Abstract
1,3-Bis[(E)-(3-bromobenzylidene)amino]propan-2-ol Schiff base was synthesized in an acceptable yield by condensation of 3-bromobenzaldehyde with 1,3-diaminopropan-2-ol in methanol. The structure of the desired Schiff base compound was spectroscopically analyzed by EI-MS, CHN-elemental analysis, FT-IR, UV-visible, and 1H and 13C-NMR. The structure was also computed by DFT-optimization, MEP, Mulliken, NPA, IR- B3LYP/6-311++G(d), and SCF-TD-DFT.
1. Introduction
Schiff bases as azomethine compounds are well-known versatile molecules that have recently received great attention in different fields [1,2,3]. Such compounds have been used as dyes and pigments, corrosion inhibitors, thermo-stable materials, and catalysts [3,4,5,6] and in medical applications as antifungal, anticancer, and antibacterial agents [7,8,9,10,11,12].
The presence of the unsaturated nitrogen atom (>C=N) together with its lone pair of electrons makes the Schiff base an attractive e-donor ligand [11,12,13,14]. Such donation ability plays a critical role in the complexation with several metal ions centers [10,11,12,13,14,15,16,17].
Metal-Schiff based drugs are highly promising and of great interest in biology and chemistry. Biologically active nitrogen of (>C=N-) upon coordination with a transition metal center lead to complexes with improved pharmacological and physicochemical properties [7,8,9,10,11,12,13,14,15]. Despite the large number of the prepared Schiff base ligands together with their complexes, there remains an urgent need for novel ligands with new applications and properties.
In connection with previous work [12,13,14,15,16,17,18], we report the preparation of 1,3-bis[(E)-(3-bromobenzylidene)amino]propan-2-ol, its spectroscopic characterization, and computational studies using DFT techniques.
