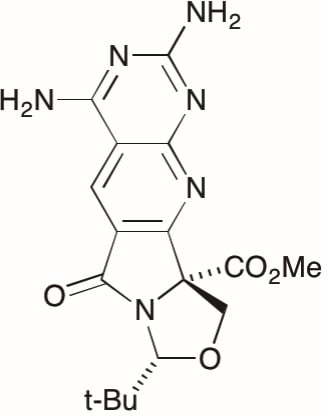
(8R,10aS)-Methyl 2,4-diamino-8-(tert-butyl)-6-oxo-6,8,10,10a-tetrahydrooxazolo[3′′,4′′:1′,5′]-pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a-carboxylate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Synthesis
2.2. Antibacterial Activity
3. Experimental Section
(8R,10aS)-Methyl 2,4-diamino-8-(tert-butyl)-6-oxo-6,8,10,10a-tetrahydrooxazolo[3'',4'':1',5']-pyrrolo[3',4':5,6]pyrido[2,3-d]pyrimidine-10a-carboxylate 4
4. Conclusions
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Supplementary File 4Author Contributions
Conflicts of Interest
References and Notes
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D. 10 × ′20 Progress—development of new drugs active against gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Pollastri, M.P.; Schiffer, C.A.; Peet, N.P. The challenge of developing robust drugs to overcome resistance. Drug Discovery Today 2011, 16, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; O’Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. Molecular mechanisms of antibiotic resistance. Chem. Commun. 2011, 47, 4055–4061. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Lister, T.; May-Dracka, T.L. Natural products as leads for antibacterial drug discovery. Biorg. Med. Chem. Lett. 2014, 24, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Royles, B.J.L. Naturally-occurring tetramic acids—Structure, isolation, and synthesis. Chem. Rev. 1995, 95, 1981–2001. [Google Scholar] [CrossRef]
- Schobert, R.; Schlenk, A. Tetramic and tetronic acids: An update on new derivatives and biological aspects. Bioorg. Med. Chem. 2008, 16, 4203–4221. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Moloney, M.G. Tetramic acids as bioactive templates: Synthesis, tautomeric and antibacterial behaviour. Synlett 2009, 2487–2491. [Google Scholar]
- Jeong, Y.-C.; Moloney, M.G. Antibacterial barbituric acid analogues inspired from natural 3-acyltetramic acids; synthesis, tautomerism and structure and physicochemical property-antibacterial activity relationships. Molecules 2015, 20, 3582–3627. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Moloney, M.G.; Bikadi, Z.; Hazai, E. A detailed study of antibacterial 3-acyltetramic acids and 3-acylpiperidine-2,4-diones. ChemMedChem 2014, 9, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Anwar, M.; Moloney, M.G.; Bikadi, Z.; Hazai, E. Synthesis, antibiotic activity and structure-activity relationship study of some 3-enaminetetramic acids. Biorg. Med. Chem. Lett. 2014, 24, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Moloney, M.G. Synthesis and antibacterial activity of monocyclic 3-carboxamidotetramic acids. Beilstein J. Org. Chem. 2013, 9, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Anwar, M.; Moloney, M.G.; Bikadi, Z.; Hazai, E. Natural product inspired antibacterial tetramic acid libraries with dual enzyme target activity. Chem. Sci. 2013, 4, 1008–1015. [Google Scholar] [CrossRef]
