Abstract
Over the past decades, studies of cyclic diacyl peroxides have shown superior or even fundamentally new reactivity compared to their acyclic counterparts in various reactions. Previously, the scope of cyclic diacyl peroxides was limited to the mono peroxy compounds. The first doubled cyclic diacyl peroxide is presented herein. The diperoxide was characterized by NMR spectroscopy, mass spectrometry, and IR spectroscopy. The structure of 4,4′-(butane-1,4-diyl)bis(4-methyl-1,2-dioxolane-3,5-dione) was confirmed by X-ray diffraction analysis. The novel diperoxide was prepared in a 55% overall yield in three steps from dibromobutane and diethyl methylmalonate.
1. Introduction
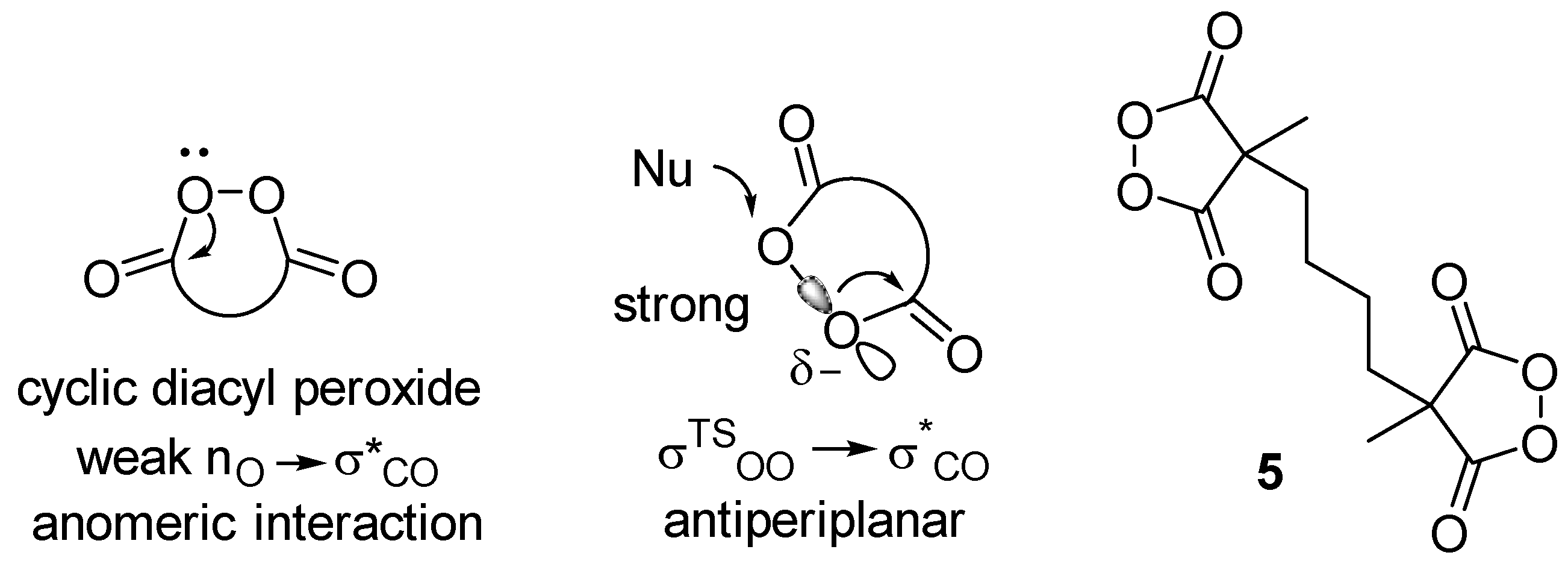
Cyclic diacyl peroxides are compounds with a five- to six-membered rings containing a C(O)-O-O-C(O) moiety. The chemistry of cyclic diacyl peroxides, first synthesized in the 1950s [1,2], has been actively studied in the last decade [3,4]. Due to their O-electrophilic nature, cyclic diacyl peroxides provide an umpolung approach for the introduction of OR- moiety into organic molecules as an electrophilic “+OR” synthon instead of the usual nucleophilic “-OR” species. The methods for the dihydroxylation, deoxygenation [5,6,7,8,9,10,11], and oxyamination [12] of alkenes were developed. Using cyclic diacyl peroxides, the oxyfunctionalization of arenes [13,14,15,16,17,18,19], as well as arene dearomatization [20], were achieved. The oxidative acyloxylation of dicarbonyl compounds [21,22,23], heterocycles [24], and the derivatives of monocarbonyl compounds [25,26,27] were realized. Such rich reactivity was explained mainly by the weak anomeric nO → σ*C=O interactions as compared to those in acyclic diacyl peroxides (Scheme 1). Additionally, the transition state in SN2 processes with cyclic diacyl peroxides has a more favorable antiperiplanar arrangement of the breaking O-O bond to the σ*CO bond of the carbonyl [28]. In this work, we presented the doubled cyclic diacyl peroxide 5. One can expect the possibility of synthesizing complex structures of oligomeric and macrocyclic nature using the reactivity of two cyclic diacyl peroxide fragments.