2. Results
1,3-Bis[(E)-(3-bromobenzylidene)amino]propan-2-ol was synthesized by condensing 2.1 equiv of 3-bromobenzaldehyde with 1 equiv 1,3-diaminopropan-2-ol in absolute methanol under reflux conditions, as shown in Scheme 1. The compound was a white powder with m.p. = 145.2 °C as a final product. The product was soluble in chloroform at RT, soluble in EtOH at 50 °C, and insoluble in n-hexane (non-polar) or water even at high temperature.
Scheme 1.
Synthesis of the 1,3-bis[(E)-(3-bromobenzylidene)amino]propan-2-ol ligand.
The elemental analysis of the 1,3-bis[(E)-(3-bromobenzylidene)amino]propan-2-ol is consistent with its proposed molecular formula (Calcd. for C17H16Br2N2O: C, 48.14; H, 3.80 and N, 6.60. Found: C, 48.02; H, 3.71 and N, 6.53). EI-MS reflected an excellent agreement with the expected structure, the experimental molecular ion [M+] m/z = 424.1 (424.9 theoretical).
2.1. Optimization, MEP, Mulliken, and NPA Analysis
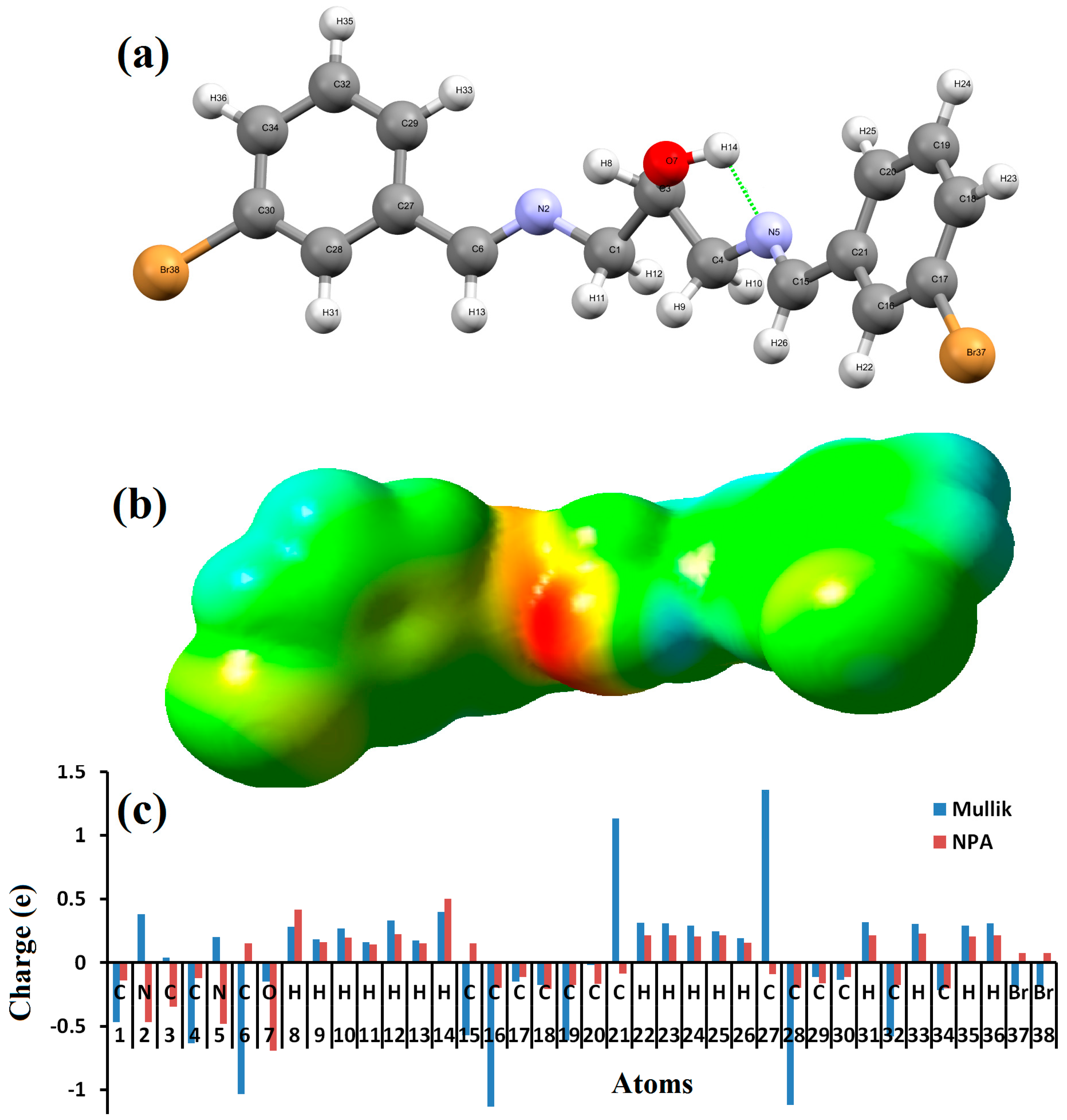
The B3LYP/6-311++G(d)-optimized molecular structure (bonds and angles lengths) of 1,3-bis[(E)-(3-bromobenzylidene)amino]propan-2-ol ligand is shown in Figure 1a and Table 1. The compound exists as E,E-conformation with respect to the imine functions. The bond lengths of N2=C and N5=C are found to be 1.2733 and 1.2739 Å, respectively, which is clearly consistent with C=N. The C–N=C bond angle of 117.82 and 118.87 (o) confirms the sp2 hybridization character of both N atoms, as seen in Table 1. The calculation indicated a short intramolecular hydrogen bond of the type O–H…N (2.233 Å) with a pseudo S5-heterocyclic formation (in Figure 1a). Moreover, the phenyl rings are oriented in perpendicular planes, which minimized the internal repulsion energy in the desired molecule.
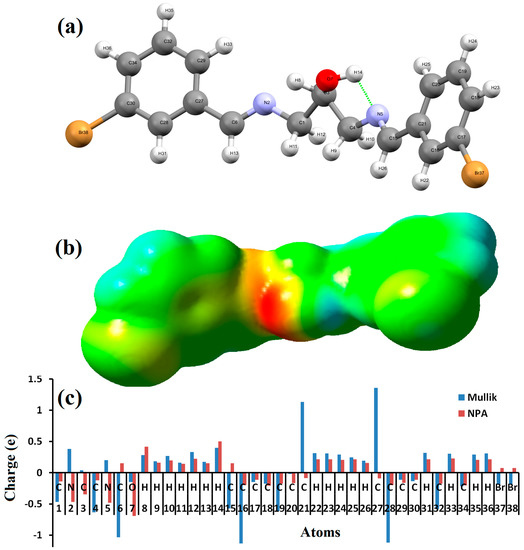
Figure 1.
