Characterization and Functional Analysis of Trim38 in the Immune Response of the Large Yellow Croaker (Larimichthys crocea) Against Pseudomonas plecoglossicida Infection
Abstract
1. Introduction
2. Results
2.1. Sequence Characteristics of Lctrim38
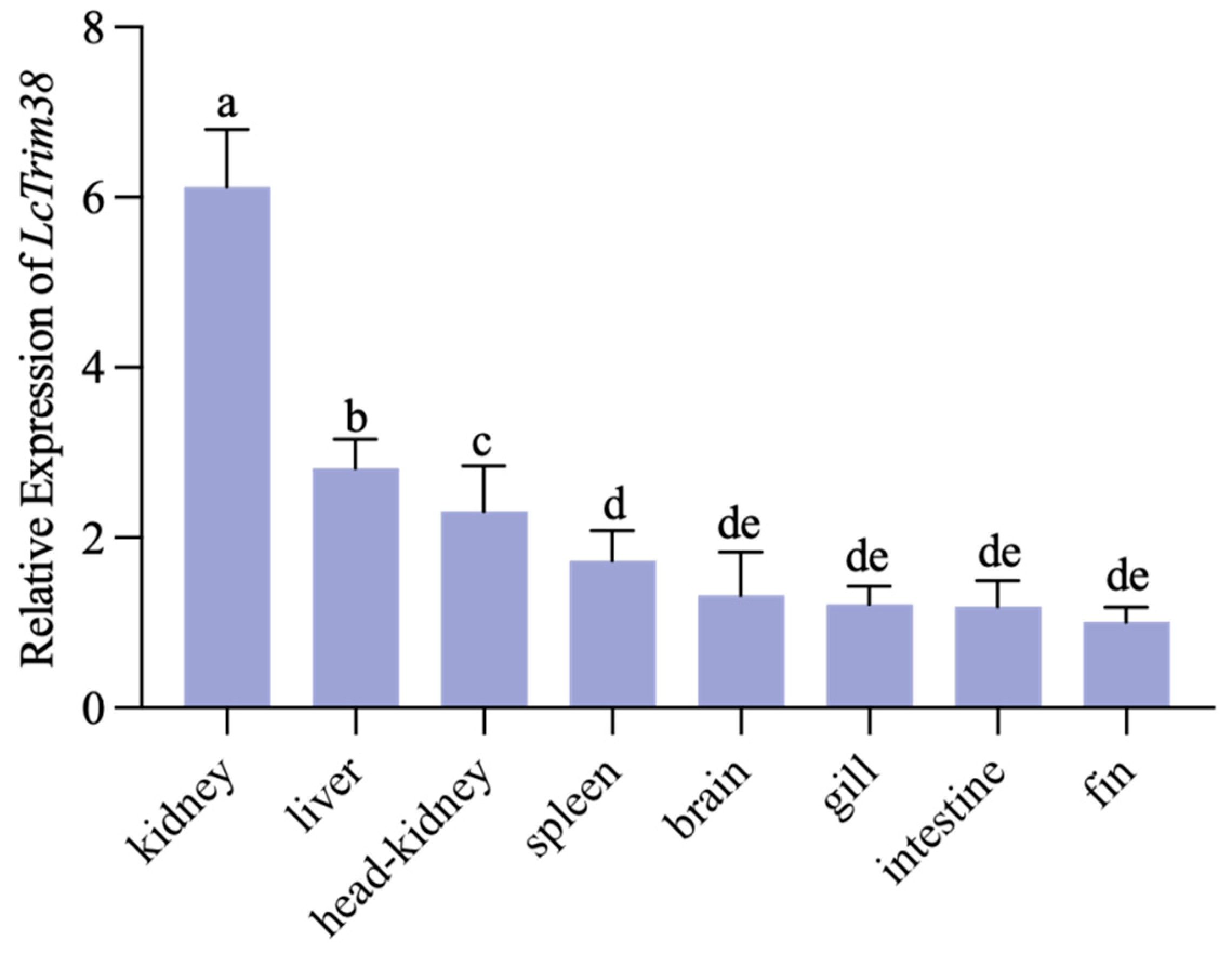
2.2. Tissue Expression Pattern
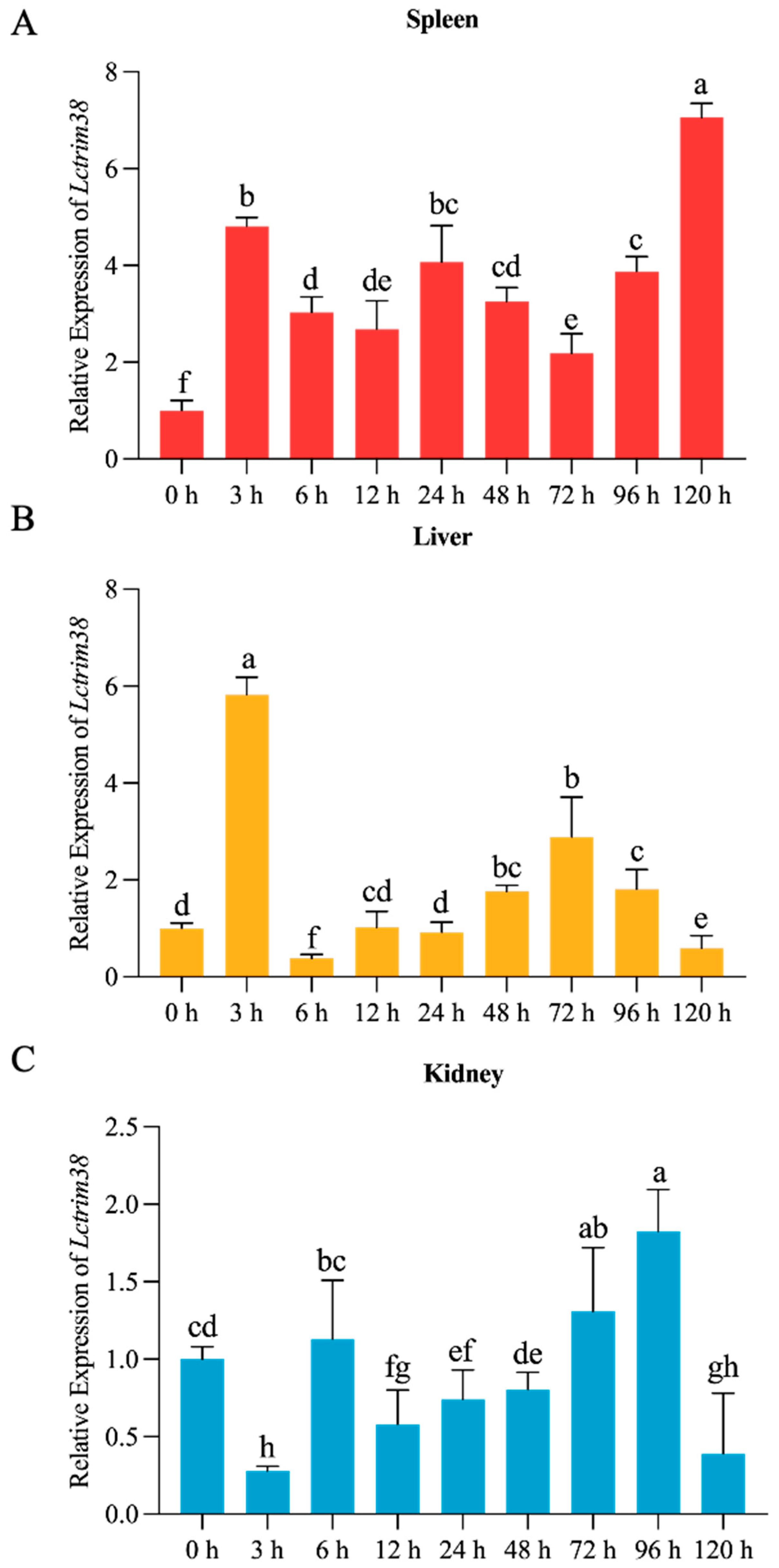
2.3. Lctrim38 Expression Profiles in Response to P. plecoglossicida Infection
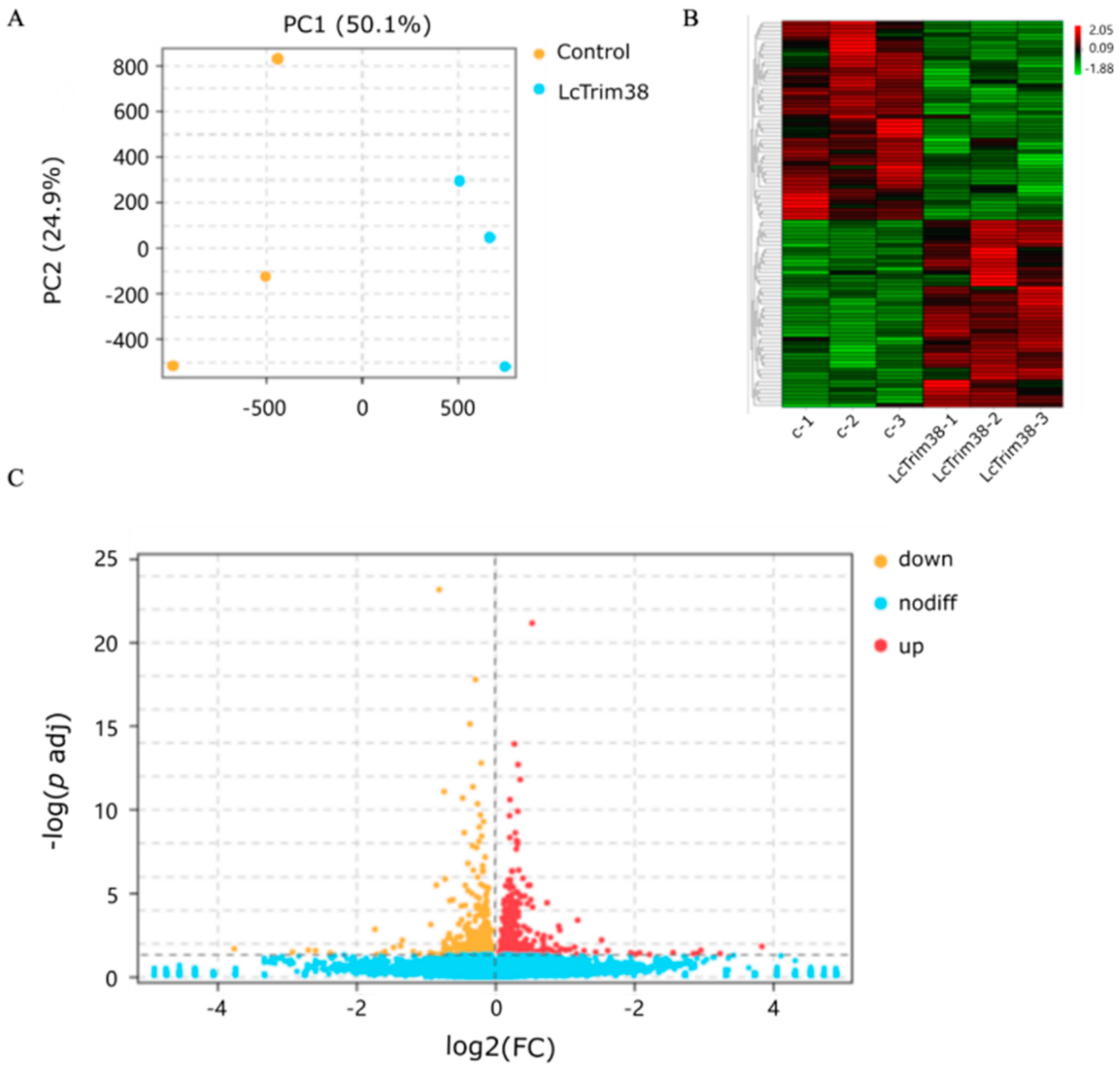
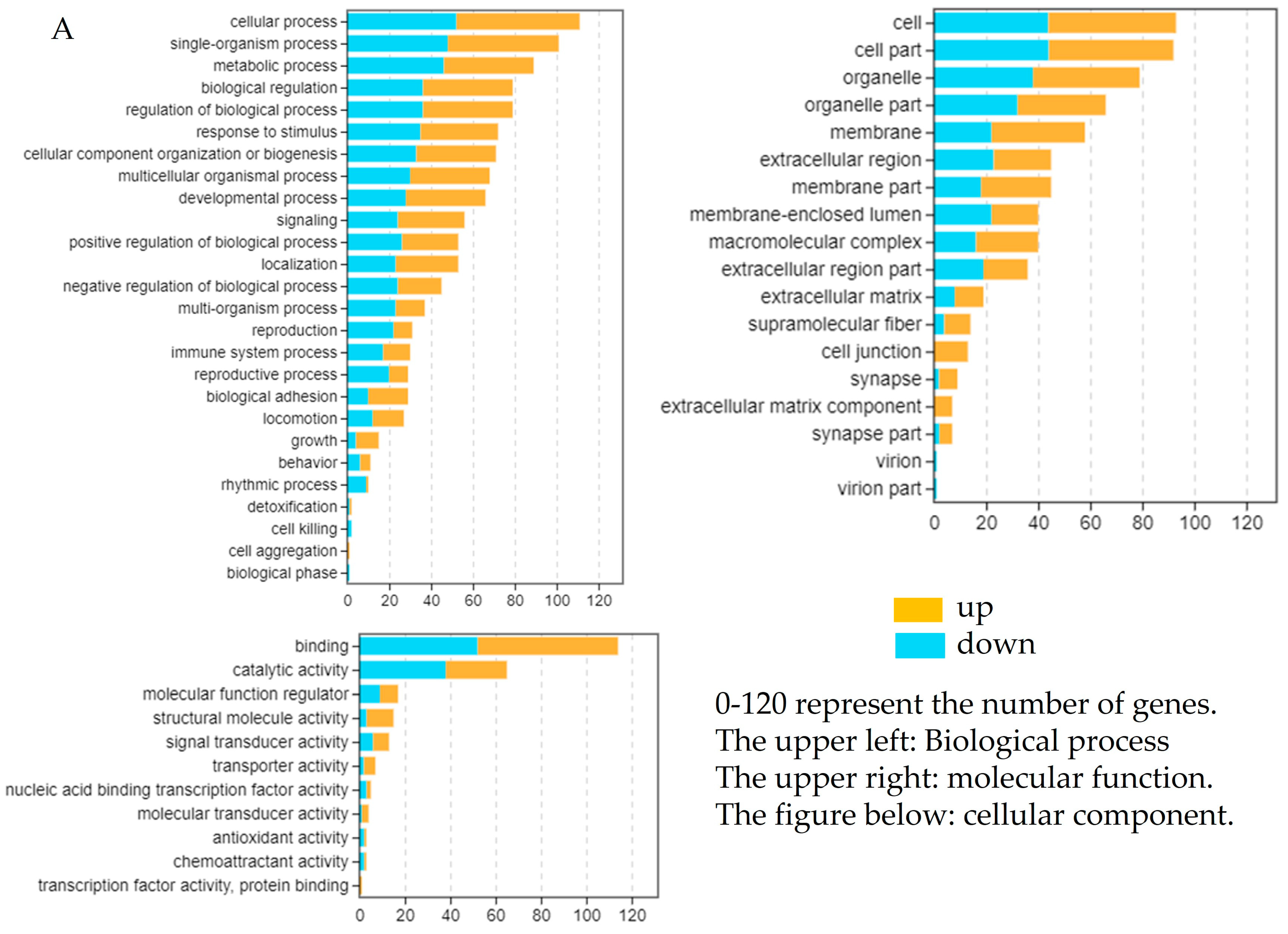
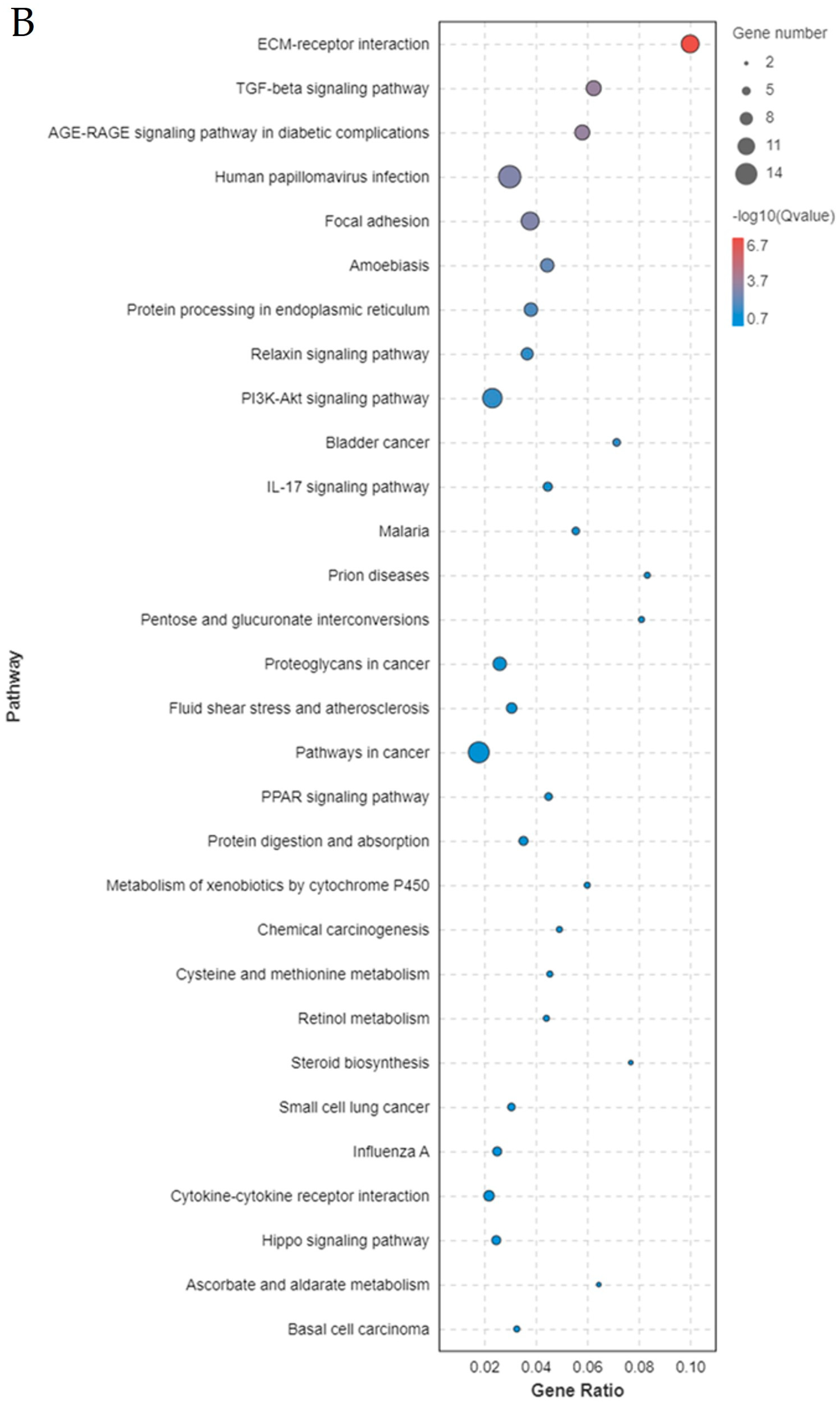
2.4. Transcriptome Data Analysis and Identification of DEGs
2.5. Enrichment Analysis of DEGs After Overexpression
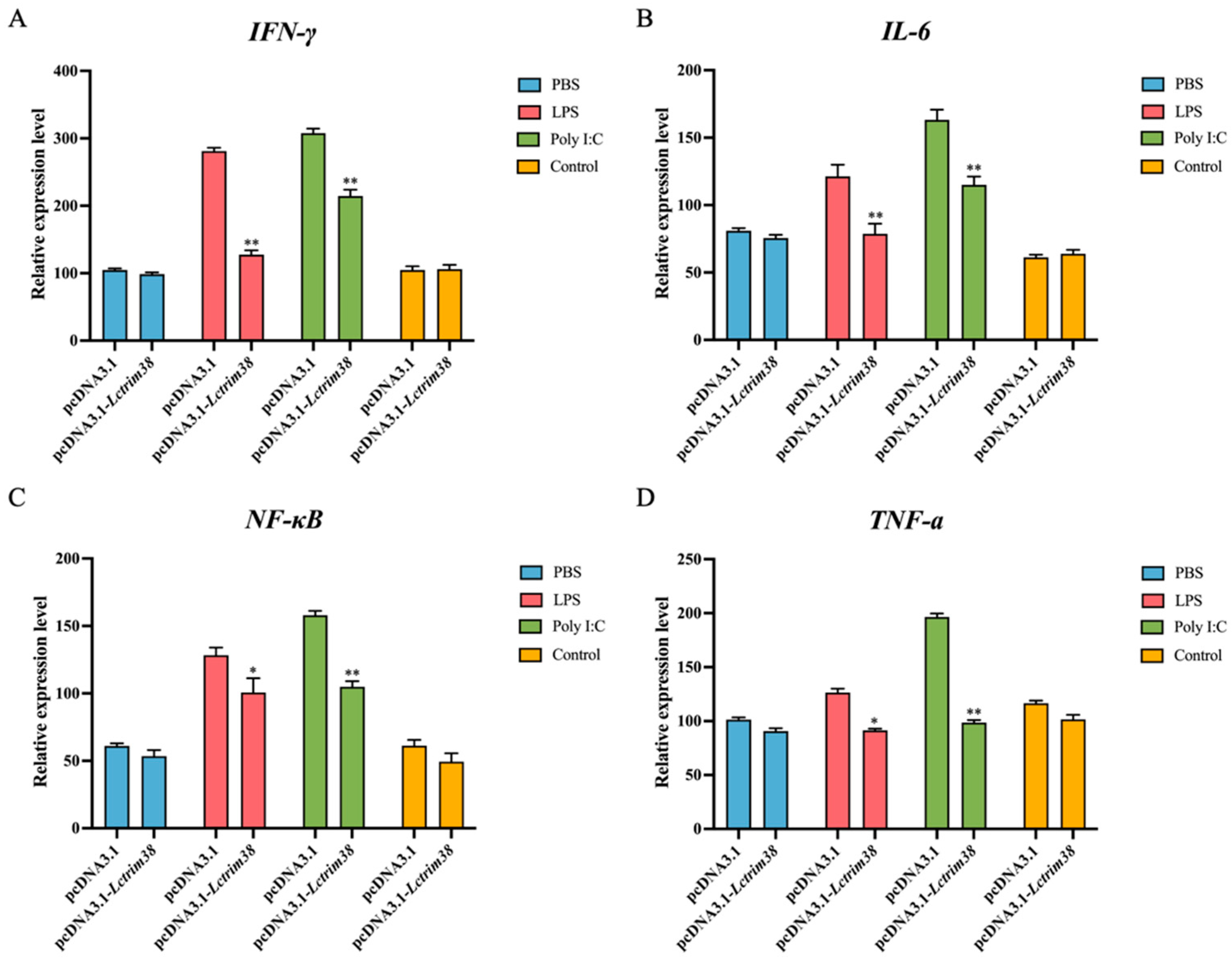
2.6. Effect of Lctrim38 on Cytokine Response Triggered by LPS and Poly I:C
3. Discussion
4. Materials and Methods
4.1. Experimental Fish
4.2. RNA Extraction and Reverse Transcription
4.3. Cloning and Bioinformatics Analysis of Lctrim38
4.4. Quantitative Real-Time PCR (RT-qPCR)
4.5. Lctrim38 Overexpression
4.6. cDNA Library Construction and Sequencing
4.7. Transcriptomic Analysis
4.8. Effect of Overexpressed Lctrim38 on Cytokine Expression
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaturvedi, P.; Bhat, R.A.H.; Pande, A. Antimicrobial Peptides of Fish: Innocuous Alternatives to Antibiotics. Rev. Aquac. 2020, 12, 85–106. [Google Scholar] [CrossRef]
- Wein, T.; Sorek, R. Bacterial Origins of Human Cell-Autonomous Innate Immune Mechanisms. Nat. Rev. Immunol. 2022, 22, 629–638. [Google Scholar] [CrossRef]
