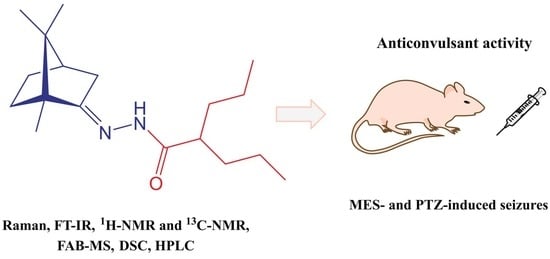
2-Propyl-N′-[1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]pentanehydrazide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Anticonvulsant Activity
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 2-Propyl-N′-[1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]pentanehydrazide (3)
- White crystals (80%). 1H-NMR (300 MHz, DMSO-d6) δ: 0.65 (s, 3H, CH3), 0.82 (s, 6H, 2CH3), 0.86 (s, 3H, CH3), 0.90 (s, 3H, CH3), 1.21 (m, 8H, 2CH2-CH2), 1.46‒1.51 (m, 2H, CH2), 1.65 (t, 1H, CH), 1.77 (m, 1H, CH), 1.89 (m, 2H, CH2), 2.30 (m, 1H, CH), 9.51 (s, 1H, NH). 13C-NMR (100 MHz, DMSO-d6) δ: 177.4 (C=O), 171.3 (C-2), 52.3 (C-1), 47.8 (C-7), 47.6 (C-4), 43.9 (CH), 35.1 (C-3), 34.5 (CH2), 32.7 (C-6), 27.3 (C-5), 20.5 (CH2), 18.8 (C-8,9), 14.2 (C-10), 11.5 (CH3). FT-IR (νmax, CM−1): 3179 (N-H), 2970‒2834 (C-H), 1697 (C=O). Raman (νmax, CM−1): 2943‒2872 (C-H), 1646 (C=N). MS (FAB) m/z: 293 [M + H]+. HPLC: tr = 23.43 min. M.p. (DSC) onset: 154.26 °C, peak max: 155.46 °C (Supplementary Materials).
3.3. Anticonvulsant Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Romoli, M.; Mazzocchetti, P.; D’Alonzo, R.; Siliquini, S.; Rinaldi, V.E.; Verrotti, A.; Calabresi, P.; Costa, C. Valproic acid and epilepsy: From molecular mechanisms to clinical evidences. Curr. Neuropharmacol. 2019, 17, 926–946. [Google Scholar] [CrossRef]
- Gayam, V.; Mandal, A.K.; Khalid, M.; Shrestha, B.; Garlapati, P.; Khalid, M. Valproic acid induced acute liver injury resulting in hepatic encephalopathy- a case report and literature review. J. Community Hosp. Intern. Med. Perspect. 2018, 8, 311–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Almeida, R.N.; Agra, M.; Maior, F.N.; De Sousa, D.P. Essential oils and their constituents: Anticonvulsant activity. Molecules 2011, 16, 2726–2742. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesterkina, M.V.; Kravchenko, I.A. Synthesis and anticonvulsant activity of menthyl γ-aminobutyrate. Chem. Nat. Compd. 2016, 52, 237–239. [Google Scholar] [CrossRef]
- Agrawal, S.; Jain, J.; Kumar, A.; Gupta, P.; Garg, V. Synthesis molecular modeling and anticonvulsant activity of some hydrazone, semicarbazone, and thiosemicarbazone derivatives of benzylidene camphor. Res. Rep. Med. Chem. 2014, 4, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, E.T.; da Silva Araújo, A.; Moraes, A.M.; de Souza, L.A.; Silva Lourenço, M.C.; de Souza, M.V.; Wardell, J.L.; Wardell, S.M. Synthesis and biological activities of camphor hydrazone and imine derivatives. Sci. Pharm. 2016, 84, 467–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Li, J.; Zeng, X.D.; Hu, X.M.; Zhou, X.; Hong, X. Synthesis and pharmacological evaluation of novel benzenesulfonamide derivatives as potential anticonvulsant agents. Molecules 2015, 20, 17585–17600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nesterkina, M.V.; Alekseeva, E.A.; Kravchenko, I.A. Synthesis, physicochemical properties, and anticonvulsant activity of the gaba complex with a calix[4]arene derivative. Pharm. Chem. J. 2014, 48, 82–84. [Google Scholar] [CrossRef]
- Nesterkina, M.; Barbalat, D.; Konovalova, I.; Shishkina, S.; Atakay, M.; Salih, B.; Kravchenko, I. Novel (–)-carvone derivatives as potential anticonvulsant and analgesic agents. Nat. Prod. Res 2020, (in press). [Google Scholar] [CrossRef] [PubMed]


| Compound | Control | Camphor | VPA | Compound 3 |
|---|---|---|---|---|
| 3 h after single oral administration | ||||
| % Mortality protection | 0 | 60 | 80 | 80 |
| 24 h after single oral administration | ||||
| % Mortality protection | 0 | 20 | 60 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nesterkina, M.; Barbalat, D.; Rakipov, I.; Kravchenko, I. 2-Propyl-N′-[1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]pentanehydrazide. Molbank 2020, 2020, M1164. https://doi.org/10.3390/M1164
Nesterkina M, Barbalat D, Rakipov I, Kravchenko I. 2-Propyl-N′-[1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]pentanehydrazide. Molbank. 2020; 2020(4):M1164. https://doi.org/10.3390/M1164
Chicago/Turabian StyleNesterkina, Mariia, Dmytro Barbalat, Ildar Rakipov, and Iryna Kravchenko. 2020. "2-Propyl-N′-[1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]pentanehydrazide" Molbank 2020, no. 4: M1164. https://doi.org/10.3390/M1164
APA StyleNesterkina, M., Barbalat, D., Rakipov, I., & Kravchenko, I. (2020). 2-Propyl-N′-[1,7,7-trimethylbicyclo[2.2.1]hept-2-ylidene]pentanehydrazide. Molbank, 2020(4), M1164. https://doi.org/10.3390/M1164