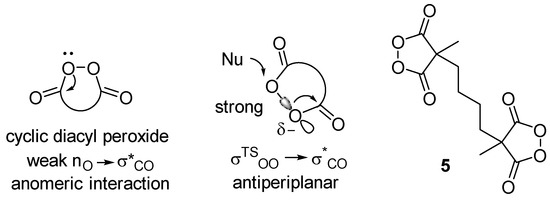
Scheme 1.
Cyclic diacyl peroxides.
2. Results and Discussion
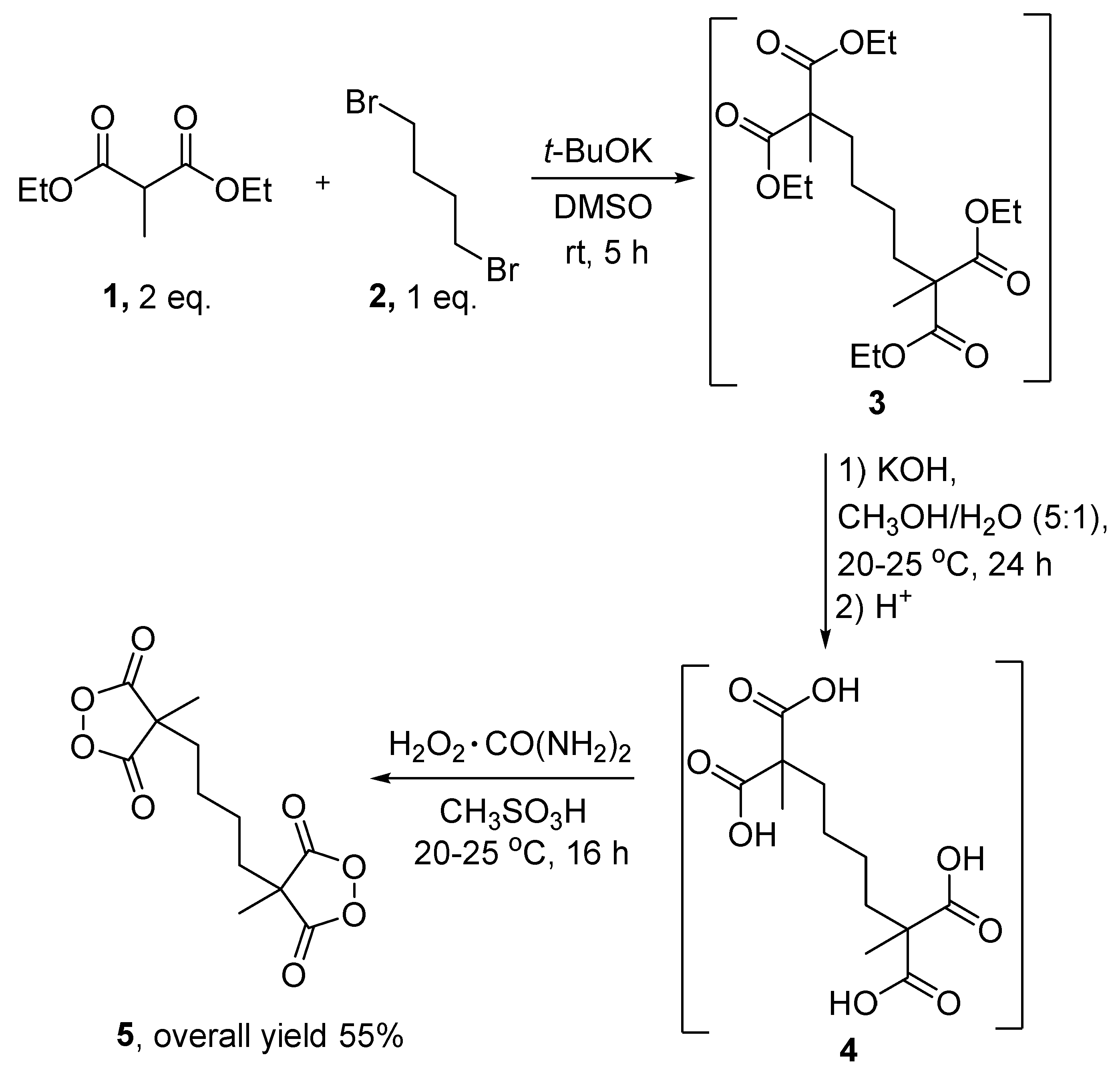
The target peroxide 5 was synthesized in three steps from readily available diethyl methylmalonate 1 and dibromobutane 2 (Scheme 2).
