Abstract
In this paper, we report on the synthesis and spectroscopic characterization of the novel nucleoside 5′-chloro-5′-deoxy-2′,3′-O-isopropylidene-6-fluoro nebularine, obtained as a side product during the second step of the synthesis of 5′-fluoro-5′-deoxy-5-aminoimidazole-4-carboxamide-β-d-riboside (5′-F-AICAR), a non-phosphorylable analogue of 5-aminoimidazole-4-carboxamide-β-d-riboside (AICAR).
1. Introduction
Nucleoside and nucleotide analogues are synthetic modified compounds that have been developed to mimic their physiological counterparts [1]. Considering that several nucleoside and nucleotide analogues have been approved by the Food and Drug Administration (FDA) for the treatment of viral and cancer diseases and others have entered clinical trials [2,3], many research groups have focused their attention on the preparation of novel compounds to expand the pool of molecules with potential biological activities, with the aim of discovering “leads” that are safer and more effective. For example, the replacement of OH groups of sugar moieties with the isosteric F atom has generated life-saving drugs for the treatment of infectious diseases, such as HIV [4], HBV [5], and HCV [6].
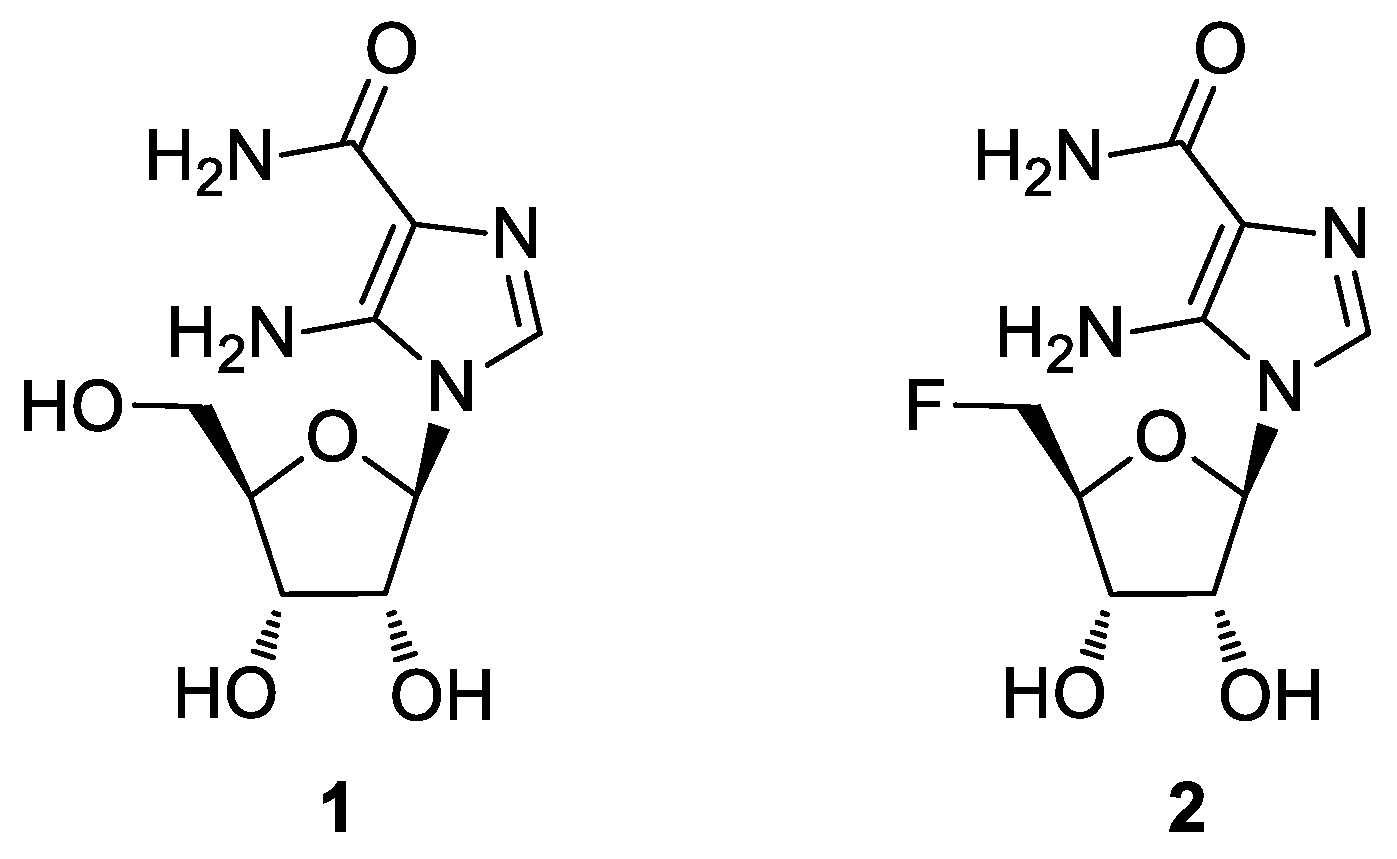
The nucleoside AICAR (1, Figure 1), besides being an intermediate involved in purine biosynthesis, is an activator of the enzyme adenosine monophosphate-activated protein kinase (AMPK) in the 5′-phosphorylated form [7,8]. This activation leads to a cascade of metabolic events, such as the inhibition of basal and insulin-stimulated glucose uptake, lipogenesis, and glucose oxidation [9]. The AMPK pathway is also implicated in the regulation of cell proliferation, and activation by AICAR could result in pro-apoptotic effects [10]. Given the importance of such a molecule, the synthesis of novel AICAR analogues is an appealing goal to better understand its mechanism of action [11,12,13,14,15]. Considering AICAR’s low intestinal absorption and poor penetration of the blood–brain barrier, we synthesized a more lipophilic analogue, where the 5′-OH group was replaced by a fluorine atom (2, 5′-F-AICAR) [16].