(a) DFT-optimized structure; (b) MEP; and (c) Mulliken and NPA analysis.

Table 1.
Structure parameters (bond length and bond angle) of the compound.
The nucleophilic and electrophilic positions of one molecule are represented here by an MEP map (Figure 1b). The O and one of the N atoms are indicated as nucleophilic centers, since it appears in red (electron-rich). The H of hydroxyl group together with its phenyl rings, indicated by the blue color, exhibit electrophilic behavior. The second N atom was not red since it was bonded to the H of the hydroxyl through the intramolecular hydrogen bond. The other functional groups in green were found to lack a nucleophilic or electrophilic character.
The Mulliken and neutral population charge analysis (NPA) of the compound along with the MPE result are shown in Figure 1c. The analysis revealed the presence of a negative charge on the donor atoms (nucleophilic positions) such as O, 2N, 2Br, and most of the C atoms. The hydrogen atoms together with the other C atoms reflected a positive character as electrophilic positions (acceptor atoms).
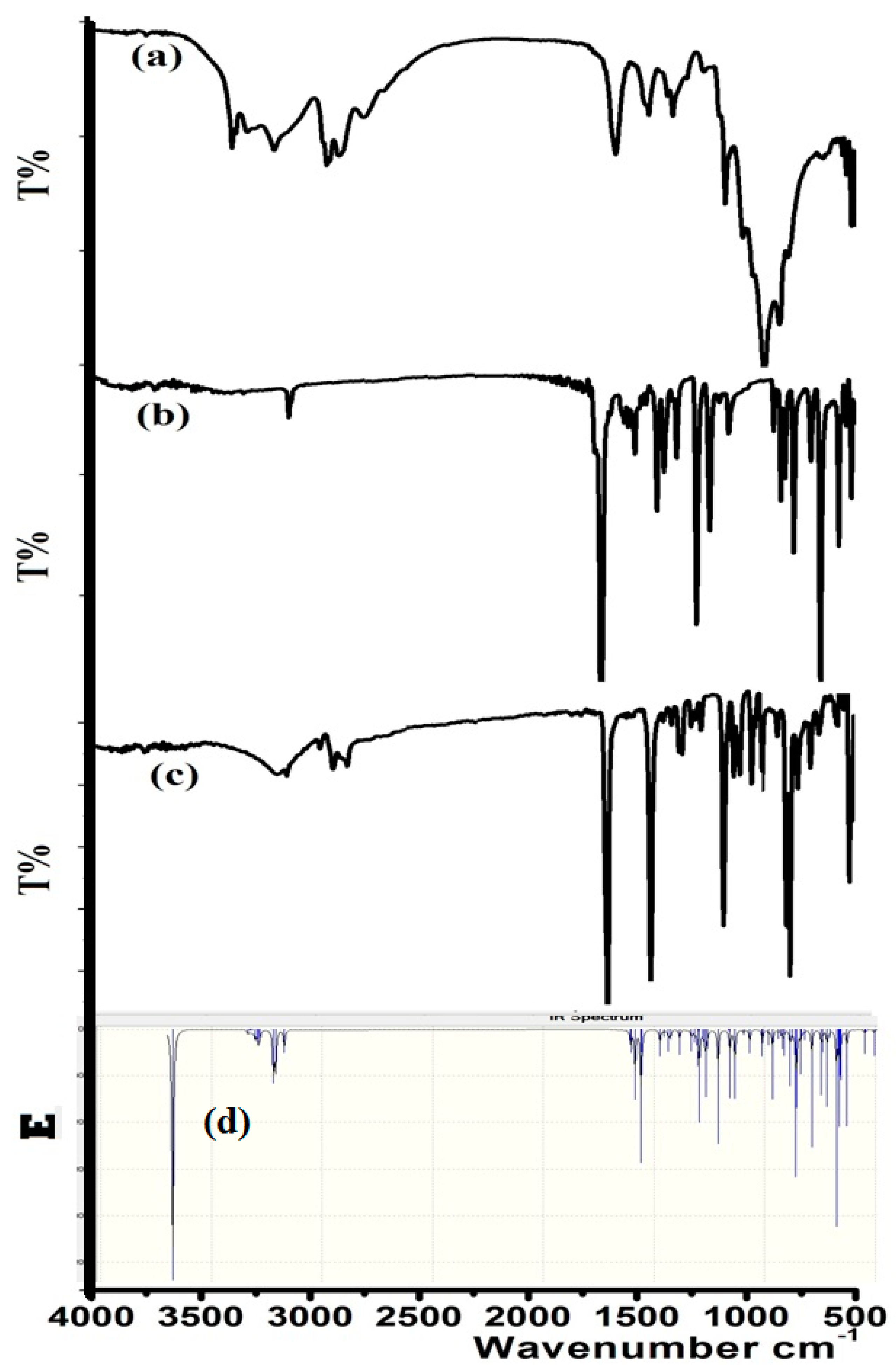
The condensation reaction was monitored by FT-IR, by measuring the IR of the starting materials before and after dehydration, as seen in Figure 2. The formation of the product mainly can be confirmed by the N–H amine disappearing at 3245 cm−1 (Figure 2a) and aldehyde C=O at 1685 cm−1 (Figure 2b) shifting to C=N at 1625 cm−1 (Figure 2c).
