4,6-Dinitro-7-(thiazol-2-ylamino)benzo[c][1,2,5]oxadiazole 1-oxide
Abstract
:1. Introduction
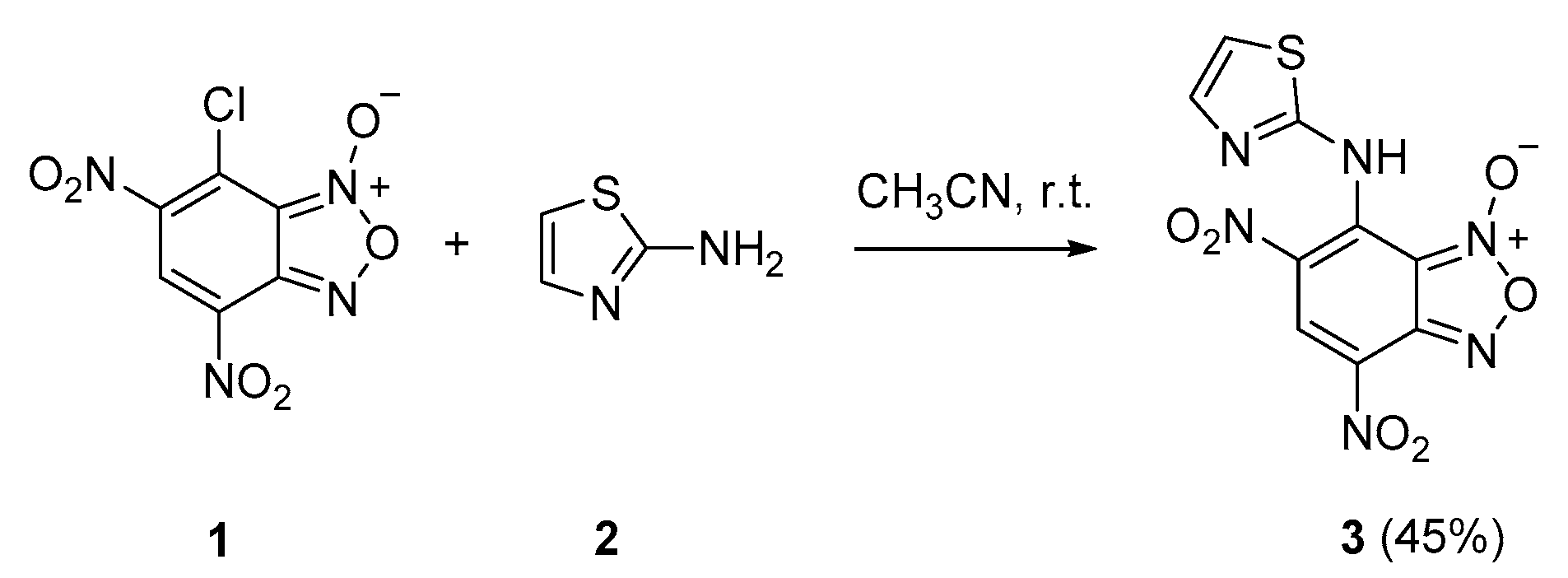
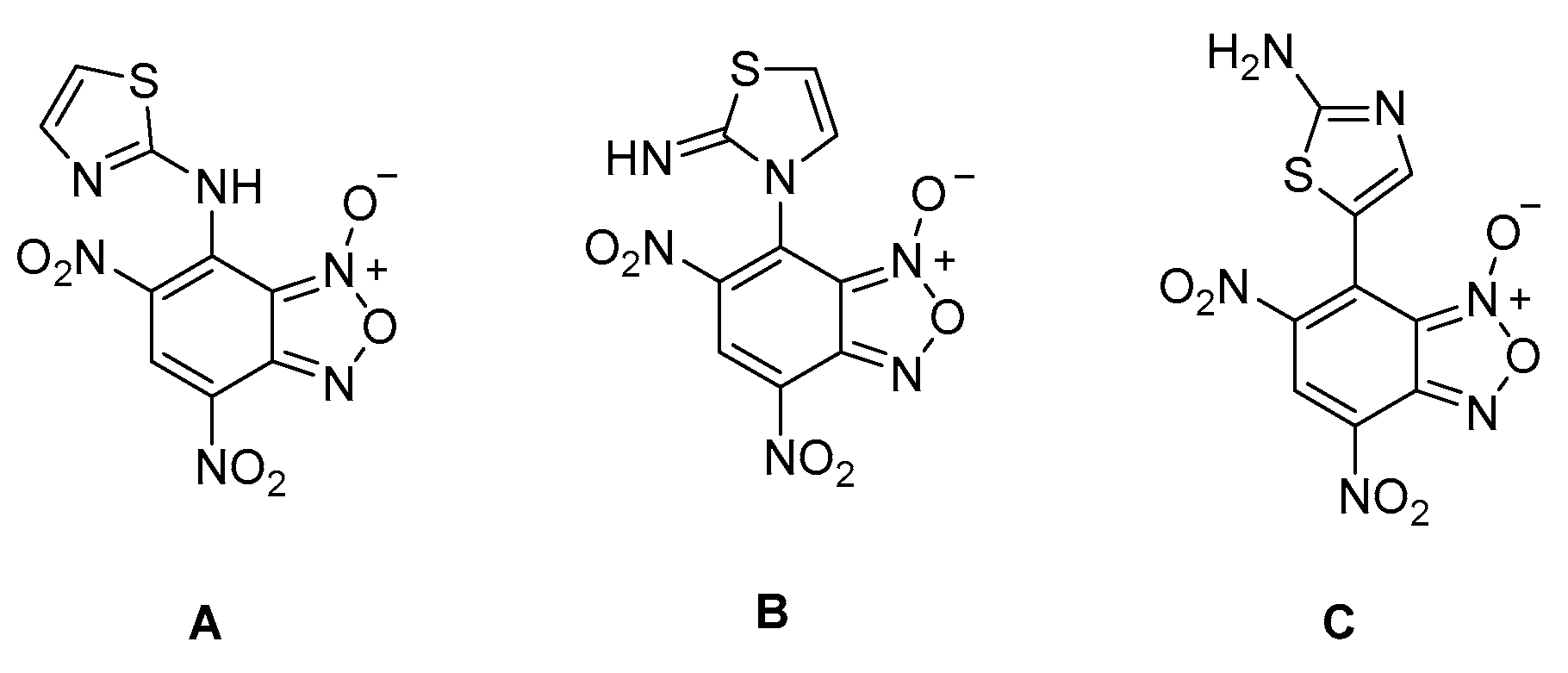
2. Results
3. Discussion
4. Materials and Methods
4,6-Dinitro-7-(thiazol-2-ylamino)benzo[c][1,2,5]oxadiazole 1-oxide (3)
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Terrier, F. Modern Nucleophilic Aromatic Substitution; Wiley VCH: Weinheim, Germany, 2013. [Google Scholar]
- Terrier, F. Nucleophilic Aromatic Displacement; VCH: New York, NY, USA, 1991. [Google Scholar]
- Ouellette, R.J.; Rawn, J.D. Electrophilic Aromatic Substitution. In Organic Chemistry: Structure, Mechanism and Synthesis, 1st ed.; Elsevier: San Diego, CA, USA, 2014; Chapter 13; pp. 417–451. [Google Scholar]
- Ouellette, R.J.; Rawn, J.D. Electrophilic Aromatic Substitution. In Organic Chemistry: Structure, Mechanism and Synthesis, 2nd ed.; Elsevier: San Diego, CA, USA, 2018; Chapter 13; pp. 375–407. [Google Scholar]
- Micheletti, G.; Boga, B. Nucleophile/Electrophile Combinations in Aromatic Substitution: From Wheland to Wheland-Meisenheimer Intermediates Using Strongly Activated Arenes. Synthesis 2017, 49, 3347–3356. [Google Scholar]
- Del Vecchio, E.; Boga, C.; Forlani, L.; Tozzi, S.; Micheletti, G.; Cino, S. Ring Closure of Azo Compounds to 1,2-Annulated Benzimidazole Derivatives and Further Evidence of Reversibility of the Azo-Coupling Reaction. J. Org. Chem. 2015, 80, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, G.; Boga, C.; Pafundi, M.; Pollicino, S.; Zanna, N. New electron-donor and -acceptor architectures from benzofurazans and sym-triaminobenzenes: Intermediates, products and an unusual nitro group shift. Org. Biomol. Chem. 2016, 14, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Boga, C.; Micheletti, G.; Cino, S.; Fazzini, S.; Forlani, L.; Zanna, N.; Spinelli, D. C–C coupling between trinitrothiophenes and triaminobenzenes: Zwitterionic intermediates and new all-conjugated structures. Org. Biomol. Chem. 2016, 14, 4267–4275. [Google Scholar] [CrossRef] [PubMed]