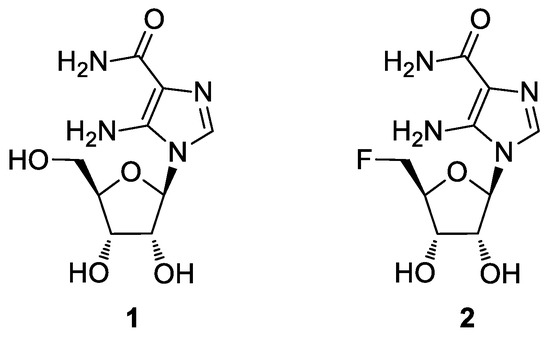
Figure 1.
Structures of AICAR (1) and 5′-F-AICAR (2).
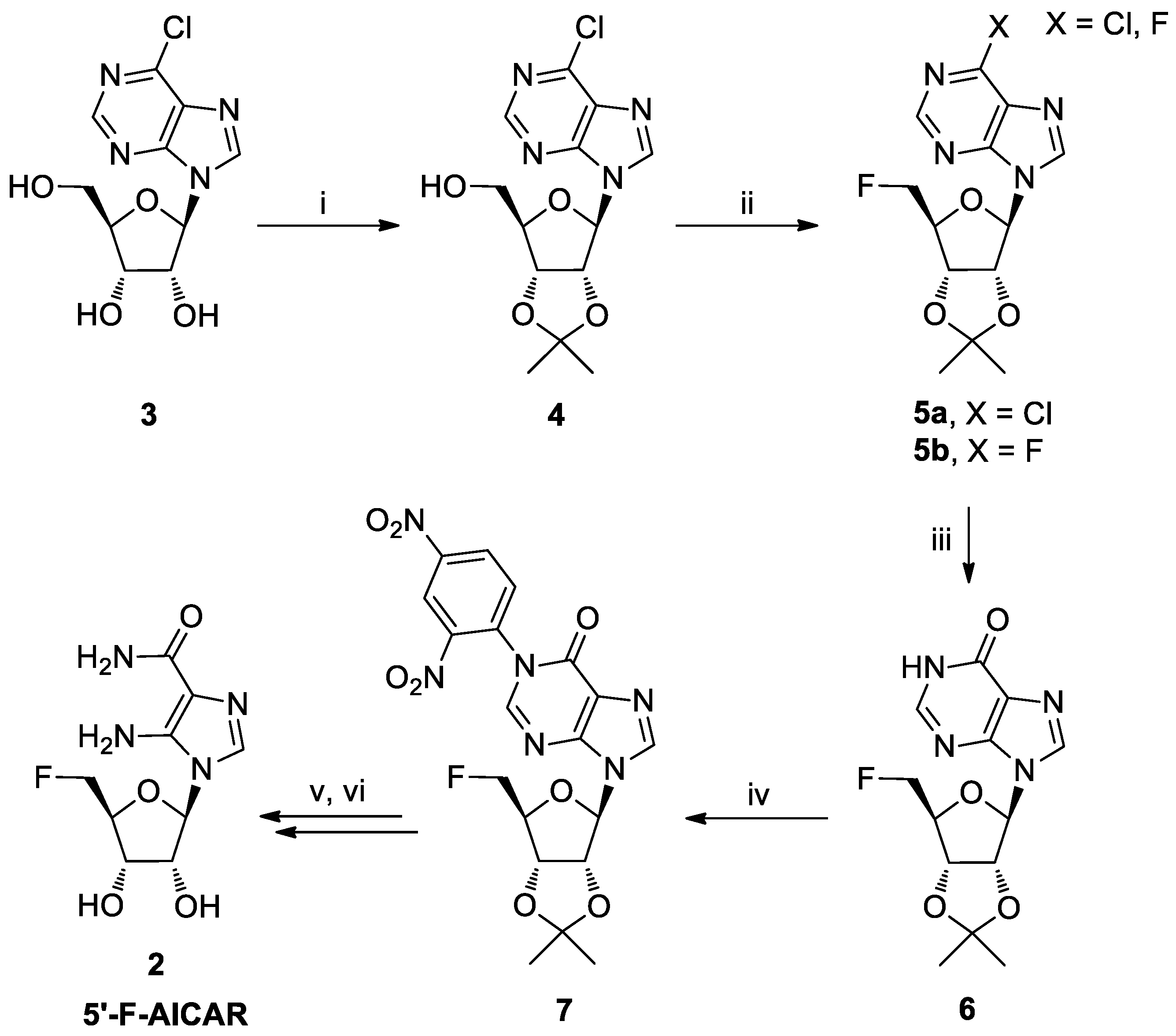
For the preparation of nucleoside 2, a key step was the 5′-fluorination of compound 4 (Scheme 1) [16]. In this paper, we report on the synthesis and structural characterization of the novel nucleoside 8 (Scheme 2), obtained by a side reaction during the fluorination step. This nucleoside could represent a valuable intermediate for a modular derivatization of the purine base moiety and the sugar residue.
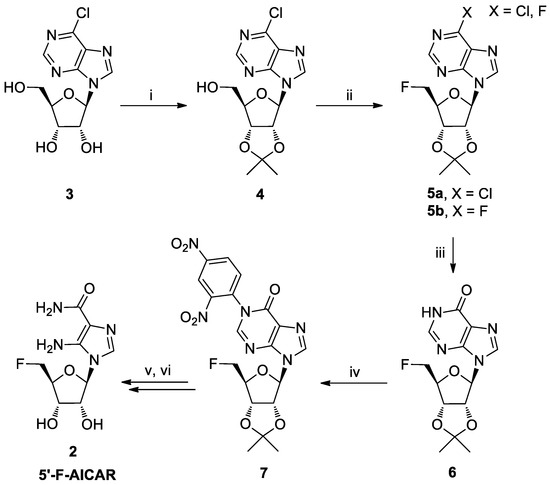
Scheme 1.
Reagents and conditions: (i) acetone, 2,2-dimethoxypropane (DMP), p-toluensulfonic acid (p-TsOH), 2 h, r.t.; (ii) TsF, TBAF, THF, reflux, 18 h; (iii) 0.1 M NaOH, 4 h, r.t.; (iv), K2CO3, 1-chloro-2,4-dinitrobenzene, N,N-dimethylformamide (DMF), 3 h, 80 °C; (v) 1,2-diaminoethane, DMF, 16 h, 50 °C; (vi) 50% trifluoracetic acid (TFA) in H2O, 4 h.