- Mokhtar, D.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.; Sayed, R. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Wang, L.; Ning, S. TRIMming Type I Interferon-Mediated Innate Immune Response in Antiviral and Antitumor Defense. Viruses 2021, 13, 279. [Google Scholar] [CrossRef]
- Versteeg, G.A.; Rajsbaum, R.; Sánchez-Aparicio, M.T.; Maestre, A.M.; Valdiviezo, J.; Shi, M.; Inn, K.-S.; Fernandez-Sesma, A.; Jung, J.; García-Sastre, A. The E3-Ligase TRIM Family of Proteins Regulates Signaling Pathways Triggered by Innate Immune Pattern-Recognition Receptors. Immunity 2013, 38, 384–398. [Google Scholar] [CrossRef]
- Hu, M.-M.; Shu, H.-B. Multifaceted Roles of TRIM38 in Innate Immune and Inflammatory Responses. Cell. Mol. Immunol. 2017, 14, 331–338. [Google Scholar] [CrossRef]
- Hatakeyama, S. TRIM Family Proteins: Roles in Autophagy, Immunity, and Carcinogenesis. Trends Biochem. Sci. 2017, 42, 297–311. [Google Scholar] [CrossRef]
- Hatakeyama, S. TRIM Proteins and Cancer. Nat. Rev. Cancer 2011, 11, 792–804. [Google Scholar] [CrossRef]
- Nisole, S.; Stoye, J.P.; Saïb, A. TRIM Family Proteins: Retroviral Restriction and Antiviral Defence. Nat. Rev. Microbiol. 2005, 3, 799–808. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Fiorentini, F.; Esposito, D.; Rittinger, K. Does It Take Two to Tango? RING Domain Self-Association and Activity in TRIM E3 Ubiquitin Ligases. Biochem. Soc. Trans. 2020, 48, 2615–2624. [Google Scholar] [CrossRef]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The Cytoplasmic Body Component TRIM5α Restricts HIV-1 Infection in Old World Monkeys. Nature 2004, 427, 848–853. [Google Scholar] [CrossRef]