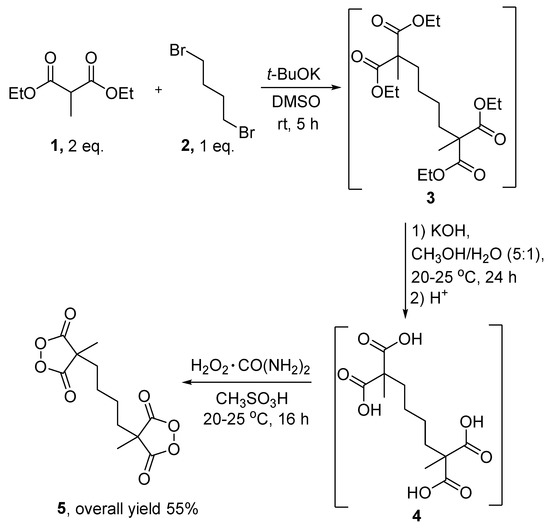
Scheme 2.
Three-step synthesis of the doubled cyclic diacyl peroxide 5.
In the first step, diethyl methylmalonate 1 was alkylated with dibromobutane 2 using potassium tert-butoxide in DMSO to form tetra ester 3. After the workup procedure, the crude tetra ester 3 was obtained in almost quantitative yield and was used in the next step without additional purification (Scheme 2). The tetra ester 3 was transformed into tetra acid 4 via base-catalyzed hydrolysis. A typical experimental procedure for relative carboxylic acids includes the acidification of the aqueous solution of the crude carboxylic acid salt and extraction with an organic solvent [29,30]. Because tetra acid 4 is highly soluble in water, extraction is not recommended for isolating the target substance. The isolation procedure of tetra acid 4 included the evaporation of a major volume of a CH3OH/H2O mixture, acidification with conc. HCl, the dissolving of tetra acid 4 in CH3CN, filtration from inorganic salts, and the evaporation of organic solvents. Tetra acid 4 was used in the next step without additional purification. The peroxidation of tetra acid 4 with urea hydrogen peroxide in methanesulfonic acid led to doubled cyclic diacyl peroxide 5 in a 55% overall yield in three steps [6,31]. Despite the presence of two peroxide groups, compound 5 is quite thermostable (mp = 113–114 °C without decomposition) and can also be stored in a refrigerator for a year without degradation.
