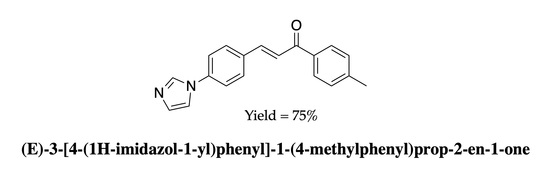
(E)-3-[4-(1H-Imidazol-1-yl)phenyl]-1-(4-methylphenyl)prop-2-en-1-one
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dagenais, T.R.T.; Keller, N.P. Pathogenesis of Aspergillus fumigatus in Invasive Aspergillosis. Clin. Microbiol. Rev. 2009, 22, 447–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazaz, R.; Dening, D.W. Aspergillosis: Cause, types and treatment. Pharm. J. 2019, 303, 7927. [Google Scholar]
- Latgé, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Tischler, B.Y.; Hohl, T.M. Menacing Mold: Recent Advances in Aspergillus Pathogenesis and Host Defense. J. Mol. Biol. 2019, 431, 4229–4246. [Google Scholar] [CrossRef] [PubMed]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Vuong, M.F.; Waymack, J.R. Aspergillosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Rudramurthy, S.M.; Paul, R.A.; Chakrabarti, A.; Mouton, J.W.; Meis, J.F. Invasive Aspergillosis by Aspergillus flavus: Epidemiology, Diagnosis, Antifungal Resistance, and Management. J. Fungi 2019, 5, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, M.; Messina, F.; Marin, E.; Arechavala, A.; Depardo, R.; Walker, L.; Negroni, R.; Santiso, G. Antifungal Resistance in Clinical Isolates of Aspergillus spp.: When Local Epidemiology Breaks the Norm. J. Fungi 2019, 5, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldeck, F.; Boroli, F.; Suh, N.; Garcia, P.D.W.; Flury, D.; Notter, J.; Iten, A.; Kaiser, L.; Schrenzel, J.; Boggian, K.; et al. Influenza-Associated Aspergillosis in Critically-Ill Patients a Retrospective Bicentric Cohort Study. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Platt, A.; Tornheim, J.A.; Zhang, S.X.; Datta, K.; Cardozo, C.; Garcia-Vidal, C. Aspergillosis Complicating Sever Coronavirus Disease. Emerg. Infect. Dis. 2021, 27, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. Pulmonary Fungal Infections Affect Patients With COVID-19. JAMA 2020, 324, 2248. [Google Scholar] [PubMed]
- Steenwyk, J.L.; Mead, M.E.; Castro, P.A.; Valero, C.; Damasio, A.; Santos, R.A.C.; Labella, A.L.; Li, Y.; Knowles, S.L.; Raja, H.A.; et al. Genomic and Phenotypic Analysis of COVID-19-Associated Pulmonary Aspergillosis isolates of Aspergillus fumigatus. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Zheng, X.; Ma, Z.; Zhang, D. Synthesis of Imidazole-Based Medicinal Molecules Utilizing the van Leusen Imidazole Synthesis. Pharmaceuticals 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, N.; Atanes, A.; Ashburn, B.O. (E)-3-(4-chlorophenyl)-1-(2-fluoro-4-methoxyphenyl)-2-propen-1-one. Molbank 2021, 2021, M1184. [Google Scholar] [CrossRef]
- Amato-Ocampo, J.; Carrillo, R.; Kae, H.; Ashburn, B.O. Synthesis and Antimicrobial Evaluation of a Series of Chlorinated Chalcone Derivatives. IJPPR Human 2018, 13, 112–119. [Google Scholar]
- Hussain, T.; Zia-ur-Rehman, M.; Zaheer, M.; Ashraf, M.; Bolte, M. 1-[4-(1H-Imidazol-1-yl)phenyl]-3-phenylprop-2-en-1-ones—A potential pharmacophore bearing anti-leishmanial activity. J. Chem. Res. 2016, 40, 199–204. [Google Scholar] [CrossRef]
- Hussain, T.; Siddiqui, H.L.; Zia-ur-Rehman, M.; Yasinzai, M.M.; Parvez, M. Anti-oxidant, anti-fungal and nti-leishmanial activities of novel 3-[4-(1H-imidazol-1-yl) phenyl]prop-2-en-1-ones. Eur. J. Med. Chem. 2009, 40, 4654–4660. [Google Scholar] [CrossRef] [PubMed]
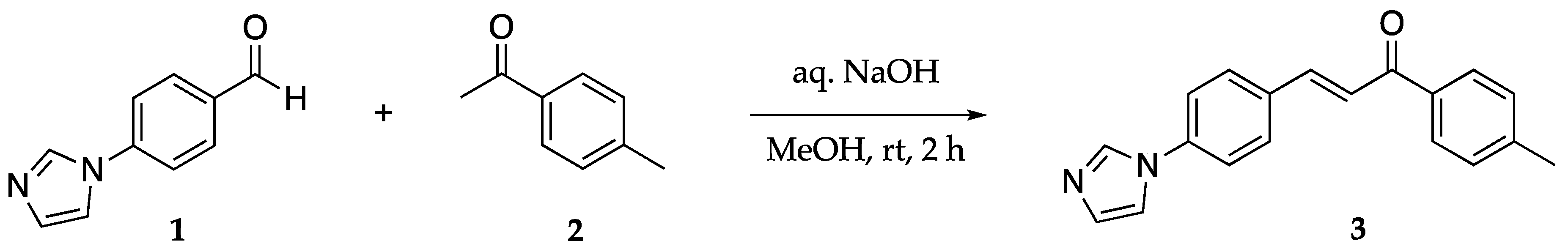

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailey, N.; Ashburn, B.O. (E)-3-[4-(1H-Imidazol-1-yl)phenyl]-1-(4-methylphenyl)prop-2-en-1-one. Molbank 2021, 2021, M1269. https://doi.org/10.3390/M1269
Bailey N, Ashburn BO. (E)-3-[4-(1H-Imidazol-1-yl)phenyl]-1-(4-methylphenyl)prop-2-en-1-one. Molbank. 2021; 2021(3):M1269. https://doi.org/10.3390/M1269
Chicago/Turabian StyleBailey, Nicholas, and Bradley O. Ashburn. 2021. "(E)-3-[4-(1H-Imidazol-1-yl)phenyl]-1-(4-methylphenyl)prop-2-en-1-one" Molbank 2021, no. 3: M1269. https://doi.org/10.3390/M1269