- Sun, Q.; Han, X.; Meng, L.; Li, H.; Chen, Y.; Yin, L.; Wang, C.; Wang, J.; Li, M.; Gao, X.; et al. TRIM38 Induced in Respiratory Syncytial Virus-Infected Cells Downregulates Type I Interferon Expression by Competing with TRIM25 to Bind RIG-I. Inflammation 2024, 47, 1328–1343. [Google Scholar] [CrossRef]
- Shang, Z.; Zhang, S.; Wang, J.; Zhou, L.; Zhang, X.; Billadeau, D.D.; Yang, P.; Zhang, L.; Zhou, F.; Bai, P.; et al. TRIM25 Predominately Associates with Anti-Viral Stress Granules. Nat. Commun. 2024, 15, 4127. [Google Scholar] [CrossRef]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Fu, R.; Zhu, X.; Ren, P.; Zhang, L.; Ai, Q.; Sun, Y.; Wang, Z. Intestinal Microbiota Was Closely Related to Feed Efficiency of Larimichthys crocea Fed Two Fishmeal-Free Diets. Aquaculture 2025, 594, 741367. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, Z.; Zhao, J.; Zhou, T.; Bai, H.; Ke, Q.; Pu, F.; Zheng, W.; Xu, P. Genome-Wide Association Study Identifies Genomic Loci of Sex Determination and Gonadosomatic Index Traits in Large Yellow Croaker (Larimichthys crocea). Mar. Biotechnol. 2021, 23, 127–139. [Google Scholar] [CrossRef]
- Huang, Y.; Gou, T.; Li, W.; Han, F. Unraveling the Immune Functions of Large Yellow Croaker Tmem208 in Response to Pseudomonas plecoglossicida: Insights from Cloning, Expression Profiling, and Transcriptome Analysis. Fish Shellfish Immunol. 2024, 149, 109584. [Google Scholar] [CrossRef]