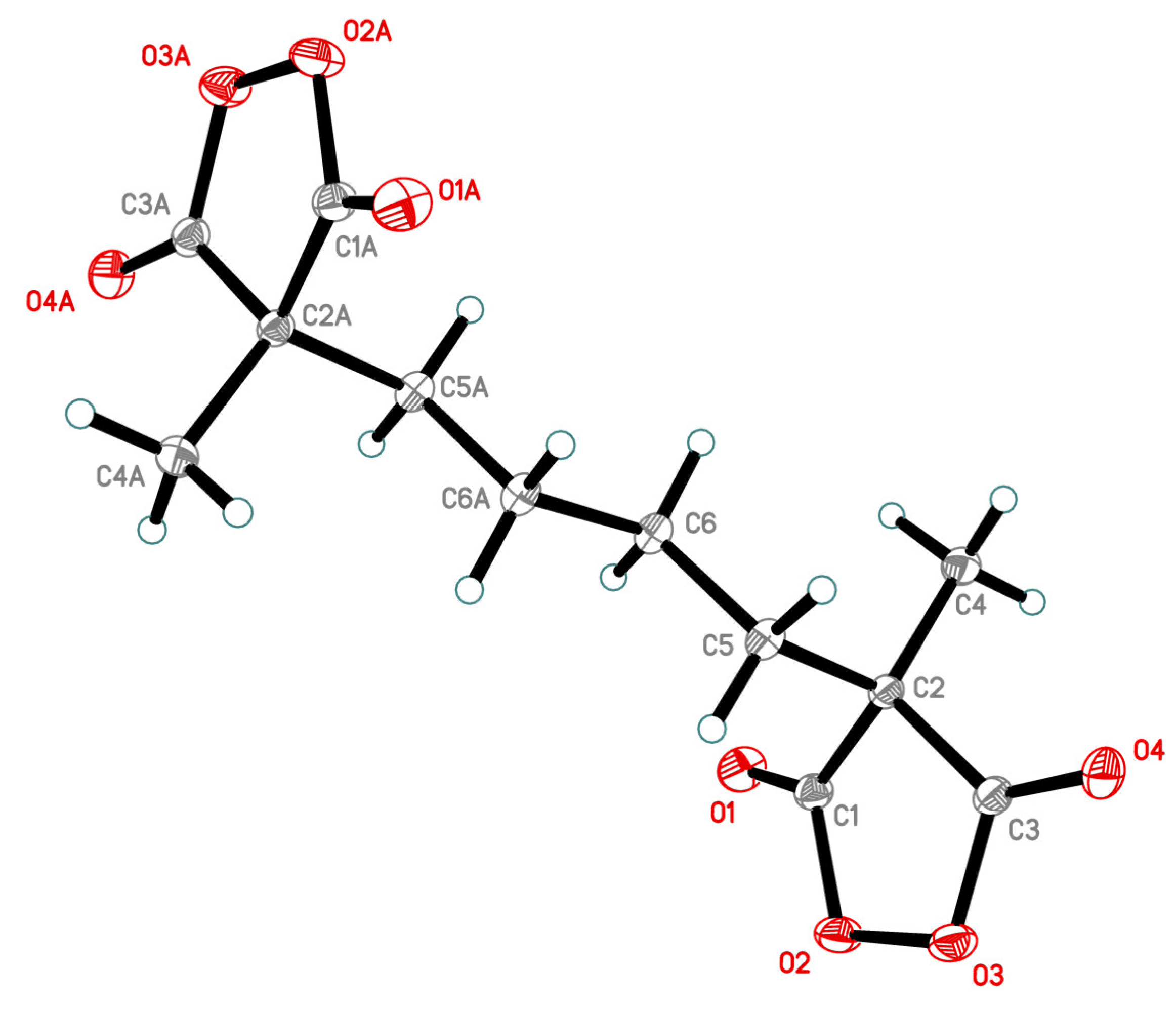
The compound 5 was fully characterized by NMR, IR spectroscopy, and mass spectrometry (Figures S1–S4, Tables S1–S6, Supplementary Materials). The structure of peroxide 5 was confirmed by X-ray diffraction analysis (Figure 1).
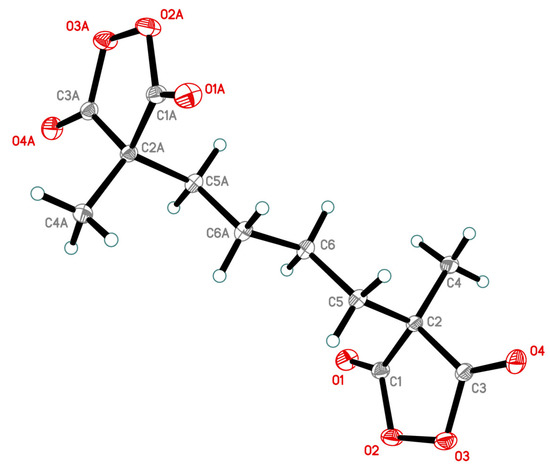
Figure 1.
The structure of compound 5 obtained by X-ray diffraction analysis.
3. Materials and Methods
3.1. General Information
Caution: Peroxides are high energy compounds. All reactions using these substances should be conducted within a fume hood and behind a safety-shield. These procedures should be carried out by knowledgeable laboratory workers.