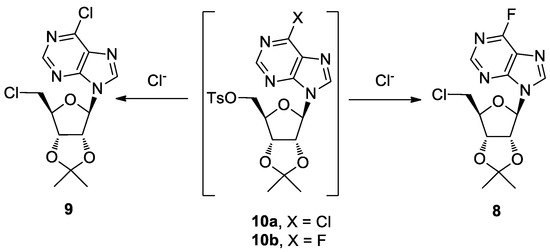
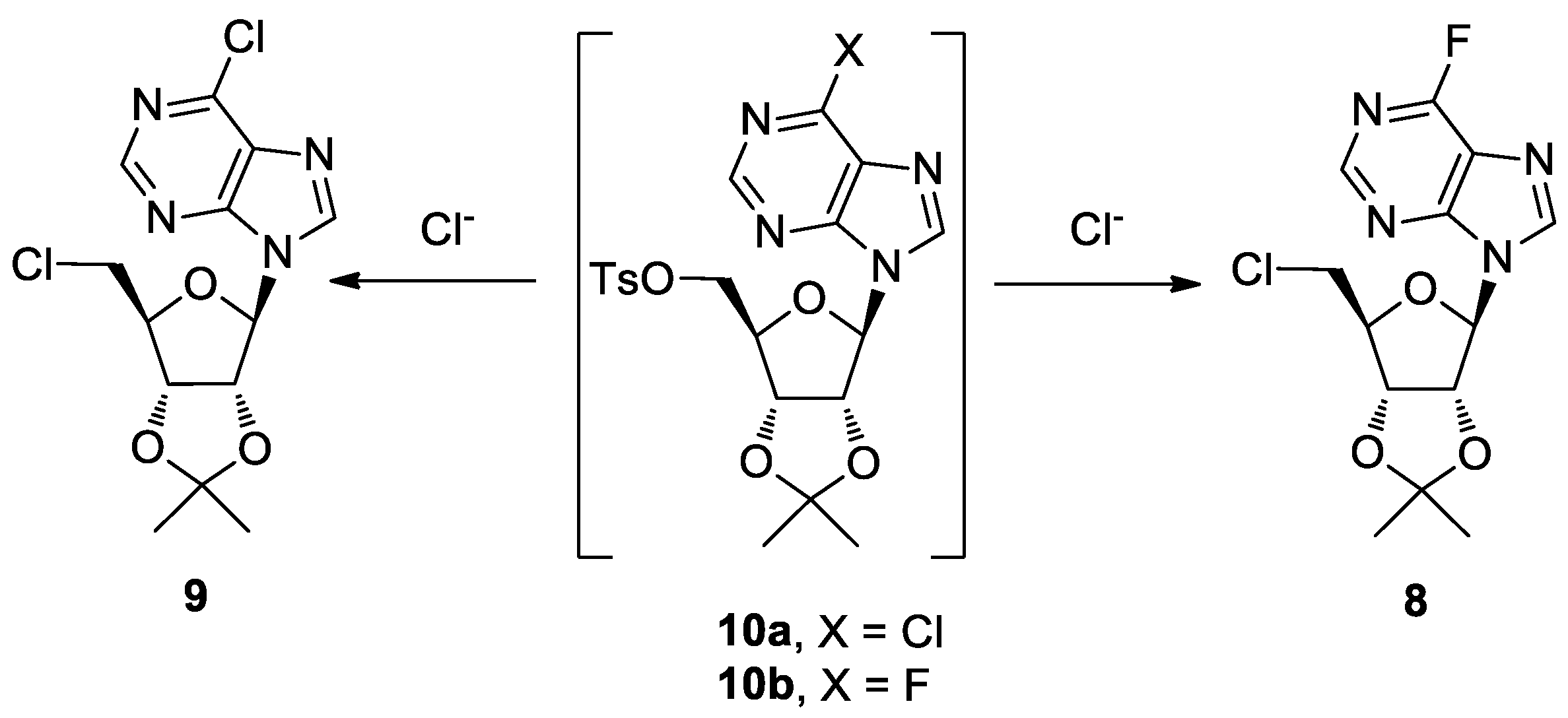
Scheme 2.
Proposed mechanisms for the formation of nucleosides 8 and 9.
2. Results and Discussion
For the synthesis of 5′-F-AICAR (2), we used as the starting material the commercially available 6-chloronebularine (3, Scheme 1), which was readily transformed into its 2′,3′-O-isopropylidene derivative 4 [17]. After 5′-fluorination, the 6-chloropurine was converted into hypoxanthine and the obtained nucleoside 6 transformed into the derivative 7. The strong electron-withdrawing 2,4-dinitrophenyl group, introduced at the N1 hypoxanthine position, allowed us to obtain the 5′-F-AICAR 2 through a 1,2-diaminoethane-mediated purine ring degradation [18].
