Abstract
A thermochemical rather simple experimental technique is applied to determine the enthalpy of formation of Diperoxide of ciclohexanone. The study is complemented with suitable theoretical calculations at the semiempirical and ab initio levels. A particular satisfactory agreement between both ways is found for the ab initio calculation at the 6–311G basis This set level. Some possible extensions of the present procedure are pointed out.
1. Introduction
Ketone and aldehyde diperoxides (1,2,4,5-tetroxanes) contain two peroxy groups in a six membered heterocycle and they are readily formed by acid-catalyzed oxidation of carbonyl compounds with hydrogen peroxide. Carbonyl oxide dimers are also used to describe these compounds since they can be generated by dimerization of a carbonyl oxide intermediate derived from ozonolysis of olefins [1–3].
Thermolysis of tetroxanes derived from cycloalkanones finds industrial use in the production of macrocyclic hydrocarbons and lactones [4–6]. Tetroxanes are capable of initiating polymerization of olefins and their utility as high temperature initiators is under examination [7]. More recently, tetroxanes have been found to posses an impressive antimalarial activity [8]. As a result of an apparent association between the peroxide functional group and antimalarial activity, a substantial effort has been devoted to developing new peroxide antimalarials [8–10].
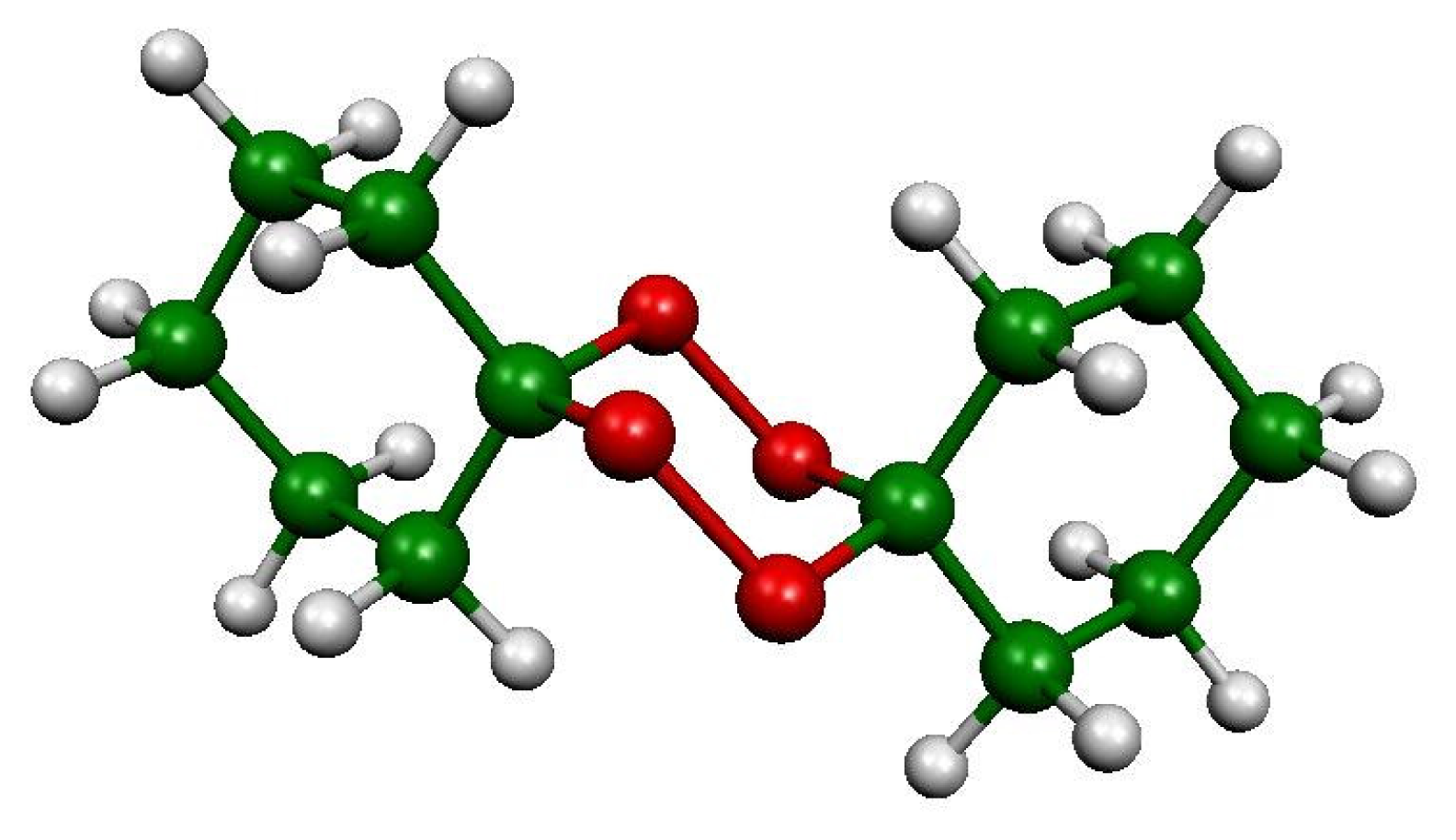
In this article, we report the experimental determination of the enthalpy of formation of the diperoxide of ciclohexanone (DPCH) (Figure 1), since this compound posses antimalarial activity, as well as the theoretical results calculated from semiempirical and ab-initio Hartree-Fock methods.