NMR spectra were recorded on commercial instrument (300.13 MHz for 1H, 75.48 MHz for 13C) in CDCl3. The IR spectrum was recorded with a Bruker (Moscow, Russia) “Alpha-T” instrument in a KBr pellet. High-resolution mass spectrum (HRMS) was measured using electrospray ionization (ESI-TOF) [32]. The measurement was done in a positive ion mode (interface capillary voltage—4500 V); mass range from m/z 50 to m/z 1600 Da; and external/internal calibration was done with Electrospray Calibrant Solution. A syringe injection was used for solutions in CH3CN (flow rate 3 µL/min). Nitrogen was applied as a dry gas; interface temperature was set at 180 °C.
The TLC analysis was carried out on standard silica gel chromatography plates. The melting point was determined on a Kofler hot-stage apparatus.
X-ray diffraction data were collected at 100 K on a Bruker Quest D8 diffractometer equipped with a Photon III area detector (graphite monochromator, shutterless φ- and ω-scan technique), using Mo Ka-radiation. The intensity data were integrated by the SAINT program [33] and corrected for absorption and decay using SADABS [34]. The structure was solved by direct methods using SHELXT [35] and refined on F2 using SHELXL-2018 [36]. All non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms were placed in ideal calculated positions and refined as riding atoms with relative isotropic displacement parameters. The SHELXTL program suite [33] was used for molecular graphics.
3.2. Synthesis of Tetraethyl Octane-2,2,7,7-tetracarboxylate (3)
Potassium tert-butoxide (2.96 g, 26.4 mmol) was added to a solution of diethyl 2-methylmalonate 1 (4.18 g, 24.0 mmol) in 20 mL of DMSO. After 20 min stirring at 20–25 °C, 1,4-dibromobutane 2 (2.59 g, 12.0 mmol) was added. The resulting suspension was stirred for 5 h at 20–25 °C. The mixture was quenched with H2O (50 mL), and the product was extracted with Et2O (3 × 40 mL). Combined organic layers were washed with H2O (2 × 10 mL), dried over MgSO4, and filtered and concentrated under reduced pressure using a rotary evaporator (15–20 mmHg, a water bath temperature ca. 20–25 °C) to yield 3 (4.54 g) as a yellow oil. Crude product 3 was used in the next step without purification.
3.3. Synthesis of Octane-2,2,7,7-tetracarboxylic Acid (4)
KOH (7.41 g, 132.0 mmol) was added to a solution of the crude product 3 (4.54 g) in 60 mL of CH3OH/H2O mixture (5:1, V/V). The resulting solution was stirred for 24 h at 20–25 °C. After that, the reaction mixture was concentrated under reduced pressure using a rotary evaporator (15–20 mmHg, a water bath temperature 60 °C). CH3CN (30 mL) was added to the residue, and the mixture was cooled to 0 °C with an ice–water bath. Then, concentrated hydrochloric acid (10.9 M aqueous solution, 142.0 mmol, 13.0 mL) was added dropwise. The mixture was stirred for 10 min and concentrated under reduced pressure using a rotary evaporator (15–20 mmHg, a water bath temperature 60 °C). CH3CN (30 mL) was added to the residue to dissolve the crude organic product. The insoluble inorganic solids were filtered and washed with acetonitrile (3 × 15 mL). The filtrate was concentrated under reduced pressure using a rotary evaporator (15–20 mmHg, a water bath temperature 40 °C) to yield 4 (3.24 g) as a pale yellow solid. Crude product 4 was used in the next step without purification.
3.4. Synthesis of 4,4′-(Butane-1,4-diyl)bis(4-methyl-1,2-dioxolane-3,5-dione) (5)
Compound 4 (3.24 g) was dissolved in CH3SO3H (25 mL), and the solution was cooled to 0 °C with an ice–water bath. Urea hydrogen peroxide (11.28 g, 112.0 mmol) was added portion wise, and the resulting mixture was stirred for 24 h at 20–25 °C. After that, the reaction mixture was poured onto 30 mL ice. Cold water (100 mL) was added and stirred, and the precipitate was filtered, washed with water (3 × 10 mL), and dried under reduced pressure until no mass change was observed. The product 5 (1.90 g, 6.60 mmol, and 55% overall yield) was obtained as a white powder, mp 113–114 °C.
1H NMR (300.13 MHz, CDCl3): δ 1.95–1.86 (m, 4H), 1.56 (s, 6H), and 1.42–1.31 (m, 4H). 13C{1H} NMR (75.48 MHz, CDCl3): δ 174.1, 43.8, 35.7, 24.1, and 20.5. IR (KBr), ν, cm−1: 2974 (C-H), 1840 (C=O), 1796 (C=O), 1468 (C-H), 1387, 1289, 1210, 1136, 1071, 1047, 923, 910, 857 (O-O), 740, 680, 669, 604, and 576. HRMS (ESI-TOF) m/z [M + Na]+. Calcd for [C12H14O8Na]+: 309.0581. Found: 309.0583.
4. Conclusions
The first doubled cyclic diacyl peroxide was prepared in a 55% overall isolated yield via a three-step synthesis from dibromobutane and diethyl methylmalonate. The synthesis was characterized by simplicity and the availability of the reagents.
Supplementary Materials
Figure S1: 1H NMR spectrum of 4,4′-(butane-1,4-diyl)bis(4-methyl-1,2-dioxolane-3,5-dione) (5); Figure S2: 13C NMR spectrum of 4,4′-(butane-1,4-diyl)bis(4-methyl-1,2-dioxolane-3,5-dione) (5); Figure S3: IR spectrum of 4,4′-(butane-1,4-diyl)bis(4-methyl-1,2-dioxolane-3,5-dione) (5); Figure S4: HRMS spectrum of 4,4′-(butane-1,4-diyl)bis(4-methyl-1,2-dioxolane-3,5-dione) (5); Table S1: crystal data and structure refinement for 4,4′-(butane-1,4-diyl)bis(4-methyl-1,2-dioxolane-3,5-dione) (5); Table S2: atomic coordinates (×104) and equivalent isotropic displacement parameters (Å2 × 103) for compound (5). U(eq) is defined as one-third of the trace of the orthogonalized Uij tensor; Table S3: bond lengths [Å] and angles [°] for compound (5); Table S4: anisotropic displacement parameters (Å2 × 103) for compound (5). The anisotropic displacement factor exponent takes the form: −2π2[h2 a* 2U11 + … + 2 h k a* b* U12]; Table S5: Hydrogen coordinates (×104) and isotropic displacement parameters (Å2 × 103) for compound (5); Table S6: torsion angles [°] for compound (5).
Author Contributions
E.S.G. designed the experiments, prepared the compound, and analyzed the data; V.A.V. supervised the progress of work and wrote the manuscript; A.O.T. designed the study and reviewed and edited the final manuscript to publish. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation (grant no. 21-13-00205).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Crystal structure determination was performed in the Department of Structural Studies of Zelinsky Institute of Organic Chemistry, Moscow.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Greene, F.D. Cyclic diacyl peroxides. I. Monomeric phthaloyl peroxide1. J. Am. Chem. Soc. 1956, 78, 2246–2250. [Google Scholar] [CrossRef]
- Adam, W.; Rucktaeschel, R. Cyclic peroxides. V. α-Lactone intermediate via photodecarboxylation of a monomeric malonyl peroxide. J. Am. Chem. Soc. 1971, 93, 557–559. [Google Scholar] [CrossRef]
- Barsegyan, Y.A.; Vil’, V.A. Malonyl peroxides in organic synthesis (microreview). Chem. Heterocycl. Compd. 2019, 55, 1035–1037. [Google Scholar] [CrossRef]
- Zhao, R.; Chang, D.; Shi, L. Recent advances in cyclic diacyl peroxides: Reactivity and selectivity enhancement brought by the cyclic structure. Synthesis 2017, 49, 3357–3365. [Google Scholar] [CrossRef]
- Greene, F.D. Cyclic diacyl peroxides. II. Reaction of phthaloyl peroxide with cis-and trans-stilbene. J. Am. Chem. Soc. 1956, 78, 2250–2254. [Google Scholar] [CrossRef]