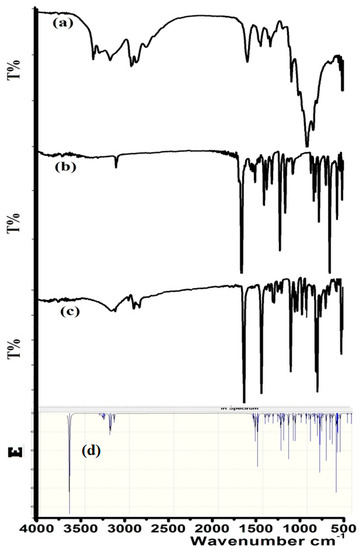
Figure 2.
IR spectra of (a) 1,3-diaminopropan-2-ol; (b) 3-bromobenzaldehyde; (c) 1,3-bis[(E)-(3-bromobenzylidene)amino]propan-2-ol; and (d) the B3LYP/6-311++G(d)-IR of the product.
Theoretical-DFT-IR calculation was performed at the B3LYP/6-311++G(d) level of theory, as shown in Figure 2d. Experimental and theoretical FT-IR spectra showed acceptable agreement. There was a small expected discrepancy: DFT-calculation reflected higher functional-group frequencies compared to the experimental one [16].
2.2. Electronic Transfer and TD-SCF Analysis