- Xia, L.; Idhayadhulla, A.; Lee, Y.R.; Kim, S.H.; Wee, Y.-J. Microwave-assisted synthesis of diverse pyrrolo[3,4-c]quinoline-1,3-diones and their antibacterial activities. ACS Comb. Sci. 2014, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.D.; Brewster, A.G.; Crapnell, K.M.; Ibbett, A.J.; Jones, T.; Moloney, M.G.; Prout, K.; Watkin, D. Regioselective Dieckmann cyclisations leading to enantiopure highly functionalised tetramic acid derivatives. J. Chem. Soc., Perkin Trans. 1 1998, 223–235. [Google Scholar] [CrossRef]
- Oshega, J.S.; Paponov, B.V.; Omelchenko, I.V.; Shishkin, O.V. One-pot three-component synthesis of 3-cyano-4-methyl-2,6-dioxopyridine amino enones. Mendeleev Commun. 2015, 25, 133–134. [Google Scholar] [CrossRef]
- Taylor, E.C.; Fletcher, S.R. Condensation of 2,4-diamino-6(1H)-pyrimidinone with 2-(aminomethylene)cyclopentanone. J. Org. Chem. 1984, 49, 3226–3227. [Google Scholar] [CrossRef]
- Data for compound 2: δH (500 MHz, CDCl3) Major: 0.88 (9H, s, -C(CH3)3), 3.46 (1H, d, J = 8.8, C-4HH'), 3.72 (3H, s, -OCH3), 4.78 (1H, d, J = 8.8, C-4HH'), 4.81 (1H, s, C-2H), 7.00–7.05 (2H, m, ArH), 7.46–7.51 (2H, m, ArH), 8.10 (1H, d, J = 13.2, C-10H), 10.93 (1H, d, J = 12.9, -NHAr), Minor: 0.87 (9H, s, -C(CH3)3), 3.45 (1H, d, J = 8.8, C-4HH'), 3.73 (3H, s, -OCH3), 4.77 (1H, d, J = 8.8, C-4HH'), 4.84 (1H, s, C-2H), 7.00–7.05 (2H, m, ArH), 7.46–7.51 (2H, m, ArH), 8.02 (1H, s, C-10H), 10.82 (1H, br s, -NHAr); δC (500 MHz, CDCl3): Major: 24.73 (-C(CH3)3), 35.29 (-C(CH3)3), 53.23 (-OCH3), 68.42 (C-4), 78.04 (C-5), 98.18 (C-2), 99.06 (C-7), 119.1 (C-13), 120.1 (C-15), 133.3 (C-14), 136.7 (C-12), 146.3 (C-10), 168.3 (C-9), 177.6 (C-8), 189.0 (C-6), Minor: 24.77 (-C(CH3)3), 35.35 (-C(CH3)3), 53.24 (-OCH3), 68.32 (C-4), 77.29 (C-5), 98.28 (C-2), 99.05 (C-7), 119.3 (C-13), 120.3 (C-15), 133.3 (C-14), 136.7 (C-12), 147.0 (C-10), 168.3 (C-9), 174.2 (C-8), 192.5 (C-6); m/z (ESI+) 437.1 ([M + H]+ 100%); HRMS (ESI+) found 436.0777, 438.0661 ([M + H]+) requires 436.0634, 438.0616; νmax 3214 (N-H), 2959 (C-H), 2871 (C-H), 1747 (C=O), 1708, (C=O) 1619 (C=O), 1474, 1300, 1205, 731; m.p. 116–117 °C.


© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, G.; Moloney, M.G. (8R,10aS)-Methyl 2,4-diamino-8-(tert-butyl)-6-oxo-6,8,10,10a-tetrahydrooxazolo[3′′,4′′:1′,5′]-pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a-carboxylate. Molbank 2015, 2015, M875. https://doi.org/10.3390/M875
Clarke G, Moloney MG. (8R,10aS)-Methyl 2,4-diamino-8-(tert-butyl)-6-oxo-6,8,10,10a-tetrahydrooxazolo[3′′,4′′:1′,5′]-pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a-carboxylate. Molbank. 2015; 2015(4):M875. https://doi.org/10.3390/M875
Chicago/Turabian StyleClarke, Georgia, and Mark G. Moloney. 2015. "(8R,10aS)-Methyl 2,4-diamino-8-(tert-butyl)-6-oxo-6,8,10,10a-tetrahydrooxazolo[3′′,4′′:1′,5′]-pyrrolo[3′,4′:5,6]pyrido[2,3-d]pyrimidine-10a-carboxylate" Molbank 2015, no. 4: M875. https://doi.org/10.3390/M875