- Zhang, J.T.; Zhou, S.M.; An, S.W.; Chen, L.; Wang, G.L. Visceral Granulomas in Farmed Large Yellow Croaker, Larimichthys crocea (Richardson), Caused by a Bacterial Pathogen, Pseudomonas plecoglossicida. J. Fish Dis. 2014, 37, 113–121. [Google Scholar] [CrossRef]
- Li, Z.; Fang, M.; Tang, X.; Zhang, D.; Wang, Z. Disentangling Genetic Variation for Endurance and Resistance to Visceral White-Nodules Disease in Large Yellow Croaker (Larimichthys crocea) Using Genome Information. Aquaculture 2023, 564, 739045. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, L.; Zhang, M.; Yuan, C.; Gao, C. E3 Ubiquitin Ligase Tripartite Motif 38 Negatively Regulates TLR-Mediated Immune Responses by Proteasomal Degradation of TNF Receptor-Associated Factor 6 in Macrophages. J. Immunol. 2012, 188, 2567–2574. [Google Scholar] [CrossRef]
- Ribet, D.; Cossart, P. Ubiquitin, SUMO, and NEDD8: Key Targets of Bacterial Pathogens. Trends Cell Biol. 2018, 28, 926–940. [Google Scholar] [CrossRef] [PubMed]
- Nenasheva, V.V.; Stepanenko, E.A.; Tarantul, V.Z. Multi-Directional Mechanisms of Participation of the TRIM Gene Family in Response of Innate Immune System to Bacterial Infections. Biochemistry 2024, 89, 1283–1299. [Google Scholar] [CrossRef]
- Barr, S.D.; Smiley, J.R.; Bushman, F.D. The Interferon Response Inhibits HIV Particle Production by Induction of TRIM22. PLoS Patho. 2008, 4, e1000007. [Google Scholar] [CrossRef]