Figure 1.
Structure of DPCH.
2. Results and Discussion
2.1. Experimental enthalpy of formation
Table 1 gives the results for a typical combustion experiment on compound DPCH. Table 2 gives the standard molar energy and enthalpy of combustion and formation of diperoxide of ciclohexanone, in the crystalline state at T = 298.15 K, and it corresponds to the chemical reaction:

Table 1.
Results from typical combustion experiments at 298.15 K.

Table 2.
Summary of experimental specific heats of combustion and standard molar thermodynamic function of DPCH at T = 298.15 K.
The uncertainties of the standard molar energy is four times the final overall standard deviation of the mean and they were estimated as outlined by statistical methods. Vapor pressure was determined at different temperatures and the enthalpy of sublimation was calculated as pointed out before.
The standard molar enthalpies of formation for both crystalline and gaseous states of the DPCH at T = 298.15 K are also given in Table 2. No combustion enthalpy and enthalpy of sublimation have been found in the standard literature for comparison purposes.
2.2. Theoretical calculation of the enthalpy of formation
In the case of the diperoxide of ciclohexanone, the suitable isodesmic chemical reaction, is:
The calculation were computed at the semiempirical, ab initio and Functional Density Theory (DFT), with corrections for zero-point energies and differences in H°298 - H°0.
In order to calculate enthalpy values at 298 K, the difference between the enthalpy at temperature T and 0 K can be evaluated according to standard thermodynamics. The sum of electronic and enthalpies energies at 298 K at the Hartree Fock level using the semiempirical, ab initio and Functional Density Theory (DFT) procedures with different basis sets for the studied compounds, are also collected in Table 3. We have resorted to the semiempirical calculations at the AM1 and PM3 levels since it is well know that in these procedures a suitable adjustment of the elements of the F matrix is used to bring the calculated results into the best possible agreement with standard thermochemical results, largely enthalpy of formation [11]. Semiempirical methods like AM1 [12] and PM3 [13–15] provide a quite effective compromise between the accuracy of the results and the expense of computer time required. A calculation performed with AM1 and PM3 is able to reflect the experiment as effectively as an ab initio calculation using a small basis set [16].

Table 3.
Calculated electronic energy and heat of reaction (in Hartree units).
The heats of formation calculated through isodesmic reaction (2) are given in Table 4. The analysis of theoretical results show that ab initio procedures yield better results than semiempirical methods. Besides, the best agreement am among experimental data and theoretical predictions happens for the 3–21G basis set.

Table 4.
Enthalpy of formation of DPCH.
3. Experimental Section
3.1. Material
Diperoxide of ciclohexanone (DPCH) was prepared and purified as described elsewhere [17].
3.2. Thermochemical measurements
The measurement of the heat of combustion of diperoxide of ciclohexanone was made with an isoperibol macrocalorimeter fitted with a stirred water bath. The substance was burned in oxygen at p = 25atm.. The current of ignition was determined to be 2 Amp. The heat capacity of the calorimeter (E) was determined with a standard reference sample of benzoic acid (Sample SRM 39i, NIST) for all experiments. E was measured to be (856.17 ± 1.5) cal/K. The crystalline compounds were pressed into gelatine capsules of masses ≈ 1 x 10−3 g. The reduction of the data to standard conditions was made through conventional procedures [18]. The atomic weights used were those recommended by the IUPAC Commission [19,20].