In the second step of the synthesis, the 5′-OH group of nucleoside 4 was replaced by a 5′-F in a one-pot tosylation/fluorination sequence, through the treatment with the tosyl fluoride/tetrabutylammonium fluoride (TsF/TBAF) reagent system with tetrahydrofuran (THF) as solvent, according to Shimizu’s procedure [19]. As previously observed by Ashton and Scammells [19], we noted (TLC monitoring in n-hexane/AcOEt; 1:1) that the process led not only to the formation of the expected fluorinated product 5a with Rf = 0.48 (Scheme 1), but also to the formation of the bis-fluorinated product 5b with Rf = 0.42, as a consequence of the substitution in species 4 of the C6 chlorine atom by a fluoride ion.
The TLC also showed a third spot with Rf = 0.52, whose corresponding product was isolated (10% yield) and analyzed by 1H-NMR. The spectrum evidenced the presence of two compounds in a ratio of 1:8, which were resolved only by means of high-performance liquid chromatography (HPLC). The nucleoside in lower amounts and with Rt = 21.2 min was assigned as the novel 5′-chloro-5′-deoxy-2′,3′-O-isopropylidene-6-fluoro nebularine (8, Scheme 2). Its structure was established through 1D-, 2D-NMR, and HRESI-MS experiments (see Supplementary Materials). In detail, in the 1H-NMR spectrum the 5′-Ha,b protons resonated as two doublets of doublets at 3.81 and 3.70 ppm, whereas in the 13C-NMR spectrum the 5′C carbon resonated at 43.4 ppm. These data were consistent with the presence of a 5′C–Cl bond [20]. In the HMBC spectrum, the 2-H proton at 8.69 ppm correlated with the C6 purine carbon at 159.9 ppm, which appeared as a doublet with J = 261.8 Hz, as a consequence of the coupling with the F atom.
A similar compound, with the 2′- and 3′-ribose hydroxyls protected as acetates, was synthesized by Robins et al. [21] and used to obtain fluorescent probes for detecting the cellular uptake of the drug gemcitabine.
The main component with Rt = 24.2 min was instead identified as the known 5′-chloro-5′-deoxy-2′,3′-O-isopropylidene-6-chloro nebularine 9 [20].
A plausible mechanism for the formation of compounds 8 and 9 is described in Scheme 2. The Cl− ions, derived from the partial Cl−/F− exchange at the C6 purine position during the 5′-fluorination step, yielded the nucleosides 8 and 9 by SN2 displacement of the tosylate groups in the intermediates 10b and 10a, respectively.
3. Materials and Methods
All the reagents and solvents for the chemical syntheses were obtained from commercial sources and used without further purification. The 1H-, 19F- and 13C-NMR spectra were acquired on 400/700 MHz instruments (Bruker, Billerica, MA, USA) using CDCl3 as the solvent. The chemical shifts were reported in parts per million (δ) relative to the residual solvent signal (1H: CHCl3 7.27; 13C: CDCl3 77.0) and assigned by 2D-NMR experiments. All the NMR spectra were processed using the iNMR software package (Nucleomatica, Molfetta, Italy). The HRESI-MS spectra were recorded in positive mode on a Thermo Orbitrap XL mass spectrometer (ThermoFisher, Waltham, MA, USA). The column chromatography was performed by using silica gel 60, 70–230 mesh (Merck, Darmstadt, Germany). The TLC analyses were performed using 0.2 mm thick F254 silica gel plates (Merck, Darmstadt, Germany). The TLC spots were detected under UV light (254 nm). The high-performance liquid chromatography (HPLC) was performed on a UP-2075 Plus pump equipped with a UV-2075 Plus UV detector (Jasco, Cremella, Italy) using a 5 µm, 250-10 Si column (Purosphere® STAR, Merck, Darmstadt, Germany) eluted with n-hexane/AcOEt, 6:4 with a flow rate of 2.0 mL/min.