- Langevin, C.; Aleksejeva, E.; Houel, A.; Briolat, V.; Torhy, C.; Lunazzi, A.; Levraud, J.-P.; Boudinot, P. FTR83, a Member of the Large Fish-Specific finTRIM Family, Triggers IFN Pathway and Counters Viral Infection. Front. Immunol. 2017, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Sardiello, M.; Cairo, S.; Fontanella, B.; Ballabio, A.; Meroni, G. Genomic Analysis of the TRIM Family Reveals Two Groups of Genes with Distinct Evolutionary Properties. BMC Evol. Biol. 2008, 8, 225. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Ren, Q.; He, T.; Chen, X. An Outbreak of Visceral White Nodules Disease Caused by Pseudomonas plecoglossicida at a Water Temperature of 12°C in Cultured Large Yellow Croaker (Larimichthys crocea) in China. J. Fish Dis. 2020, 43, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, S.; Han, F.; Wang, Z. C1QBP Is a Critical Component in the Immune Response of Large Yellow Croaker (Larimichthys crocea) against Visceral White Spot Disease Caused by Pseudomonas plecoglossicida. Fish Shellfish Immunol. 2024, 146, 109372. [Google Scholar] [CrossRef]
- Dhingra, V.; Li, X.; Liu, Y.; Fu, Z.F. Proteomic Profiling Reveals That Rabies Virus Infection Results in Differential Expression of Host Proteins Involved in Ion Homeostasis and Synaptic Physiology in the Central Nervous System. J. Neurovirol. 2007, 13, 107–117. [Google Scholar] [CrossRef]
- Lu, Z.; Deng, M.; Ma, G.; Chen, L. TRIM38 Protects H9c2 Cells from Hypoxia/Reoxygenation Injury via the TRAF6/TAK1/NF-κB Signalling Pathway. PeerJ 2022, 10, e13815. [Google Scholar] [CrossRef]
