Synthesis and Inhibition of Influenza H1N1 Virus by Propargylaminoalkyl Derivative of Lithocholic Acid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
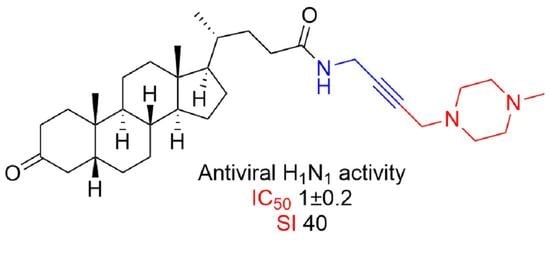
2.2. Biological Assay
3. Materials and Methods
3.1. N-(Prop-2-yn-1-yl)-5β-Cholan-24-Amide (2)
3.2. N-(4-(4-Methylpiperazin-1-yl)but-2-yn-1-yl)-5β-Cholan-24-Amide (3)
3.3. Biological Activity
3.3.1. Cytotoxicity Assay
3.3.2. CPE Reduction Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Global Influenza Strategy 2019–2030; World Health Organization: Geneva, Switzerland, 2019. Available online: https://apps.who.int/iris/handle/10665/311184 (accessed on 20 March 2019).
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, T. Baloxavir Marboxil: The First Cap-dependent Endonuclease inhibitor for the treatment of influenza. Ann. Pharmacother. 2019, 53, 754–759. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin. Infect. