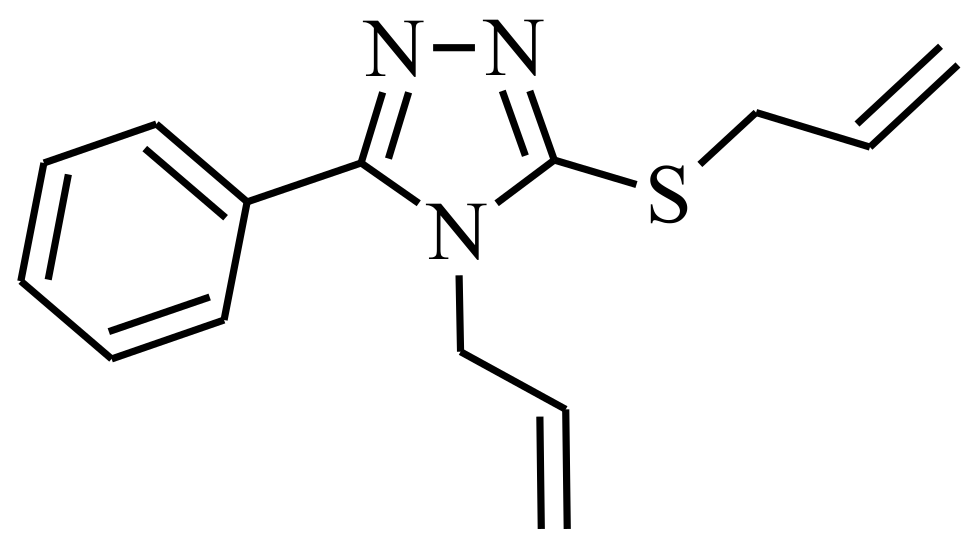
3-Phenyl-4-(prop-2-en-1-yl)-5-[(prop-2-en-1-yl)sulfanyl]-4H-1,2,4-triazole
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Synthesis of Atr
3.3. Single Crystal X-ray Diffraction Studies
3.4. Computational Study
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ji Ram, V.; Sethi, A.; Nath, M.; Pratap, R. Five-membered heterocycles. In The Chemistry of Heterocycles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–478. [Google Scholar]
- Abdelli, A.; Azzouni, S.; Plais, R.; Gaucher, A.; Efrit, M.L.; Prim, D. Recent advances in the chemistry of 1,2,4-triazoles: Synthesis, reactivity and biological activities. Tetrahedron Lett. 2021, 86, 153518. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Ranjan Dwivedi, A.; Kumar, B.; Kumar, V. Recent developments on 1,2,4-triazole nucleus in anticancer compounds: A Review. Anticancer Agents Med. Chem. 2016, 16, 465–489. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Hooda, M.; Kumar, P.; Sumran, G. Vision on synthetic and medicinal facets of 1,2,4-triazolo[3,4-b][1,3,4]thiadiazine scaffold. Top. Curr. Chem. 2022, 380, 10. [Google Scholar] [CrossRef]
- Guo, H.-Y.; Chen, Z.-A.; Shen, Q.-K.; Quan, Z.-S. Application of triazoles in the structural modification of natural products. J. Enzym. Inhib. Med. Chem. 2021, 36, 1115–1144. [Google Scholar] [CrossRef]
- Tratrat, C. 1,2,4-Triazole: A privileged scaffold for the development of potent antifungal agents—A brief review. Curr. Top. Med. Chem. 2020, 20, 2235–2258. [Google Scholar] [CrossRef]
- Gupta, D.; Jain, D. Synthesis, antifungal and antibacterial activity of novel 1,2,4-triazole derivatives. J. Adv. Pharm. Technol. Res. 2015, 6, 141. [Google Scholar] [CrossRef]
- Russell, P.E. A century of fungicide evolution. J. Agric. Sci. 2005, 143, 11–25. [Google Scholar] [CrossRef]
- Aromí, G.; Barrios, L.A.; Roubeau, O.; Gamez, P. Triazoles and tetrazoles: Prime ligands to generate remarkable coordination materials. Coord. Chem. Rev. 2011, 255, 485–546. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal azolate frameworks: From crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef]
- Roubeau, O. Triazole-based one-dimensional spin-crossover coordination polymers. Chem.-A Eur. J. 2012, 18, 15230–15244. [Google Scholar] [CrossRef]
- Yang, L.; Shen, Y.; Chen, Y.; Pan, X.; Wang, X.; Wang, X. A Novel octamolybdate-based metal-organic complex constructed from a bis(tetrazole)-functionalized thioether ligand and an anderson-type polyoxometalate. Inorg. Chem. Commun. 2019, 108, 107493. [Google Scholar] [CrossRef]
- Vinogradova, K.A.; Pishchur, D.P.; Komarov, V.Y.; Lavrenova, L.G.; Bushuev, M.B. Cooperative spin transition in a 1D-polymeric complex [Fe(4-Ethyl-1,2,4-Triazole)3]SiF6·nH2O. Inorg. Chim. Acta 2020, 506, 119560. [Google Scholar] [CrossRef]
- Li, J.; Ren, G.-Y.; Zhang, Y.; Yang, M.-Y.; Ma, H.-X. Two Cu(II) complexes of 1,2,4-triazole fungicides with enhanced antifungal activities. Polyhedron 2019, 157, 163–169. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Nguyen, H.H.; Do, H.H. 1,2,4-Triazole-derived N-heterocyclic carbene complexes of platinum(II) as catalysts for hydroamination reactions and active anticancer agents. Inorg. Chem. Commun. 2020, 121, 108173. [Google Scholar] [CrossRef]
