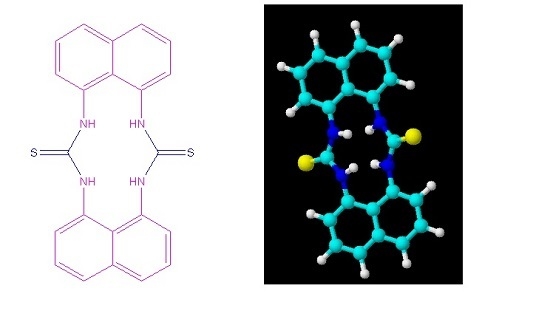
1,3,1′,3′-(Dinaphthalene-1,8-diyl)bisthiourea
Abstract
:1. Introduction
2. Results
3. Experimental Section
3.1. Materials and Measurements
3.2. Synthesis of 1,3,1′,3′-(Dinaphthalene-1,8-diyl)bisthiourea 3
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Katritzky, A.R.; Kirichenko, N.; Rogovoy, B.V. Synthesis of mono- and N,N-disubstitutedureas. Arkivoc 2003, viii, 8–14. [Google Scholar]
- Biaolin, Y.; Zhaogui, L.; Mingjun, Y.; Jiancun, Z. An efficient method for the synthesis of disubstitutedthioureas via the reaction of N,N′-di-Boc-substituted with alkyl and aryl amines under mild conditions. Tetrahedron Lett. 2008, 49, 3687–3690. [Google Scholar]
- Silvio, C.; Fernando, C.M., Jr.; Giselle, A.N.C.; Manoel, T.R., Jr.; Rosival, B.V.V.; Lourdes, C.S.N.; Ivo, V.; Carlito, L.; Fernando, P.S. Antimicrobial activity and structural study of disubstitutedthiourea derivatives. Mon. Chem. 2007, 138, 511–516. [Google Scholar]
- Khalid, M.K.; Farzana, N.; Muhammad, T.; Ajmal, K.; Shahnaz, P.; Muhammad, I.C.; Wolfgang, V. Synthesis and in vitro urease inhibitory activity of N,N′-disubstitutedthioureas. Eur. J. Med. Chem. 2014, 74, 314–323. [Google Scholar]
- Azeem, S.; Ataf, A.A.; Ashfaq, M.Q.; Amin, B. Thiourea derivatives in drug design and medicinal chemistry: A short review. J. Drug Des. Med. Chem. 2016, 2, 10–20. [Google Scholar]
- Truong, P.; Thai, K.-M.; Nguyen Thi, V.H.; Huynh Thi, N.P. Synthesis and antifungal activities of phenylenedithioureas. Bioorg. Med. Chem. Lett. 2004, 14, 653–656. [Google Scholar]
- Jennifer, C.S.; Jae, W.C.; Robert, C.; Mark, A.S.; Jann, N.S.; Abdul, F.; Richard, J.B. A novel synthetic 1,3-phenyl bis-thiourea compound targets microtubule polymerization to cause cancer cell death. Cancer Biol. Ther. 2014, 15, 895–905. [Google Scholar]
- Gary, M.C.; Robert, E.D.; James, B.E.; Dennis, S.F.; James, R.P., Jr. 1-Hydroxyalkyl-3-phenylthioureas as novel HDL-elevating agents. Bioorg. Med. Chem. Lett. 2005, 15, 809–812. [Google Scholar]
- Bianca, K.V.; Jandali, N.; Janette, S.; Shiy, K.S.; Ross, J.B.; Patrick, M.W.; Lyn-Marie, B. Discovery of novel alkylated (bis)urea and (bis)thiourea polyamine analogues with potent anti-malarial activities. J. Med. Chem. 2011, 54, 6624–6633. [Google Scholar]
- Muthu, K.; Meenatchi, V.; Rajasekar, M.; Aditya, P.; Meena, K.; Agilandeshwari, R.; Kanagarajan, V.; Meenakshisundaram, S.P. Combined theoretical and experimental studies on the molecular structure, spectral and hirshfeld surface studies of NLO tris(thiourea)zinc(II) sulfate crystals. J. Mol. Struct. 2015, 1091, 210–221. [Google Scholar] [CrossRef]
- Sivakumar, N.; Kanagathara, N.; Varghese, B.; Bhagavannarayana, G.; Gunasekaran, S.; Anbalagan, G. Structure, crystal growth, optical and mechanical studies of poly bis(thiourea) silver (I) nitrate single crystal: A new semi organic NLO material. Spectrochim. Acta A 2014, 118, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Jose, D.A.; Kumar, D.K.; Ganguly, B.; Das, A. Efficient and simple colorimetric fluoride ion sensor based on receptors having urea and thiourea binding sites. Org. Lett. 2004, 6, 3445–3448. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.S.; Gavalas, V.G.; Cammers, A.; Andrada, P.S.; Alajarin, M.; Bachas, L.G. Nitrate-selective electrode based on a cyclic bis-thiourea ionophore. Sens. Actuators B 2007, 121, 200–207. [Google Scholar] [CrossRef]
- Aydin, F.; Tunoglu, N.; Aykac, D.; Arslan, N.B.; Kazak, C. Synthesis and structural X-ray analysis of 1,1′-(naphthalene-1,8-diyl)-3,3′-dibenzoyl-bisthiourea and its use as anion-binding receptor. Turk. J. Chem. 2012, 36, 764–777. [Google Scholar]



© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydin, F. 1,3,1′,3′-(Dinaphthalene-1,8-diyl)bisthiourea. Molbank 2016, 2016, M917. https://doi.org/10.3390/M917
Aydin F. 1,3,1′,3′-(Dinaphthalene-1,8-diyl)bisthiourea. Molbank. 2016; 2016(4):M917. https://doi.org/10.3390/M917
Chicago/Turabian StyleAydin, Fatma. 2016. "1,3,1′,3′-(Dinaphthalene-1,8-diyl)bisthiourea" Molbank 2016, no. 4: M917. https://doi.org/10.3390/M917
APA StyleAydin, F. (2016). 1,3,1′,3′-(Dinaphthalene-1,8-diyl)bisthiourea. Molbank, 2016(4), M917. https://doi.org/10.3390/M917