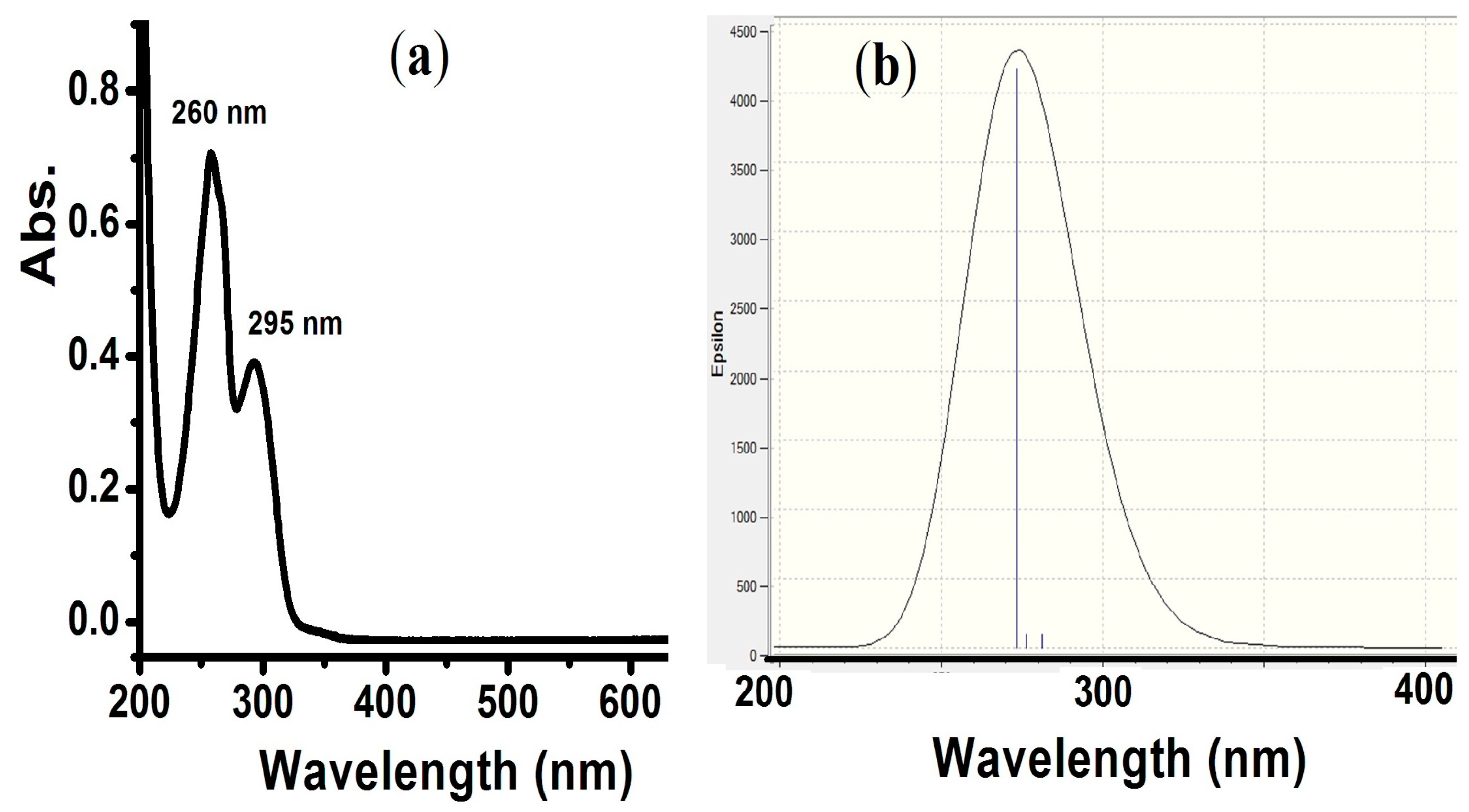
TD-SCF theoretical and experimental electron transfer spectral analysis was performed in an MeOH solvent. The experimental and TD-SCF/DFT analysis revealed no bands in the visible area. The λmax = 260 nm (π→π*) and 295 nm (n→π*) electronic transition were detected experimentally (Figure 3a). In the DFT calculation, a broad peak mainly at λmax = 270 nm was predicted (Figure 3b).
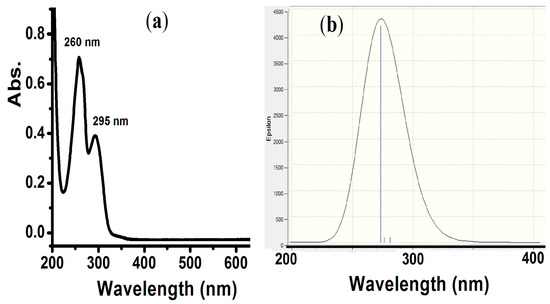
Figure 3.
(a) UV-Visible spectrum and (b) TD-SCF-DFT of the compound in MeOH at RT.
