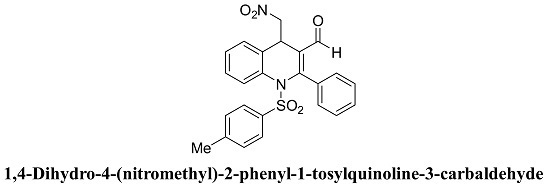
1,4-Dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde
Abstract
:1. Introduction
2. Results
3. Experimental Section
3.1. General Information
3.2. Syntheis of 1,4-Dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde (3)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kouznetsov, V.; Palma, A.; Ewert, C.; Varlamov, A. Some aspects of reduced quinoline chemistry. J. Heterocycl. Chem. 1998, 35, 761–785. [Google Scholar] [CrossRef]
- Zhou, Y.-G. Asymmetric Hydrogenation of Heteroaromatic Compounds. Acc. Chem. Res. 2007, 40, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, E.; Manian, R.D.R.S.; Raghunathan, R.; Sainath, S.; Raghunathan, M. Synthesis and antibacterial property of quinolines with potent DNA gyrase activity. Bioorg. Med. Chem. 2009, 17, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.Z.; Hunt, J.T.; Ricca, C.; Manne, V. 3-Imidazolylmethylaminophenylsulfonyl- tetrahydroquinolines, a novel series of farnesyltransferase inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 273–275. [Google Scholar] [CrossRef]
- Fotie, J.; Kaiser, M.; Delfin, D.A.; Manley, J.; Reid, C.S.; Paris, J.-M.; Wenzler, T.; Maes, L.; Mahasenan, K.V.; Li, C.; Werbovetz, K.A. Antitrypanosomal Activity of 1,2-Dihydroquinolin-6-ols and Their Ester Derivatives. J. Med. Chem. 2010, 53, 966–982. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Suryavanshi, P.A.; Menendez, J.C. Advances in the Chemistry of Tetrahydroquinolines. Chem. Rev. 2011, 111, 7157–7259. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Heo, S.; Kim, S.-G. Asymmetric one-pot synthesis of 1,4-dihydroquinolines via an organocatalytic aza-Michael/Michael cascade strategy. Adv. Synth. Catal. 2015, 357, 1545–1550. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.-G. One-pot organocatalytic enantioselective Michael addition and aza-cyclization/dehydration cascade reaction strategy: Asymmetric synthesis of highly functionalized 1,4-dihydroquinolines. Tetrahedron Lett. 2015, 56, 4819–4823. [Google Scholar] [CrossRef]
- Yu, M.; Kim, S.-G. Asymmetric organocatalytic Michael addition/aza-cyclization coupled with sequential Michael addition for synthesizing densely polycyclic-fused dihydroquinolines. Tetrahedron Lett. 2015, 56, 4159–4162. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, S.-G. Asymmetric organocatalytic cascade reaction of aldehydes with 2-amino-β-nitrostyrenes: Synthesis of chiral tetrahydroquinolines and dihydroquinolines. J. Org. Chem. 2014, 79, 8234–8243. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, K.M.; Kim, S.-G. 1,4-Dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde. Molbank 2016, 2016, M918. https://doi.org/10.3390/M918
Ko KM, Kim S-G. 1,4-Dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde. Molbank. 2016; 2016(4):M918. https://doi.org/10.3390/M918
Chicago/Turabian StyleKo, Kwang Min, and Sung-Gon Kim. 2016. "1,4-Dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde" Molbank 2016, no. 4: M918. https://doi.org/10.3390/M918
APA StyleKo, K. M., & Kim, S.-G. (2016). 1,4-Dihydro-4-(nitromethyl)-2-phenyl-1-tosylquinoline-3-carbaldehyde. Molbank, 2016(4), M918. https://doi.org/10.3390/M918