5′-Chloro-5′-deoxy-2′,3′-O-isopropylidene-6-fluoro nebularine (8). To a stirred solution of 4 (0.28 g, 0.86 mmol) in dry THF (7.5 mL), TsF (0.30 g, 1.7 mmol) and TBAF (2.6 mL of a 1.0 M solution in dry THF, 2.6 mmol) were added and the mixture refluxed for 16 h (TLC monitoring: n-hexane/AcOEt; 1:1). The solvent was removed under reduced pressure and the crude residue purified by silica gel flash chromatography (n-hexane/AcOEt, 6:4) to afford, in addition to the two expected compounds 5a and 5b [19], the inseparable mixture of the two nucleosides 8 and 9 with Rf = 0.52 (10% yield). The mixture was purified by HPLC (see above), thus obtaining the pure compound 8. Colorless foam (3.5 mg, 1.2% yield). 1H-NMR (400 MHz; CDCl3): δ 8.69 (s, 1H, 2-H), 8.29 (s, 1H, 8-H), 6.24 (d, J = 2.6 Hz, 1H, 1′-H), 5.40 (dd, J = 6.4, 2.7 Hz, 1H, 2′-H), 5.13 (dd, J = 6.4, 3.1 Hz, 1H, 3′-H), 4.58-4.55 (m, 1H, 4′-H), 3.82 (dd, J = 11.6, 6.1 Hz, 1H, 5′-Ha), 3.70 (dd, J = 11.6, 4.9 Hz, 1H, 5′-Hb), 1.66 (s, 3H, CH3), 1.42 (s, 3H, CH3); 19F-NMR (377 MHz; CDCl3): δ −68.4 (s); 13C-NMR (176 MHz; CDCl3): δ 159.9 (d, J = 261.8, 6-C), 154.5 (d, J = 11.1, 4-C), 152.2 (d, J = 14.3, 2-C), 144.0 (8-C), 121.15 (d, J = 29.2, 5-C), 115.1 (Cq acetonide), 91.3 (1′-C), 86.2 (4′-C), 84.2 (2′-C), 82.3 (3′-C), 43.9 (CH2Cl), 27.1 (CH3), 25.3 (CH3); HRESI-MS m/z 329.0809, ([M + H]+ calcd. for C13H15ClFN4O3 329.0817).
Supplementary Materials
The following are available online, Figure S1: copies of 1H- and 19F-NMR spectra of compound 8; Figure S2: copy of 13C-NMR spectrum of compound 8; Figure S3: copy of HMBC spectrum of compound 8; Figure S4: copy of HRESI-MS spectrum of compound 8.
Author Contributions
S.D. conceived and designed the experiments; A.P.F. performed the synthetic experiments; M.M. performed the spectroscopic experiments; M.T. analyzed the data; S.D. wrote the paper. All authors read and approved the final manuscript.
Funding
This research was funded by “Campania Onco Terapie – Combattere la resistenza tumorale: piattaforma integrata multidisciplinare per un approccio tecnologico innovativo alle oncoterapie” – POR Campania FESR 2014/2020 O.S. 1.2 Az. 1.2.2 Avviso per Manifestazione di interesse per la Realizzazione di Technology Platform nell’ambito della lotta alle patologie oncologiche CUP B61G18000470007”.
Acknowledgments
The authors are grateful to Luisa Cuorvo for her technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burke, M.; Borland, K.; Litosh, V. Base-modified nucleosides as chemotherapeutic agents: Past and future. Curr. Top. Med. Chem. 2016, 16, 1231–1241. [Google Scholar] [CrossRef]
- Berdis, A.J. Inhibiting DNA polymerases as a therapeutic intervention against cancer. Front. Mol. Biosci. 2017, 4, 78. [Google Scholar] [CrossRef]
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef]
- Andreatta, K.; Willkom, M.; Martin, R.; Chang, S.; Wei, L.; Liu, H.; Liu, Y.-P.; Graham, H.; Quirk, E.; Martin, H.; et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide maintained HIV-1 RNA suppression in participants with archived antiretroviral resistance including M184V/I. J. Antimicrob. Chemother. 2019, 74, 3555–3564. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Peng, Y.; Qin, C.; Liu, Y.; Li, J.; Jiang, J.; Zhou, Y.; Chang, J.; Wang, Q. Novel fluoronucleoside analog NCC inhibits lamivudine-resistant hepatitis B virus in a hepatocyte model. Brazilian J. Infect. Dis. 2018, 22, 477–486. [Google Scholar] [CrossRef]
- Sofia, M.J.; Bao, D.; Chang, W.; Du, J.; Nagarathnam, D.; Rachakonda, S.; Reddy, P.G.; Ross, B.S.; Wang, P.; Zhang, H.R.; et al. Discovery of a β-d-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem. 2010, 53, 7202–7218. [Google Scholar] [CrossRef]
