(9):4148. https://doi.org/10.3390/ijms26094148
Chicago/Turabian StyleVaseva, Irina I., Heorhii Balzhyk, Maria Trailova, Tsvetina Nikolova, Zornitsa Katerova, Simona Galabova, Dessislava Todorova, Iskren Sergiev, and Valya Vassileva. 2025. "Ethylene Signaling Modulates Dehydrin Expression in Arabidopsis thaliana Under Prolonged Dehydration" International Journal of Molecular Sciences 26, no. 9: 4148. https://doi.org/10.3390/ijms26094148
APA StyleVaseva, I. I., Balzhyk, H., Trailova, M., Nikolova, T., Katerova, Z., Galabova, S., Todorova, D., Sergiev, I., & Vassileva, V. (2025). Ethylene Signaling Modulates Dehydrin Expression in Arabidopsis thaliana Under Prolonged Dehydration. International Journal of Molecular Sciences, 26(9), 4148. https://doi.org/10.3390/ijms26094148