- Rajsbaum, R.; García-Sastre, A.; Versteeg, G.A. TRIMmunity: The Roles of the TRIM E3-Ubiquitin Ligase Family in Innate Antiviral Immunity. J. Mol. Biol. 2014, 426, 1265–1284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Xu, M.; Wei, C.; Qin, A.-P.; Liu, C.-F.; Hong, L.-Z.; Zhao, X.-Y.; Liu, J.; Qin, Z.-H. Neuroprotective Effects of Pioglitazone in a Rat Model of Permanent Focal Cerebral Ischemia Are Associated with Peroxisome Proliferator-Activated Receptor Gamma-Mediated Suppression of Nuclear Factor-κB Signaling Pathway. Neuroscience 2011, 176, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Chen, Z.-L.; Zhang, T.; Wei, C.; Shi, W.-Y. TGF-β and NF-κB Signaling Pathway Crosstalk Potentiates Corneal Epithelial Senescence through an RNA Stress Response. Aging 2016, 8, 2337–2354. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, J.; Guan, Y.; Guan, H.; Mu, Y.; Ding, Y.; Zou, J.; Ouyang, S.; Chen, X. Molecular and Structural Basis of Receptor Binding and Signaling of a Fish Type I IFN with Three Disulfide Bonds. J. Immunol. 2022, 209, 806–819. [Google Scholar] [CrossRef]

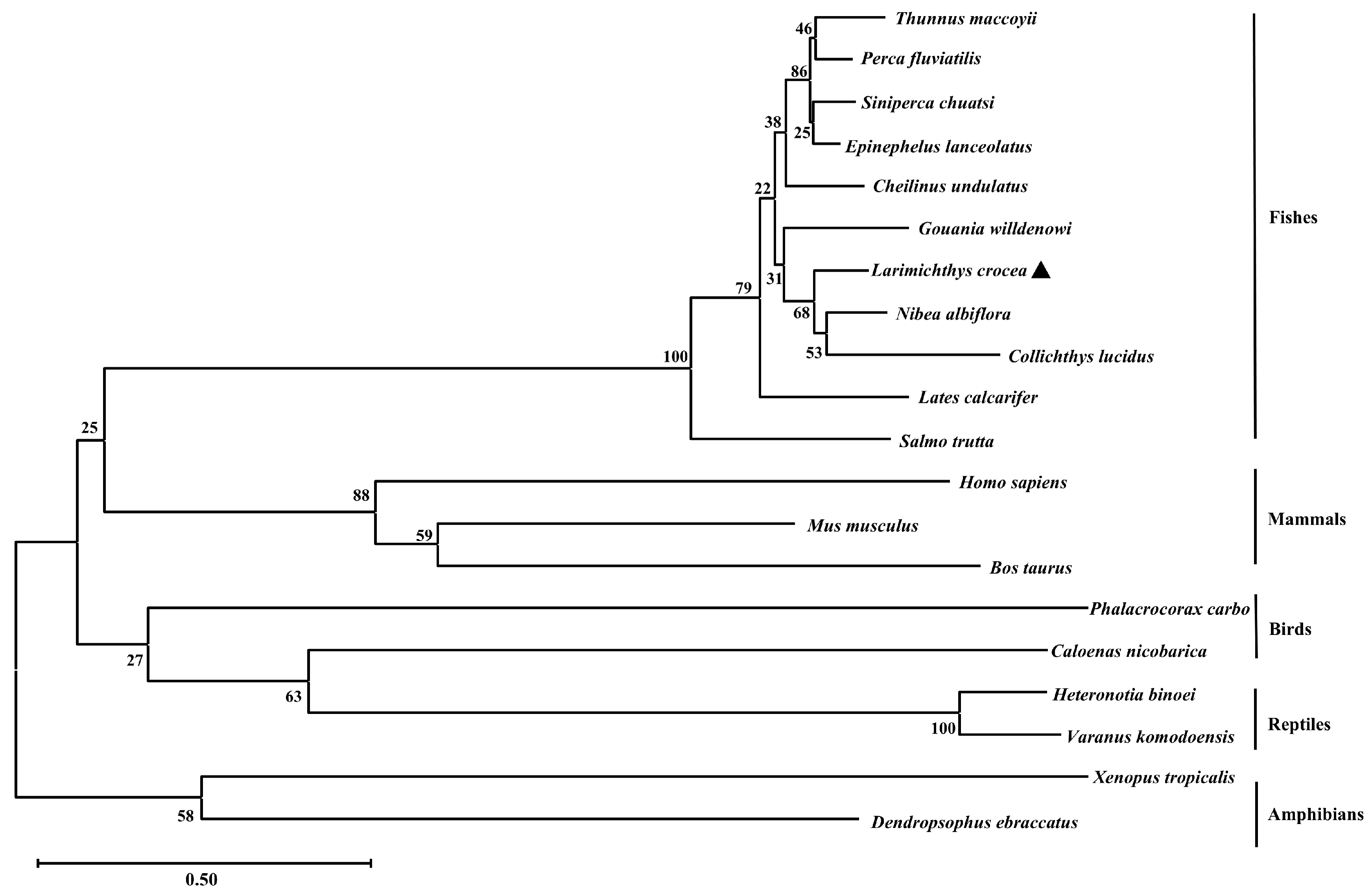

| Latin Name | Foreign Name | Identity (%) | GenBank Accession |
|---|---|---|---|
| Larimichthys crocea | large yellow croaker | 100 | XP_027127989.1 |
| Nibea albiflora | yellow drum | 86.86 | KAG8005608.1 |
| Collichthys lucidus | spinyhead croaker | 84.96 | TKS65205.1 |
| Siniperca chuatsi | aucha perch | 68.63 | XP_044041494.1 |
| Perca fluviatilis | english perch | 66.67 | XP_039648391.1 |
| Epinephelus lanceolatus | giant grouper | 66.31 | XP_033470412.1 |