- Forlani, L.; Boga, C.; Mazzanti, A.; Zanna, N. Trapping and analysing Wheland-Meisenheimer σ complexes, usually labile and escaping intermediates. Eur. J. Org. Chem. 2012, 6, 1123–1129. [Google Scholar] [CrossRef]
- Boga, C.; Cino, S.; Micheletti, G.; Padovan, D.; Prati, L.; Mazzanti, A.; Zanna, N. New azo-decorated N-pyrrolidinylthiazoles: Synthesis, properties and an unexpected remote substituent effect transmission. Org. Biomol. Chem. 2016, 14, 7061–7068. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, G.; Iannuzzo, L.; Calvaresi, M.; Bordoni, S.; Telese, D.; Chugunova, E.; Boga, C. Intriguing Enigma of Nitrobenzofuroxan’s ‘Sphinx’: Boulton–Katritzky rearrangement or unusual evidence of the N-1/N-3-oxide rearrangement? RSC Adv. 2020, 10, 34670–34680. [Google Scholar] [CrossRef]
- Boga, C.; Del Vecchio, E.; Forlani, L.; Goumont, R.; Terrier, F.; Tozzi, S. Evidence for the intermediacy of Wheland-Meisenheimer complexes in SEAr reactions of aminothiazoles with 4,6-dinitrobenzofuroxan. Chem. Eur. J. 2007, 13, 9600–9607. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, H.; Porcal, W. Pharmacological properties of furoxans and benzofuroxans: Recent developments. Mini Rev. Med. Chem. 2005, 5, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Medana, C.; Di Stilo, A.; Visentin, S.; Fruttero, R.; Gasco, A.; Ghigo, D.; Bosia, A. NO donor and biological properties of different benzofuroxans. Pharm. Res. 1999, 16, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Chugunova, E.; Boga, C.; Sazykin, I.; Cino, S.; Micheletti, G.; Mazzanti, A.; Sazykina, M.; Burilov, A.; Khmelevstova, L.; Kostina, N. Synthesis and antimicrobial