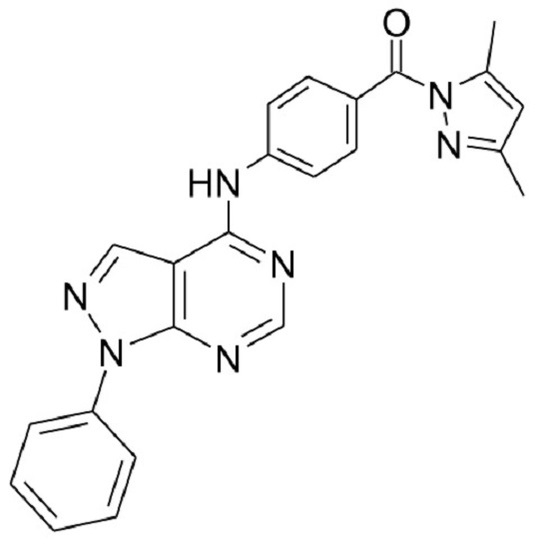
(3,5-Dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenyl]methanone
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Pharmacological Screening
3. Materials and Methods
3.1. Chemistry
(3,5-Dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenyl]methanone) (4)
3.2. Pharmacological Studies
3.2.1. Cell Viability Analysis
3.2.2. Kinases Inhibitory Activity
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase-role and significance in cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, I.N. Mechanisms of activation of receptor tyrosine kinases: Monomers or dimers. Cells 2014, 3, 304–330. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem. Biophys. Res. Commun. 2004, 319, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Na, I.-K.; le Coutre, P. Emerging Role of Tyrosine Kinases as Drugable Targets in Cancer. Biomark. Insights 2015, 10, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Sachsenmaier, C. Targeting protein kinases for tumor therapy. Oncol. Res. Treat. 2001, 24, 346–355. [Google Scholar] [CrossRef]
- Diana, P.; Carbone, A.; Barraja, P.; Montalbano, A.; Parrino, B.; Lopergolo, A.; Pennati, M.; Zaffaroni, N.; Cirrincione, G. Synthesis and Antitumor Activity of 3-(2-Phenyl-1,3-thiazol-4-yl)-1H-indoles and 3-(2-Phenyl-1,3-thiazol-4-yl)-1H-7-azaindoles. ChemMedChem 2011, 6, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Barraja, P.; Montalbano, A.; Parrino, B.; Spanò, V.; Lopergolo, A.; Sbarra, S.; Doldi, V.; Zaffaroni, N. Synthesis and antiproliferative activity of substituted 3[2-(1H-indol-3-yl)-1,3-thiazol-4-yl]-1H-pyrrolo[3,2-b]pyridines, marine alkaloid nortopsentin analogues. Curr. Med. Chem. 2014, 21, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Pennati, M.; Parrino, B.; Lopergolo, A.; Barraja, P.; Montalbano, A.; Spanò, V.; Sbarra, S.; Doldi, V.; De Cesare, M. Novel 1H-pyrrolo[2,3-b]pyridine derivative nortopsentin analogues: Synthesis and antitumor activity in peritoneal mesothelioma experimental models. J. Med. Chem. 2013, 56, 7060–7072. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; Ragab, F.A.; Alqasoumi, S.I.; Alafeefy, A.M.; Aboulmagd, S.A. Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives of expected anticancer and radioprotective activity. Eur. J. Med. Chem. 2010, 45, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Traxler, P.; Bold, G.; Frei, J.; Lang, M.; Lydon, N.; Mett, H.; Buchdunger, E.; Meyer, T.; Mueller, M.; Furet, P. Use of a pharmacophore model for the design of EGF-R tyrosine kinase inhibitors: 4-(phenylamino)pyrazolo[3,4-d]pyrimidines. J. Med. Chem. 1997, 40, 3601–3616. [Google Scholar] [CrossRef] [PubMed]
- Bakr, R.B.; Abdelall, E.K.; Abdel-Hamid, M.K.; Kandeel, M.M. Design and synthesis of new EGFR-tyrosine kinase inhibitors containing pyrazolo[3,4-d]pyrimidine cores as anticancer agents. Bull. Pharm. Sci. Assiut. Univ. 2012, 35, 1–16. [Google Scholar]
- Abdellatif, K.R.; Abdelall, E.K.; Abdelgawad, M.A.; Ahmed, R.R.; Bakr, R.B. Synthesis, docking study and antitumor evaluation of certain newly synthesized pyrazolo[3,4-d]pyrimidine derivatives. Organ. Chem. Indian J. 2014, 10, 157–167. [Google Scholar]
