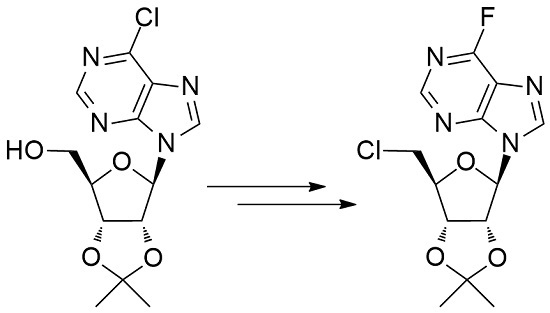
5′-Chloro-5′-deoxy-2′,3′-O-isopropylidene-6-fluoro nebularine
Abstract
:1. Introduction
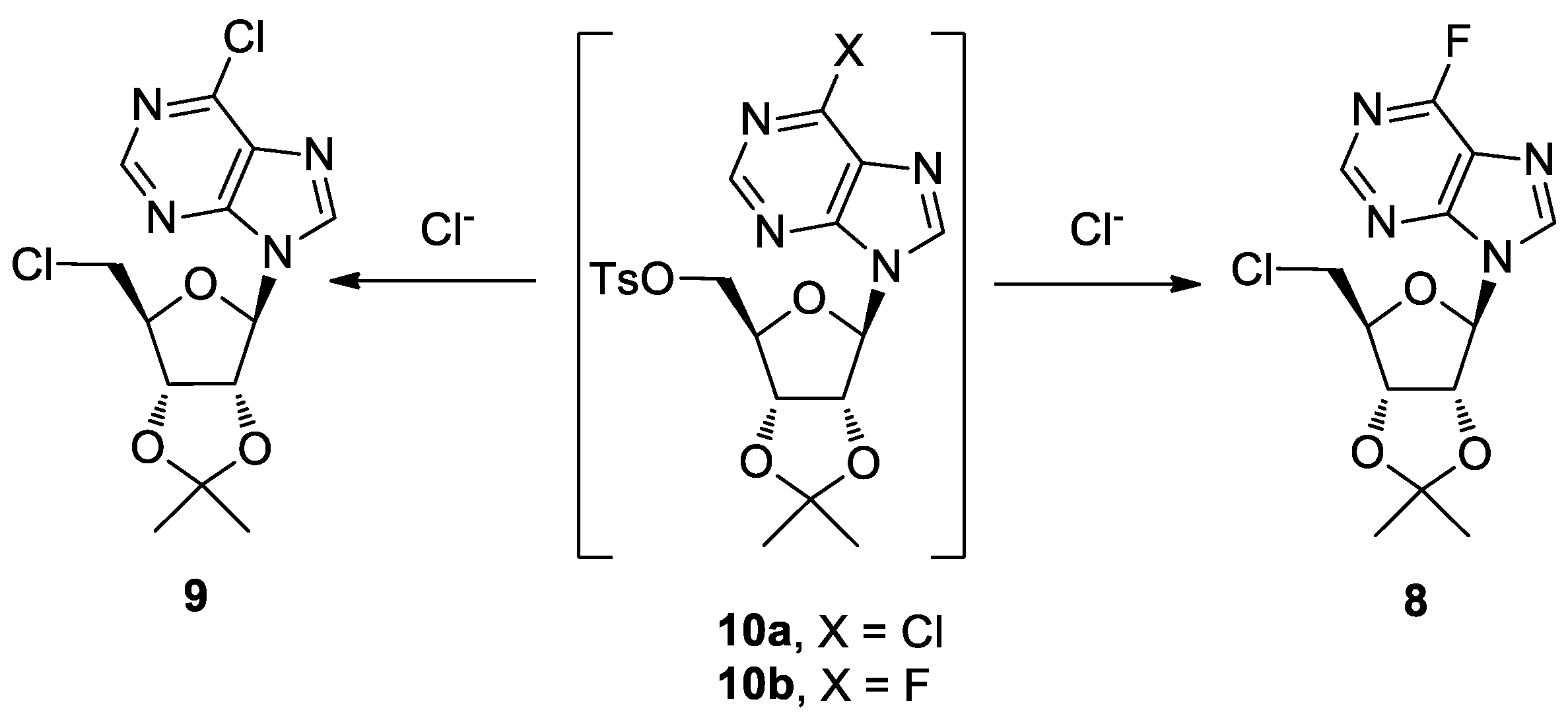
2. Results and Discussion
3. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burke, M.; Borland, K.; Litosh, V. Base-modified nucleosides as chemotherapeutic agents: Past and future. Curr. Top. Med. Chem. 2016, 16, 1231–1241. [Google Scholar] [CrossRef]
- Berdis, A.J. Inhibiting DNA polymerases as a therapeutic intervention against cancer. Front. Mol. Biosci. 2017, 4, 78. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E.; Li, G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016, 29, 695–747. [Google Scholar] [CrossRef] [Green Version]
- Andreatta, K.; Willkom, M.; Martin, R.; Chang, S.; Wei, L.; Liu, H.; Liu, Y.-P.; Graham, H.; Quirk, E.; Martin, H.; et al. Switching to bictegravir/emtricitabine/tenofovir alafenamide maintained HIV-1 RNA suppression in participants with archived antiretroviral resistance including M184V/I. J. Antimicrob. Chemother. 2019, 74, 3555–3564. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Y.; Peng, Y.; Qin, C.; Liu, Y.; Li, J.; Jiang, J.; Zhou, Y.; Chang, J.; Wang, Q. Novel fluoronucleoside analog NCC inhibits lamivudine-resistant hepatitis B virus in a hepatocyte model. Brazilian J. Infect. Dis. 2018, 22, 477–486. [Google Scholar] [CrossRef]
- Sofia, M.J.; Bao, D.; Chang, W.; Du, J.; Nagarathnam, D.; Rachakonda, S.; Reddy, P.G.; Ross, B.S.; Wang, P.; Zhang, H.R.; et al. Discovery of a β-d-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem. 2010, 53, 7202–7218. [Google Scholar] [CrossRef]
- Daignan-Fornier, B.; Pinson, B. 5-Aminoimidazole-4-carboxamide-1-beta-D-ribofuranosyl 5′-monophosphate (AICAR), a highly conserved purine intermediate with multiple effects. Metabolites 2012, 2, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Scudiero, O.; Nigro, E.; Monaco, M.L.; Oliviero, G.; Polito, R.; Borbone, N.; D’Errico, S.; Mayol, L.; Daniele, A.; Piccialli, G. New synthetic AICAR derivatives with enhanced AMPK and ACC activation. J. Enzyme Inhib. Med. Chem. 2015, 6366, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrill, G.F.; Kurth, E.J.; Hardie, D.G.; Winder, W.W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. - Endocrinol. Metab. 