- Griffith, J.C.; Jones, K.M.; Picon, S.; Rawling, M.J.; Kariuki, B.M.; Campbell, M.; Tomkinson, N.C. Alkene syn dihydroxylation with malonoyl peroxides. J. Am. Chem. Soc. 2010, 132, 14409–14411. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Axelrod, A.; Varela, M.; Danysh, L.; Siegel, D. Synthesis and reaction of phthaloyl peroxide derivatives, potential organocatalysts for the stereospecific dihydroxylation of alkenes. Tetrahedron Lett. 2011, 52, 2540–2542. [Google Scholar] [CrossRef]
- Tomkinson, N.; Jones, K.M. Metal-free dihydroxylation of alkenes using cyclobutane malonoyl peroxide. J. Org. Chem. 2012, 77, 921–928. [Google Scholar] [CrossRef]
- Rawling, M.J.; Tomkinson, N.C. Metal-free syn-dioxygenation of alkenes. Org. Biomol. Chem. 2013, 11, 1434–1440. [Google Scholar] [CrossRef]
- Alamillo-Ferrer, C.; Davidson, S.C.; Rawling, M.J.; Theodoulou, N.H.; Campbell, M.; Humphreys, P.G.; Kennedy, A.R.; Tomkinson, N.C. Alkene anti-dihydroxylation with malonoyl peroxides. Org. Lett. 2015, 17, 5132–5135. [Google Scholar] [CrossRef]
- Alamillo-Ferrer, C.; Karabourniotis-Sotti, M.; Kennedy, A.R.; Campbell, M.; Tomkinson, N.C. Alkene dioxygenation with malonoyl peroxides: Synthesis of γ-lactones, isobenzofuranones, and tetrahydrofurans. Org. Lett. 2016, 18, 3102–3105. [Google Scholar] [CrossRef] [PubMed]
- Alamillo-Ferrer, C.; Curle, J.M.; Davidson, S.C.; Lucas, S.C.; Atkinson, S.J.; Campbell, M.; Kennedy, A.R.; Tomkinson, N.C. Alkene oxyamination using malonoyl peroxides: Preparation of pyrrolidines and isoxazolidines. J. Org. Chem. 2018, 83, 6728–6740. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Liang, Y.; Hernandez, T.; Berriochoa, A.; Houk, K.N.; Siegel, D. Metal-free oxidation of aromatic carbon-hydrogen bonds through a reverse-rebound mechanism. Nature 2013, 499, 192–196. [Google Scholar] [CrossRef]
- Camelio, A.M.; Liang, Y.; Eliasen, A.M.; Johnson, T.C.; Yuan, C.; Schuppe, A.W.; Houk, K.; Siegel, D. Computational and experimental studies of phthaloyl peroxide-mediated hydroxylation of arenes yield a more reactive derivative, 4,5-dichlorophthaloyl peroxide. J. Org. Chem. 2015, 80, 8084–8095. [Google Scholar] [CrossRef] [PubMed]
- Dragan, A.; Kubczyk, T.M.; Rowley, J.H.; Sproules, S.; Tomkinson, N.C. Arene oxidation with malonoyl peroxides. Org. Lett. 2015, 17, 2618–2621. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-Z.; Li, S.; Zhang, P.-P.; Huang, Z.-H.; Zhang, W.-B.; Gong, J.; Yang, Z. A chiral pool approach for asymmetric syntheses of (−)-antrocin, (+)-asperolide C, and (−)-trans-ozic acid. Chem. Commun. 2016, 52, 12426–12429. [Google Scholar] [CrossRef]
- Pilevar, A.; Hosseini, A.; Šekutor, M.; Hausmann, H.; Becker, J.; Turke, K.; Schreiner, P.R. Tuning the reactivity of peroxo anhydrides for aromatic C-H bond oxidation. J. Org. Chem. 2018, 83, 10070–10079. [Google Scholar] [CrossRef]
- Song, Y.-K.; Liu, L.; Wang, J.-J.; Qian, F.; Yang, M.-Q.; Zhang, L.-Q.; Fu, J.-G.; Li, Y.-M.; Feng, C.-G. An asymmetric synthesis of (+)-scrodentoid A from dehydroabietic acid. Tetrahedron 2021, 85, 132031. [Google Scholar] [CrossRef]
- Tavakoli, A.; Dudley, G.B. Synthesis of coprinol and several alcyopterosin sesquiterpenes by regioselective [2 + 2 + 2] alkyne cyclotrimerization. J. Org. Chem. 2022, 87, 14909–14914. [Google Scholar] [CrossRef]