The vapor pressures as a function of temperature of DPCH were measured by a mercury manometer through a Bodestein differential equipment and the enthalpy of sublimation was deduced from the temperature dependence of the vapor pressures (Clausius-Clapeyron equation).
3.3. Theoretical calculations
Among the most important purposes of the calorimetric studies is to find out the molecular energy of a set of structurally and functionally related molecules in order to establish the corresponding structure-activity relationships and to be able to discuss the main electronic features determining the chemical reactivity.
It is well known that in order to make theoretical calculations of molecular enthalpy of formation it is necessary to find suitable isodesmic chemical reactions to optimize the corresponding molecular structure and to perform the frequency calculations from the optimized equilibrium molecular geometries applying the corresponding theoretical method to obtain the total electronic energy at 298 K. Here we have chosen the Gaussian 94 package [21] to perform the theoretical calculations at the semiempirical and ab initio levels.
When one tries to get the equilibrium molecular geometries, it is necessary to localize the absolute minimum at the potential energy hypersurface, which is not a trivial task. The optimization procedure is complete when the numerical process converges, i.e. when the forces are null and all the vibrational frequencies are real.
4. Conclusions
We have reported a rather simple and accurate enough experimental method to determine the enthalpy of formation of the title compound and we have complemented it with the theoretical calculation of the property under study via semiempirical, ab initio Molecular Orbital methods and DFT procedures. The theoretical value of the enthalpy of formation of diperoxide of ciclohexanone, - 95.52 kcal mol−1, evaluated at the Hartree Fock at the 3–21 G basis set level, is in very good agreement with the experimental value, −96.50 kcal mol−1. This methodology consisting in the experimental determination of the thermochemical property and its complementation with theoretical procedures represents a quite sensible way to study similar oxane derivatives molecules and at present further studies along this line are under development.
Acknowledgements
AHJ is member of the Research Career CIC and EAC is member of the Research Career CONICET.
References and Notes
- a)McCullough, K.J.; Morgan, A.R.; Nonhebel, D.C.; Pauson, P.L.; White, G.J. Ketones-derived Peroxides. Part. III. Decompositions of Cyclic Peroxides derived from Dialkyl Ketones. J. Chem. Res. Synop 1980, 2. [Google Scholar]McCullough, K.J.; Morgan, A.R.; Nonhebel, D.C.; Pauson, P.L. Ketones-derived Peroxides. Part. I. Synthetic Methods. J. Chem. Res. Synop 1981, 2, 34–36. [Google Scholar]
- Leiva, L.C.A.; Romero, J.M.; Jorge, N.L.; Gómez Vara, M.E.; Cafferata, L.F.R. Síntesis y Descomposición Térmica Del Diperóxido De Formaldehído. Revista Internacional Información Tecnológica 2002, 13(2), 23–26. [Google Scholar]
- Adam, W.; Hadjiarapoglou, L.P.; Curci, R.; Mello, R. Organic Peroxides; Ando, W., Ed.; John Wiley & Sons: Chichester, England, 1992; Volume Chapter 4. [Google Scholar]
- Story, P.R.; Busch, P. Advances in Organic Chemistry; Taylor, E.C., Ed.; Wiley: New York, 1972; Volume 8, pp. 67–95. [Google Scholar]