- Kim, D.C.; Lee, Y.R.; Yang, B.-S.; Shin, K.J.; Kim, D.J.; Chung, B.Y.; Yoo, K.H. Synthesis and biological evaluations of pyrazolo[3,4-d]pyrimidines as cyclin-dependent kinase 2 inhibitors. Eur. J. Med. Chem. 2003, 38, 525–532. [Google Scholar] [CrossRef]
- Fernandez, M.; Tundidor-Camba, A.; Caballero, J. Modeling of cyclin-dependent kinase inhibition by 1H-pyrazolo[3,4-d]pyrimidine derivatives using artificial neural network ensembles. J. Chem. Inf. Model. 2005, 45, 1884–1895. [Google Scholar] [CrossRef] [PubMed]
- Jorda, R.; Havlícek, L.; McNae, I.W.; Walkinshaw, M.D.; Voller, J.; Šturc, A.N.; Navrátilová, J.; Kuzma, M.; Mistrík, M.; Bártek, J. Pyrazolo[4,3-d]pyrimidine bioisostere of roscovitine: Evaluation of a novel selective inhibitor of cyclin-dependent kinases with antiproliferative activity. J. Med. Chem. 2011, 54, 2980–2993. [Google Scholar] [CrossRef] [PubMed]
- Peat, A.J.; Boucheron, J.A.; Dickerson, S.H.; Garrido, D.; Mills, W.; Peckham, J.; Preugschat, F.; Smalley, T.; Schweiker, S.L.; Wilson, J.R. Novel pyrazolopyrimidine derivatives as GSK-3 inhibitors. Bioorgan. Med. Chem. Lett. 2004, 14, 2121–2125. [Google Scholar] [CrossRef] [PubMed]
- Peat, A.J.; Garrido, D.; Boucheron, J.A.; Schweiker, S.L.; Dickerson, S.H.; Wilson, J.R.; Wang, T.Y.; Thomson, S.A. Novel GSK-3 inhibitors with improved cellular activity. Bioorgan. Med. Chem. Lett. 2004, 14, 2127–2130. [Google Scholar] [CrossRef] [PubMed]
- Ducray, R.; Ballard, P.; Barlaam, B.C.; Hickinson, M.D.; Kettle, J.G.; Ogilvie, D.J.; Trigwell, C.B. Novel 3-alkoxy-1H-pyrazolo[3,4-d]pyrimidines as EGFR and erbB2 receptor tyrosine kinase inhibitors. Bioorgan. Med. Chem. Lett. 2008, 18, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Schenone, S.; Brullo, C.; Bruno, O.; Bondavalli, F.; Mosti, L.; Maga, G.; Crespan, E.; Carraro, F.; Manetti, F.; Tintori, C. Synthesis, biological evaluation and docking studies of 4-amino substituted 1H-pyrazolo[3,4-d]pyrimidines. Eur. J. Med. Chem. 2008, 43, 2665–2676. [Google Scholar] [CrossRef] [PubMed]
- Abdelgawad, M.A.; Bakr, R.B.; Alkhoja, O.A.; Mohamed, W.R. Design, synthesis and antitumor activity of novel pyrazolo[3,4-d]pyrimidine derivatives as EGFR-TK inhibitors. Bioorgan. Chem. 2016, 66, 88–96. [Google Scholar] [CrossRef] [PubMed]
- El Hamid, M.K.A.; Mihovilovic, M.D.; El-Nassan, H.B. Synthesis of novel pyrazolo[3,4-d]pyrimidine derivatives as potential anti-breast cancer agents. Eur. J. Med. Chem. 2012, 57, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Draghici, C.; Missir, A.V. Synthesis of new pyrazole derivatives and their anticancer evaluation. Eur. J. Med. Chem. 2010, 45, 4914–4919. [Google Scholar] [CrossRef] [PubMed]
- Faidallah, H.M.; Rostom, S.A.; Al-Saadi, M.S. Synthesis and biological evaluation of some new substituted fused pyrazole ring systems as possible anticancer and antimicrobial agents. Science (JKAU: Sci.) 2010, 22, 177–191. [Google Scholar] [CrossRef]