activity of novel structural hybrids of benzofuroxan and benzothiazole derivatives. Eur. J. Med. Chem. 2015, 93, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.; Varela, M.; Canclini, L.; Acosta, S.; Martínez-López, W.; López, G.V.; Hernández, P. Furoxans and tocopherol analogs–furoxan hybrids as anticancer agents. Anti-Cancer Drugs 2019, 30, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Prabhuling, S.; Tamboli, Y.; Choudhari, P.B.; Bhatia, M.S.; Kumar Mohanta, T.; Al-Harrasi, A.; Pudukulathan, Z.K. Synthesis and Modeling Studies of Furoxan Coupled Spiro-Isoquinolino Piperidine Derivatives as NO Releasing PDE 5 Inhibitors. Biomedicines 2020, 8, 121. [Google Scholar]
- Al-Sehemi, A.G.; Pannipara, M.; Parulekar, R.S.; Patil, O.; Choudhari, P.B.; Bhatia, M.S.; Zubaidha, P.K.; Tamboli, Y. Potential of NO donor furoxan as SARS-CoV-2 main protease (Mpro) inhibitors: In silico analysis. J. Biomol. Struct. Dyn 2020, 1–15. [Google Scholar] [CrossRef]
- Norris, W.P.; Chafin, A. Synthesis and thermal rearrangement of 5-chloro-4,6-dinitrobenzofuroxan. Heterocycles 1984, 22, 271–274. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micheletti, G.; Telese, D.; Boga, C. 4,6-Dinitro-7-(thiazol-2-ylamino)benzo[c][1,2,5]oxadiazole 1-oxide. Molbank 2020, 2020, M1165. https://doi.org/10.3390/M1165
Micheletti G, Telese D, Boga C. 4,6-Dinitro-7-(thiazol-2-ylamino)benzo[c][1,2,5]oxadiazole 1-oxide. Molbank. 2020; 2020(4):M1165. https://doi.org/10.3390/M1165
Chicago/Turabian StyleMicheletti, Gabriele, Dario Telese, and Carla Boga. 2020. "4,6-Dinitro-7-(thiazol-2-ylamino)benzo[c][1,2,5]oxadiazole 1-oxide" Molbank 2020, no. 4: M1165. https://doi.org/10.3390/M1165
APA StyleMicheletti, G., Telese, D., & Boga, C. (2020). 4,6-Dinitro-7-(thiazol-2-ylamino)benzo[c][1,2,5]oxadiazole 1-oxide. Molbank, 2020(4), M1165. https://doi.org/10.3390/M1165