| Thunnus maccoyii | southern bluefin tuna | 65.23 | XP_042259368.1 |
| Lates calcarifer | barramundi | 62.54 | XP_018526461.1 |
| Cheilinus undulatus | humphead wrasse | 57.04 | XP_041636212.1 |
| Salmo trutta | brown trout | 47.64 | XP_029597735.1 |
| Gouania willdenowi | Blue tilapia | 44.12 | XP_028329838.1 |
| Homo sapiens | human | 43.48 | NP_006346.1 |
| Varanus komodoensis | komodo dragon | 41.58 | XP_044307824.1 |
| Bos taurus | cattle | 29.16 | NP_001029746.1 |
| Phalacrocorax carbo | great cormorant fairywren | 28.30 | XP_064330074.1 |
| Heteronotia binoei | binoes gecko | 28.14 | XP_060095092.1 |
| Caloenas nicobarica | nicobar igeon | 27.98 | XP_065510802.1 |
| Dendropsophus ebraccatus | hourglass tree frog | 27.55 | XP_069838323.1 |
| Xenopus tropicalis | western clawed frog | 27.27 | XP_031753047.1 |
| Mus musculus | mouse | 25.41 | NP_001025106.1 |
| Primer Name | Sequence (5′-3′) | Usage |
|---|---|---|
| Lctrim38-F | ATGGAATATCTGAGAAGTCTGCTG | ORF amplification |
| Lctrim38-R | GTGTGTTTGAGTTACAGGTGTCAT | |
| Lctrim38-qF | ACTCCAGACTCCCAACTT | |
| Lctrim38-qR | AGACCAGCGACTCTATGA | |
| β-actin-qF | TTATGAAGGCTATGCCCTGCC | |
| β-actin-qR | TGAAGGAGTAGCCACGCTCTGT | |
| INF-γ-qF | AGGTCATTCAGATGTACCGGATA | |
| INF-γ-qR | TTCCTTGATGGTCTCCACACT | RT-qPCR |
| TNF-α-qF | TAAGCAACAAGACCACCACTTC | |
| TNF-α-qR | TCTCCAGATTCCAGATGTCAGG | |
| IL6-qF | AAGCCAGAGCTGTGCAGATG | |
| IL6-qR | CTGGCATTTGTGGTTGGGTC | |
| NF-κB-qF | GAGCTCAAGATCTGCCGAGT | |
| NF-κB-qR | ATCAGCTTGCGAAAAGGAGC | |