- Eliasen, A.M.; Christy, M.; Claussen, K.R.; Besandre, R.; Thedford, R.P.; Siegel, D. Dearomatization reactions using phthaloyl peroxide. Org. Lett. 2015, 17, 4420–4423. [Google Scholar] [CrossRef] [PubMed]
- Terent’ev, A.O.; Vil’, V.A.; Nikishin, G.I.; Adam, W. Lanthanide-catalyzed oxidative C-O coupling of 1,3-dicarbonyl compounds with diacyl peroxides. Synlett 2015, 26, 802–806. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Vil’, V.A.; Gorlov, E.S.; Nikishin, G.I.; Pivnitsky, K.K.; Adam, W. Lanthanide-catalyzed oxyfunctionalization of 1,3-diketones, acetoacetic esters, and malonates by oxidative C-O coupling with malonyl peroxides. J. Org. Chem. 2016, 81, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Bityukov, O.V.; Vil’, V.A.; Merkulova, V.M.; Nikishin, G.I.; Terent’ev, A.O. Silica gel mediated oxidative C-O coupling of β-dicarbonyl compounds with malonyl peroxides in solvent-free conditions. Pure Appl. Chem. 2018, 90, 7–20. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Vil’, V.A.; Gorlov, E.S.; Rusina, O.N.; Korlyukov, A.A.; Nikishin, G.I.; Adam, W. Selective oxidative coupling of 3H-pyrazol-3-ones, isoxazol-5(2H)-ones, pyrazolidine-3,5-diones, and barbituric acids with malonyl peroxides: An effective C-O functionalization. ChemistrySelect 2017, 2, 3334–3341. [Google Scholar] [CrossRef]
- Vil’, V.A.; Gorlov, E.S.; Bityukov, O.V.; Barsegyan, Y.A.; Romanova, Y.E.; Merkulova, V.M.; Terent’ev, A.O. C-O coupling of malonyl peroxides with enol ethers via [5 + 2] cycloaddition: Non-rubottom oxidation. Adv. Synth. Catal. 2019, 361, 3173–3181. [Google Scholar] [CrossRef]
- Vil’, V.A.; Gorlov, E.S.; Yu, B.; Terent’ev, A.O. Oxidative α-acyloxylation of acetals with cyclic diacyl peroxides. Org. Chem. Front. 2021, 8, 3091–3101. [Google Scholar] [CrossRef]
- Vil’, V.A.; Gorlov, E.S.; Shuingalieva, D.V.; Kunitsyn, A.Y.; Krivoshchapov, N.V.; Medvedev, M.G.; Alabugin, I.V.; Terent’ev, A.O. Activation of O-electrophiles via structural and solvent effects: SN2@O reaction of cyclic diacyl peroxides with enol acetates. J. Org. Chem. 2022, 87, 13980–13989. [Google Scholar] [CrossRef]
- Alabugin, I.V.; Kuhn, L.; Medvedev, M.G.; Krivoshchapov, N.V.; Vil’, V.A.; Yaremenko, I.A.; Mehaffy, P.; Yarie, M.; Terent’ev, A.O.; Zolfigol, M.A. Stereoelectronic power of oxygen in control of chemical reactivity: The anomeric effect is not alone. Chem. Soc. Rev. 2021, 50, 10253–10345. [Google Scholar] [CrossRef]
- Singh, R.K.; Danishefsky, S. Preparation of activated cyclopropanes by phase transfer alkylation. J. Org. Chem. 1975, 40, 2969–2970. [Google Scholar] [CrossRef]
- Weiner, N. Malonic acid. Org. Synth. 1938, 18, 50. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Vil’, V.A.; Mulina, O.M.; Pivnitsky, K.K.; Nikishin, G.I. A convenient synthesis of cyclopropane malonyl peroxide. Mendeleev Commun. 2014, 24, 345. [Google Scholar] [CrossRef]
- Tsedilin, A.M.; Fakhrutdinov, A.N.; Eremin, D.B.; Zalesskiy, S.S.; Chizhov, A.O.; Kolotyrkina, N.G.; Ananikov, V.P. How sensitive and accurate are routine NMR and MS measurements? Mendeleev Commun. 2015, 25, 454–456. [Google Scholar] [CrossRef]
- Bruker AXS Inc. Bruker. APEX-III; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Krause, L.S.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).