1997, 273, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Hsieh, K.L.; Liu, P.L.; Yeh, H.C.; Huang, S.P.; Fang, S.H.; Cheng, W.C.; Huang, K.H.; Chiu, F.Y.; Lin, I.L.; et al. AICAR induces apoptosis and inhibits migration and invasion in prostate cancer cells through an AMPK/mTOR-dependent pathway. Int. J. Mol. Sci. 2019, 20, 1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliviero, G.; Amato, J.; Borbone, N.; D’Errico, S.; Piccialli, G.; Mayol, L. Synthesis of N-1 and ribose modified inosine analogues on solid support. Tetrahedron Lett. 2007, 48, 397–400. [Google Scholar] [CrossRef]
- Oliviero, G.; Amato, J.; Borbone, N.; D’Errico, S.; Piccialli, G.; Bucci, E.; Piccialli, V.; Mayol, L. Synthesis of 4-N-alkyl and ribose-modified AICAR analogues on solid support. Tetrahedron 2008, 64, 6475–6481. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Solid-phase synthesis of a new diphosphate 5-aminoimidazole-4-carboxamide riboside (AICAR) derivative and studies toward cyclic AICAR diphosphate ribose. Molecules 2011, 16, 8110–8118. [Google Scholar] [CrossRef] [Green Version]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; Piccialli, V.; Varra, M.; Mayol, L.; Piccialli, G. Synthesis of new acadesine (AICA-riboside) analogues having acyclic d-ribityl or 4-hydroxybutyl chains in place of the ribose. Molecules 2013, 18, 9420–9431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Piccialli, V.; Piccialli, G. Synthesis of 5-aminoimidazole-4-carboxamide riboside (AICAR) and its derivatives using inosine as starting material. Curr. Protoc. Nucleic Acid Chem. 2015, 63, 1.35.1–1.35.24. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; D’Alonzo, D.; Piccialli, V.; Mayol, L.; Piccialli, G. A facile synthesis of 5′-fluoro-5′-deoxyacadesine (5′-F-AICAR): A novel non-phosphorylable AICAR analogue. Molecules 2012, 17, 13036–13044. [Google Scholar] [CrossRef] [Green Version]