| Lctrim38-oF | AACGGGCCCTCTAGACTCGAGATGGAATATCTGAGAAGTCTGCTGTC | overexpression |
| Lctrim38-oR | TAGTCCAGTGTGGTGGAATTCTTAAGTTACAGCTGTTATGACGAGAGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wu, H.; Huang, Y.; Yekefenhazi, D.; Zou, W.; Han, F. Characterization and Functional Analysis of Trim38 in the Immune Response of the Large Yellow Croaker (Larimichthys crocea) Against Pseudomonas plecoglossicida Infection. Int. J. Mol. Sci. 2025, 26, 4150. https://doi.org/10.3390/ijms26094150
Li Q, Wu H, Huang Y, Yekefenhazi D, Zou W, Han F. Characterization and Functional Analysis of Trim38 in the Immune Response of the Large Yellow Croaker (Larimichthys crocea) Against Pseudomonas plecoglossicida Infection. International Journal of Molecular Sciences. 2025; 26(9):4150. https://doi.org/10.3390/ijms26094150
Chicago/Turabian StyleLi, Qiaoying, Hongling Wu, Ying Huang, Dinaer Yekefenhazi, Wenzheng Zou, and Fang Han. 2025. "Characterization and Functional Analysis of Trim38 in the Immune Response of the Large Yellow Croaker (Larimichthys crocea) Against Pseudomonas plecoglossicida Infection" International Journal of Molecular Sciences 26, no. 9: 4150. https://doi.org/10.3390/ijms26094150
APA StyleLi, Q., Wu, H., Huang, Y., Yekefenhazi, D., Zou, W., & Han, F. (2025). Characterization and Functional Analysis of Trim38 in the Immune Response of the Large Yellow Croaker (Larimichthys crocea) Against Pseudomonas plecoglossicida Infection. International Journal of Molecular Sciences, 26(9), 4150. https://doi.org/10.3390/ijms26094150