- Eyler, G.N.; Mateo, C.M.; Alvarez, E.E.; Cañizo, A.I. Termal Decomposition reaction of Acetone Triperoxide in Toluene solution. J. Org. Chem. 2000, 65, 2319–2321. [Google Scholar]
- Eyler, G.N.; Mateo, C.M.; Álvarez, E.E.; Cañizo, A.I.; Cafferata, L.F.R. Solvent Effect in the Thermal Decomposition Reaction of trans-3,3-Dimethyl-5,6-tetramethylene-1,2,4-trioxacyclohexane. J. Org. Chem. 1999, 64, 8457–8460. [Google Scholar]
- Morales, G.; Eyler, G.N.; Cerna, J.R.; Cañizo, A.I. Use of Cyclic Di- and Triperoxides as Initiators of Styrene Polymerization at High Temperature with a View to Their Use in Industrial Applications. Molecules 2000, 5, 549–550. [Google Scholar]
- Vennerstrom, J.L.; Fu, H.N.; Ellis, W.Y.; Ager, A.L., Jr.; Wood, J.K.; Andersen, S.L.; Gerena, L.; Milhous, W.K. Dispiro-1,2,4,5-tetraoxanes: a new class of antimalarial peroxides. J. Medical Chem 1992, 35, 3023–3027. [Google Scholar]
- Kim, H.S.; Shibata, Y.; Wataya, Y.; Tsuchiya, K.; Masuyama, A.; Nojima, M. Synthesis and Antimalarial Activity of Cyclic Peroxides, 1,2,4,5,7-Pentoxocanes and 1,2,4,5-Tetroxanes. J. Med. Chem 1999, 42, 2604–2609. [Google Scholar]
- McCullogh, K.; Nonami, Y.; Masuyama, A.; Nojima, M.; Kim, HS; Wataya, Y. Synthesis, crystal structure and antimalarial activity of novel 1,2,5,6-tetraoxacycloalkanes from 2,3-dihydroperoxy-2-phenylnorbornane. Tetrahedrom Lett. 1999, 40, 9151–9155. [Google Scholar]
- Rogers, D.W. Computational Chemistry Using the PC, Third Edition ed; Wiley: New York, 2003. [Google Scholar]
- Dewar, M.J.S.; Zoebisch, E.G.; Healy, E.F.; Stewart, J.J.P. AM1: A New General Purpose Quantum Mechanical Model. J. Am. Chem. Soc 1985, 107, 3902–3909. [Google Scholar]
- Stewart, J.J.P. Semiempirical Molecular Orbital Methods. In Reviews in Computational Chemistry; Volume 1, Lipkpwitz, K.B., Boyd, D.B., Eds.; VCH: New York, 1990; pp. 45–81. [Google Scholar]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem 1989, 10, 209–220. [Google Scholar]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods II. Applications. J. Comput. Chem 1989, 10, 221–264. [Google Scholar]
- Höltje, H.D.; Sippl, W.; Rognan, D.; Folkers, G. Molecular Modeling. In Basic Principles and Applications, Second Edition ed; Wiley: New York, 2003; p. 23. [Google Scholar]
- Story, P.R.; Lee, B.; Bishop, C.E.; Denson, D.D.; Busch, P. Macrocyclic Synthesis. II. Cyclohexanone Peroxides. J. Org. Chem 1970, 35, 3059–3062. [Google Scholar]
- Hubbart, W.N.; Scott, D.W.; Waddington, G. Experimental Thermochemistry; Rossini, F.D., Ed.; Interscience: New York, 1956; pp. 75–127. [Google Scholar]
- Cox, J.D.; Wagman, D.D.; Medvedev, V.A. (Eds.) CODATA Key Values for Thermodynamics; Hemisphere: New York, 1989.
- Wieser, M.E. Atomic Weights of The Elements 2005 (IUPAC Technical Report). Pure Appl. Chem. 2006, 78(11), 2051–2066. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Gill, P.M.W.; Johnson, B.G.; Robb, A.; Cheeseman, J.R.; Keith, T.A.; Petersson, G.A.; Montgomery, J.A.; Kaghavachari, K.; Al-Laham, M.A.; Kakrewski, V.G.; Ortiz, J.V.; Foresman, J.B.; Cioslowski, J.; Stefanov, B.B.; Nanayakkara, A.; Challacombe, M.; Peng, C.Y.; Ayala, P.Y.; Chen, W.; Wong, M.W.; Andrés, J.L.; Replogle, E.S.; Gomperts, R.; Martin, R.L.; Fox, D.J.; Binkley, J.S.; DeFrees, D.J.; Baker, J.; Stewart, J.P.; Head-Gordon, M.; González, C.; Pople, J. A. Gaussian 94; rev D3, SGI; Gaussian, Inc: Pittsburgh, PA, 1995. [Google Scholar]
© 2007 by MDPI ( http://www.mdpi.org) Reproduction is permitted for noncommercial purposes.