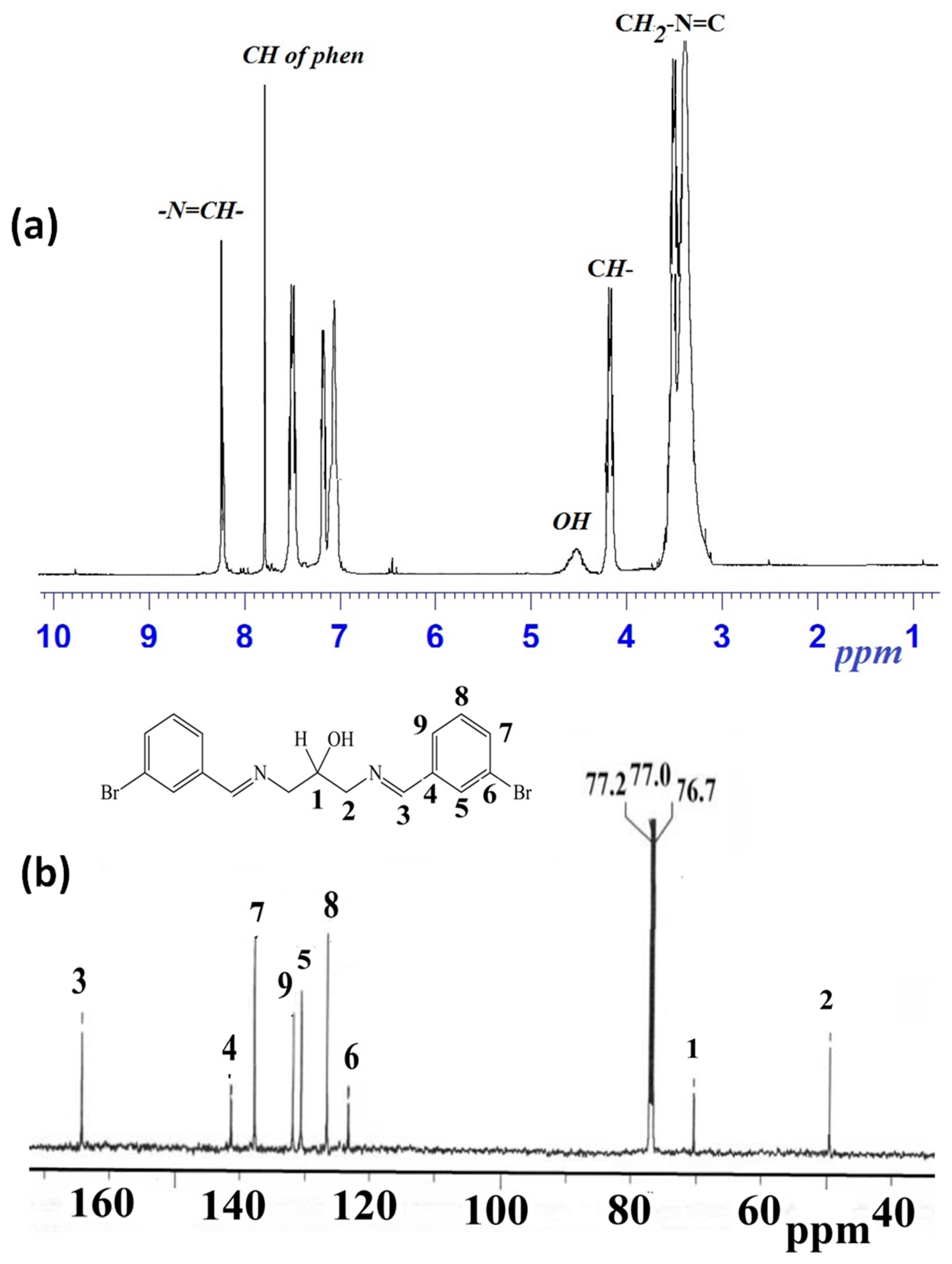
1H-NMR spectrum of the 1,3-bis[(E)-(3-bromobenzylidene)amino]propan-2-ol is simple and appeared in good agreement with its assigned structure: five aliphatic protons (δH 3.4, 3.5 and 4.2 ppm), one alcohol (δH δ 4.6 ppm), eight aromatic protons (δH 7.1–7.8 ppm), and two aldimine protons (δH 8.3 ppm) were observed as shown in Figure 4a.
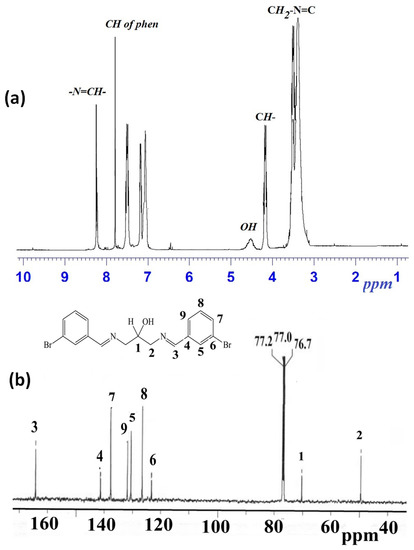
Figure 4.
(a) 250 MHz 1H-NMR and (b) 62.5 MHz 13C-NMR of 1,3-bis[(E)-(3-bromobenzylidene)amino]propan-2-ol in CDCl3.