- Diana, P.; Carbone, A.; Barraja, P.; Martorana, A.; Gia, O.; DallaVia, L.; Cirrincione, G. 3,5-Bis(3′-indolyl)pyrazoles, analogues of marine alkaloid nortopsentin: Synthesis and antitumor properties. Bioorgan. Med. Chem. Lett. 2007, 17, 6134–6137. [Google Scholar] [CrossRef] [PubMed]
- Spanò, V.; Parrino, B.; Carbone, A.; Montalbano, A.; Salvador, A.; Brun, P.; Vedaldi, D.; Diana, P.; Cirrincione, G.; Barraja, P. Pyrazolo[3,4-h]quinolines promising photosensitizing agents in the treatment of cancer. Eur. J. Med. Chem. 2015, 102, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Barraja, P.; Spanò, V.; Giallombardo, D.; Diana, P.; Montalbano, A.; Carbone, A.; Parrino, B.; Cirrincione, G. Synthesis of [1,2]oxazolo[5,4-e]indazoles as antitumour agents. Tetrahedron 2013, 69, 6474–6477. [Google Scholar] [CrossRef]
- Maggio, B.; Raimondi, M.V.; Raffa, D.; Plescia, F.; Cascioferro, S.; Cancemi, G.; Tolomeo, M.; Grimaudo, S.; Daidone, G. Synthesis and antiproliferative activity of 3-(2-chloroethyl)-5-methyl-6-phenyl-8-(trifluoromethyl)-5,6-dihydropyrazolo [3,4-f][1,2,3,5]tetrazepin-4-(3H)-one. Eur. J. Med. Chem. 2015, 96, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Maggio, B.; Raffa, D.; Raimondi, M.V.; Cascioferro, S.; Plescia, F.; Tolomeo, M.; Barbusca, E.; Cannizzo, G.; Mancuso, S.; Daidone, G. Synthesis and induction of G0–G1 phase arrest with apoptosis of 3,5-dimethyl-6-phenyl-8-(trifluoromethyl)-5,6-dihydropyrazolo[3,4-f][1,2,3,5]tetrazepin-4(3H)-one. Eur. J. Med. Chem. 2008, 43, 2386–2394. [Google Scholar] [CrossRef] [PubMed]
- Abdelall, E.; BBakr, R.; Abdel-Hamid, M.; Kandeel, M. Enhancement to synthesize, design and dock of novel EGFR inhibitors containing pyrazolo[3,4-d]pyrimidine cores of expected anticancer activity. OCAIJ 2014, 10, 470–483. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Balzano, D.; Santaguida, S.; Musacchio, A.; Villa, F. A general framework for inhibitor resistance in protein kinases. Chem. Biol. 2011, 18, 966–975. [Google Scholar] [CrossRef] [PubMed]


| Compound | IC50 (μM) | ||
|---|---|---|---|
| MCF-7 | HEPG-2 | HCT-116 | |
| 4 | 32.52 ± 0.02 | 5.00 ± 0.02 | 14.31 ± 0.05 |
| Doxorubicin | 2.60 ± 0.02 | 5.66 ± 0.12 | 8.48 ± 0.32 |
| IC50 (μM) | |||
|---|---|---|---|
| EGFR | IR | VEGFR | FGFR |
| 13.47 | 10.29 | 27.89 | 5.18 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakr, R.B.; Mehany, A.B.M. (3,5-Dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenyl]methanone. Molbank 2016, 2016, M915. https://doi.org/10.3390/M915
Bakr RB, Mehany ABM. (3,5-Dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenyl]methanone. Molbank. 2016; 2016(4):M915. https://doi.org/10.3390/M915
Chicago/Turabian StyleBakr, Rania B., and Ahmed B. M. Mehany. 2016. "(3,5-Dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenyl]methanone" Molbank 2016, no. 4: M915. https://doi.org/10.3390/M915
APA StyleBakr, R. B., & Mehany, A. B. M. (2016). (3,5-Dimethylpyrazol-1-yl)-[4-(1-phenyl-1H-pyrazolo[3,4-d]pyrimidin-4-ylamino)phenyl]methanone. Molbank, 2016(4), M915. https://doi.org/10.3390/M915