- Kappler, F.; Hampton, A. Approaches to isozyme-specific inhibitors. 17.1 Attachment of a selectivity-inducing substituent to a multisubstrate adduct. Implications for facilitated design of potent, isozyme-selective inhibitors. J. Med. Chem. 1990, 33, 2545–2551. [Google Scholar] [CrossRef]
- Oliviero, G.; D’Errico, S.; Borbone, N.; Amato, J.; Piccialli, V.; Piccialli, G.; Mayol, L. Facile solid-phase synthesis of AICAR 5′-monophosphate (ZMP) and its 4-N-alkyl derivatives. Eur. J. Org. Chem. 2010, 2010, 1517–1524. [Google Scholar] [CrossRef]
- Ashton, T.D.; Scammells, P.J. An improved synthesis of 5′-fluoro-5′-deoxyadenosines. Bioorg. Med. Chem. Lett. 2005, 15, 3361–3363. [Google Scholar] [CrossRef]
- Amiable, C.; Pochet, S. Unprecedented formation of 8(R),5′-O-cycloribonucleosides through a triflation reaction of purine ribonucleosides. Tetrahedron 2015, 71, 2525–2529. [Google Scholar] [CrossRef]
- Robins, M.J.; Peng, Y.; Damaraju, V.L.; Mowles, D.; Barron, G.; Tackaberry, T.; Young, J.D.; Cass, C.E. Improved syntheses of 5′-S-(2-aminoethyl)-6-N-(4-nitrobenzyl)-5′-thioadenosine (SAENTA), analogues, and fluorescent probe conjugates: Analysis of cell-surface human equilibrative nucleoside transporter 1 (hENT1) levels for prediction of the antitumor efficacy of gemcitabine. J. Med. Chem. 2010, 53, 6040–6053. [Google Scholar] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falanga, A.P.; Marzano, M.; Terracciano, M.; D'Errico, S. 5′-Chloro-5′-deoxy-2′,3′-O-isopropylidene-6-fluoro nebularine. Molbank 2019, 2019, M1097. https://doi.org/10.3390/M1097
Falanga AP, Marzano M, Terracciano M, D'Errico S. 5′-Chloro-5′-deoxy-2′,3′-O-isopropylidene-6-fluoro nebularine. Molbank. 2019; 2019(4):M1097. https://doi.org/10.3390/M1097
Chicago/Turabian StyleFalanga, Andrea Patrizia, Maria Marzano, Monica Terracciano, and Stefano D'Errico. 2019. "5′-Chloro-5′-deoxy-2′,3′-O-isopropylidene-6-fluoro nebularine" Molbank 2019, no. 4: M1097. https://doi.org/10.3390/M1097
APA StyleFalanga, A. P., Marzano, M., Terracciano, M., & D'Errico, S. (2019). 5′-Chloro-5′-deoxy-2′,3′-O-isopropylidene-6-fluoro nebularine. Molbank, 2019(4), M1097. https://doi.org/10.3390/M1097