13C-NMR spectrum revealed nine carbon signals. Two of them arose from aliphatic carbons at 50–70 ppm, six were aromatic carbons at 120–142 ppm, and aldimine carbons were noted at 165.5 ppm, as seen in Figure 4a.
3. Materials and Methods
NMR was performed on a DRX 250 Bruker spectrometer (Bruker, Mainz, Germany). The UV-Visible spectrum was recorded on a double beam TU-1901 spectrophotometer (Purkinje General Instrument Co., Ltd., Beijing, China). The FT-IR spectra were measured with a PerkinElmer-1000 FT-IR Spectrometer (PerkinElmer Inc., Waltham, MA, USA). EI-MS were recorded on a Finnigan 711A (8 kV) (PerkinElmer Inc., Waltham, MA, USA).
A solution of 1,3-diaminopropan-2-ol (1 mmol) and 3-bromobenzaldehyde (2.1 mmol) in MeOH (20 mL) was subjected to reflux for 4 h. The mixture volume was reduced under vacuum (2 mL) until the white precipitate product appeared. The product was filtered, washed several times with distilled water and several times with n-hexane and ethers, and then dried.
Yield: 81% as a white powder, mp = 145.2 °C, was collected; molecular formula C17H16Br2N2O; 1H-NMR (250 MHz, CDCl3): (ppm) 3.4, 3.5 (2m, 4H, =NCH2CH(OH)CH2N=), 4.2 (m, 1H, =NCH2CH(OH)CH2N=), 4.6 (br, 1H, =NCH2CH(OH)CH2N=) 7.1–7.8 (8H, Ph), 8.3 (s, 2H, –HC=N-). 13C-NMR (62.5 MHz, CDCl3): (ppm) 49.8 (2C, =NCH2CH(OH)CH2N=), 70.1 (C, =NCH2CH(OH)CH2N=), 124.8, 127.2, 130.4, 131.8, 138.1, 141.8 (12C, Ph), 165.5 (2C, –HC=N-). [M+] = 424.1 m/z. IR: 3240 cm−1 (O–H), 3040 cm−1 (ArC–H), 2985-2765 cm−1 (Aliphatic C–H), 1625 cm−1 (C=N).
Acknowledgments
The authors are thankful to DST-FIST for providing financial support under research grant scheme Project No. SR/FST/ETT-378/2014.
Author Contributions
H.A., N.A., and A.A.A. performed the experiments; N.A.-Z. measured and analyzed the NMR; N.S. measured and analyzed the MS; M.A.-N. and I.W. wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alshaheri, A.A.; Tahir, M.I.M.; Rahman, M.B.A.; Begum, T.; Saleh, T.A. Synthesis, characterization and catalytic activity of dithiocarbazate Schiff base complexes in oxidation of cyclohexane. J. Mol. Liq. 2017, 240, 486–496. [Google Scholar] [CrossRef]
- Įiįek, B.; Įalısļır, Ü.; Tavaslı, M.; Tülek, R.; Teke, A. Synthesis and optical characterization of novel carbazole Schiff bases. J. Mol. Struct. 2018, 1153, 42–47. [Google Scholar]
- Locke, J.M.; Griffith, R.; Bailey, T.D.; Crumbie, R.L. Competition between cyclisation and bisimine formation in the reaction of 1,3-diaminopropanes with aromatic aldehydes. Tetrahedron 2009, 65, 10685–10692. [Google Scholar] [CrossRef]
- Halli, M.B.; Sumathi, R.B. Synthesis, spectroscopic, antimicrobial and DNA cleavage studies of new Co(II), Ni(II), Cu(II), Cd(II), Zn(II) and Hg(II) complexes with naphthofuran-2-carbohydrazide Schiff base. J. Mol. Struct. 2012, 1022, 130–138. [Google Scholar] [CrossRef]
- Barone, G.; Terenzi, A.; Lauria, A.; Almerico, A.M.; Leal, J.M.; Busto, N.; García, B. DNA-binding of nickel (II), copper(II) and zinc(II) complexes: Structure–affinity relationships. Chem. Rev. 2013, 257, 2848–2862. [Google Scholar] [CrossRef]