- Ouellette, W.; Jones, S.; Zubieta, J. Solid state coordination chemistry of metal-1,2,4-triazolates and the related metal-4-pyridyltetrazolates. CrystEngComm 2011, 13, 4457. [Google Scholar] [CrossRef]
- Hordiichuk, O.R.; Slyvka, Y.I.; Kinzhybalo, V.V.; Goreshnik, E.A.; Bednarchuk, T.J.; Bednarchuk, O.; Jedryka, J.; Kityk, I.; Mys’kiv, M.G. Construction of heterometallic and mixed-valence copper(I/II) chloride π-complexes with 1,2,4-triazole allyl-derivative. Inorg. Chim. Acta 2019, 495, 119012. [Google Scholar] [CrossRef]
- Hordiichuk, O.R.; Kinzhybalo, V.V.; Goreshnik, E.A.; Slyvka, Y.I.; Krawczyk, M.S.; Mys’kiv, M.G. Influence of apical ligands on Cu–(C=C) interaction in copper(I) halides (Cl−, Br−, I−) π-complexes with an 1,2,4-triazole allyl-derivative: Syntheses, crystal structures and NMR spectroscopy. J. Organomet. Chem. 2017, 838. [Google Scholar] [CrossRef]
- Slyvka, Y.; Goreshnik, E.; Veryasov, G.; Morozov, D.; Fedorchuk, A.A.; Pokhodylo, N.; Kityk, I.; Mys’kiv, M. The novel copper(I) π,σ-complexes with 1-(aryl)-5-(allylthio)-1H-tetrazoles: Synthesis, structure characterization, DFT-calculation and third-order nonlinear optics. J. Coord. Chem. 2019, 72, 1049–1063. [Google Scholar] [CrossRef]
- Pavlyuk, O.V.; Slyvka, Y.I.; Goreshnik, E.A.; Mys’kiv, M.G. 6-Amino-3-(prop-2-en-1-yl)-9H-purin-3-ium tetracopper(I) hexabromide: Synthesis and X-ray structure determination. Molbank 2022, 2022, M1401. [Google Scholar] [CrossRef]
- Slyvka, Y.; Kinzhybalo, V.; Shyyka, O.; Mys’kiv, M. Synthesis, structure and computational study of 5-[(prop-2-en-1-yl)sulfanyl]-1,3,4-thiadiazol-2-amine (Pesta) and its heterometallic π,σ-complex [Cu2FeCl2(Pesta)4][FeCl4]. Acta Crystallogr. Sect. C Struct. Chem. 2021, 77, 249–256. [Google Scholar] [CrossRef]
- Slyvka, Y.; Goreshnik, E.; Veryasov, G.; Morozov, D.; Luk’yanov, M.; Mys’kiv, M. The first copper(I)-olefin complexes bearing a 1,3,4-oxadiazole core: Alternating-current electrochemical crystallization, X-ray experiment and DFT study. Polyhedron 2017, 133, 319–326. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. Rigaku CrysAlisPro Software System, Version 1.171.41.104a. 2021. Available online: http://www.rigaku.com (accessed on 1 September 2021).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]


| Bond | d, Å | Angle | ω, ° |
|---|---|---|---|
| C1—N2 | 1.310(3) | N1—C3—C4 | 114.9(2) |
| N2—N3 | 1.401(3) | C3—C4—C5 | 125.7(2) |
| C1—N1 | 1.370(3) | C1—S1—C6A | 104.0(2) |
| C4—C5 | 1.304(3) | S1—C6A—C7A | 110.6(4) |
| C7A—C8A | 1.339(11) | C6A—C7A—C8A | 122.0(9) |
| S1—C1 | 1.744(2) | C6B—C7B—C8B | 122.6(14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slyvka, Y.I.; Goreshnik, E.A.; Fedko, A.M.; Mys’kiv, M.G. 3-Phenyl-4-(prop-2-en-1-yl)-5-[(prop-2-en-1-yl)sulfanyl]-4H-1,2,4-triazole. Molbank 2022, 2022, M1405. https://doi.org/10.3390/M1405
Slyvka YI, Goreshnik EA, Fedko AM, Mys’kiv MG. 3-Phenyl-4-(prop-2-en-1-yl)-5-[(prop-2-en-1-yl)sulfanyl]-4H-1,2,4-triazole. Molbank. 2022; 2022(3):M1405. https://doi.org/10.3390/M1405
Chicago/Turabian StyleSlyvka, Yurii I., Evgeny A. Goreshnik, Andrii M. Fedko, and Marian G. Mys’kiv. 2022. "3-Phenyl-4-(prop-2-en-1-yl)-5-[(prop-2-en-1-yl)sulfanyl]-4H-1,2,4-triazole" Molbank 2022, no. 3: M1405. https://doi.org/10.3390/M1405
APA StyleSlyvka, Y. I., Goreshnik, E. A., Fedko, A. M., & Mys’kiv, M. G. (2022). 3-Phenyl-4-(prop-2-en-1-yl)-5-[(prop-2-en-1-yl)sulfanyl]-4H-1,2,4-triazole. Molbank, 2022(3), M1405. https://doi.org/10.3390/M1405