- Daignan-Fornier, B.; Pinson, B. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5′-monophosphate (AICAR), a highly conserved purine intermediate with multiple effects. Metabolites 2012, 2, 292–302. [Google Scholar] [CrossRef]
- Scudiero, O.; Nigro, E.; Monaco, M.L.; Oliviero, G.; Polito, R.; Borbone, N.; D’Errico, S.; Mayol, L.; Daniele, A.; Piccialli, G. New synthetic AICAR derivatives with enhanced AMPK and ACC activation. J. Enzyme Inhib. Med. Chem. 2015, 6366, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Merrill, G.F.; Kurth, E.J.; Hardie, D.G.; Winder, W.W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. - Endocrinol. Metab. 1997, 273, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Hsieh, K.L.; Liu, P.L.; Yeh, H.C.; Huang, S.P.; Fang, S.H.; Cheng, W.C.; Huang, K.H.; Chiu, F.Y.; Lin, I.L.; et al. AICAR induces apoptosis and inhibits migration and invasion in prostate cancer cells through an AMPK/mTOR-dependent pathway. Int. J. Mol. Sci. 2019, 20, 1647. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, G.; Amato, J.; Borbone, N.; D’Errico, S.; Piccialli, G.; Mayol, L. Synthesis of N-1 and ribose modified inosine analogues on solid support. Tetrahedron Lett. 2007, 48, 397–400. [Google Scholar] [CrossRef]
- Oliviero, G.; Amato, J.; Borbone, N.; D’Errico, S.; Piccialli, G.; Bucci, E.; Piccialli, V.; Mayol, L. Synthesis of 4-N-alkyl and ribose-modified AICAR analogues on solid support. Tetrahedron 2008, 64, 6475–6481. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Solid-phase synthesis of a new diphosphate 5-aminoimidazole-4-carboxamide riboside (AICAR) derivative and studies toward cyclic AICAR diphosphate ribose. Molecules 2011, 16, 8110–8118. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Synthesis of new acadesine (AICA-riboside) analogues having acyclic d-ribityl or 4-hydroxybutyl chains in place of the ribose. Molecules 2013, 18, 9420–9431. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Piccialli, V.; Piccialli, G. Synthesis of 5-aminoimidazole-4-carboxamide riboside (AICAR) and its derivatives using inosine as starting material. Curr. Protoc. Nucleic Acid Chem. 2015, 63, 1.35.1–1.35.24. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; D’Alonzo, D.; Piccialli, V.; Mayol, L.; Piccialli, G. A facile synthesis of 5′-fluoro-5′-deoxyacadesine (5′-F-AICAR): A novel non-phosphorylable AICAR analogue. Molecules 2012, 17, 13036–13044. [Google Scholar] [CrossRef]
- Kappler, F.; Hampton, A. Approaches to isozyme-specific inhibitors. 17.1 Attachment of a selectivity-inducing substituent to a multisubstrate adduct. Implications for facilitated design of potent, isozyme-selective inhibitors. J. Med. Chem. 1990, 33, 2545–2551. [Google Scholar] [CrossRef]
- Oliviero, G.; D’Errico, S.; Borbone, N.; Amato, J.; Piccialli, V.; Piccialli, G.; Mayol, L. Facile solid-phase synthesis of AICAR 5′-monophosphate (ZMP) and its 4-N-alkyl derivatives. Eur. J. Org. Chem. 2010, 2010, 1517–1524. [Google Scholar] [CrossRef]
- Ashton, T.D.; Scammells, P.J. An improved synthesis of 5′-fluoro-5′-deoxyadenosines. Bioorg. Med. Chem. Lett. 2005, 15, 3361–3363. [Google Scholar] [CrossRef]
- Amiable, C.; Pochet, S. Unprecedented formation of 8(R),5′-O-cycloribonucleosides through a triflation reaction of purine ribonucleosides. Tetrahedron 2015, 71, 2525–2529. [Google Scholar] [CrossRef]
- Robins, M.J.; Peng, Y.; Damaraju, V.L.; Mowles, D.; Barron, G.; Tackaberry, T.; Young, J.D.; Cass, C.E. Improved syntheses of 5′-S-(2-aminoethyl)-6-N-(4-nitrobenzyl)-5′-thioadenosine (SAENTA), analogues, and fluorescent probe conjugates: Analysis of cell-surface human equilibrative nucleoside transporter 1 (hENT1) levels for prediction of the antitumor efficacy of gemcitabine. J. Med. Chem. 2010, 53, 6040–6053. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).