- Elemike, E.E.; Nwankwo, H.U.; Onwudiwe, D.C. Experimental and theoretical studies of (Z)-N-(2-chlorobenzylidene) naphthalen-1-amine and (Z)-N-(3-nitrobenzylidene)naphthalen-1-amine, and their corrosion inhibition properties. J. Mol. Struct. 2018, 1155, 123–132. [Google Scholar] [CrossRef]
- Asadi, Z.; Nasrollahi, N. The effect of metal and substituent on DNA binding, cleavage activity, and cytotoxicity of new synthesized Schiff base ligands and Zn(II) complex. J. Mol. Struct. 2017, 1147, 582–593. [Google Scholar] [CrossRef]
- Resayes, S.; Warad, I.; Choudhary, M.; Wahab, A.; Rasheed, S. Heterocyclic Schiff’s Bases as Novel and new Antiglycation Agents. U.S. Patent 2014/0221429A1, 7 August 2014. [Google Scholar]
- Tadavi, S.K.; Yadav, A.A.; Bendre, R.S. Synthesis and characterization of a novel Schiff base of 1,2-diaminopropane with substituted salicyaldehyde and its transition metal complexes: Single crystal structures and biological activities. J. Mol. Struct. 2018, 1152, 223–231. [Google Scholar] [CrossRef]
- Rivera, A.; Miranda-Carvajal, I.; Ríos-Motta, J.; Bolte, M. Crystal structure of 1,3-bis[(E)-4-methoxybenzylideneamino] propan-2-ol. Acta Cryst. 2016, E72, 1731–1733. [Google Scholar] [CrossRef] [PubMed]
- Rivera, A.; Miranda-Carvajal, I.; Ríos-Motta, J.; Bolte, M. Crystal structure of 1,3-bis[(E)-benzylideneamino]propan-2-ol. Acta Cryst. 2017, E73, 627–629. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.; Warad, I.; Al-Resayes, S.; Alzaqri, Z.; Khan, M.; Pallepogu, R.; Dwivedi, S.; Musarrat, J.; Shakir, M. Synthesis and structural characterization of Pd(II) complexes derived from perimidine ligand and their in vitro antimicrobial studies. J. Mol. Struct. 2013, 1047, 48–54. [Google Scholar] [CrossRef]
- Warad, I.; Al-Noaimi, M.; Haddad, S.; Al-Demeri, Y.; Hammouti, B.; Ben Hadda, T. Rac-(E,E)-N,N′-Bis(2-chlorobenzylidene)-cyclohexane-1,2-diamine. Acta Cryst. 2013, E69, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Abdoh, M.; Warad, I.; Naveen, S.; Lokanath, N.; Salghi, R. Crystal structure of (1E,1′E)-N,N′-(ethane-1,2-diyl)bis[(pyridin-2-yl)-methanimine]. Acta Cryst. 2015, E71, 431–435. [Google Scholar]
- Warad, I.; Khan, A.; Azam, M.; Al-Resayes, S.; Haddad, S. Design and structural studies of diimine/CdX2 (X = Cl, I) complexes based on 2,2-dimethyl-1,3-diaminopropane ligand. J. Mol. Struct. 2014, 1062, 167–173. [Google Scholar] [CrossRef]
- Warad, I.; Khan, A.; Azam, M.; Al-Resayes, S.; Khan, M.; Ahmad, P.; Al-Nuri, M.; Jodeh, S.; Husein, A.; Haddad, S.; et al. Structural studies on Cd(II) complexes incorporating di-2-pyridyl ligand and the X-ray crystal structure of the chloroform solvated DPMNPH/CdI2 complex. Inorg. Chem. Comm. 2014, 43, 155–161. [Google Scholar] [CrossRef]
- Warad, I.; Musameh, S.; Badran, I.; Nassar, N.N.; Brandao, P.; Tavares, C.J.; Barakat, A. Synthesis, solvatochromism and crystal structure of trans-[Cu(Et2NCH2CH2NH2)2.H2O](NO3)2 complex: Experimental with DFT combination. J. Mol. Struct. 2017, 1148, 328–338. [Google Scholar] [CrossRef]
- Warad, I.; Al-Demeri, Y.; Al-Nuri, M.; Suleiman, M.; Al-Ali, A.; Amereih, S. N-[(1E)-(3-Bromophenyl)methylene]-N-(2-piperidin-1-ylethyl)amine. Molbank 2016, 3, M903. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).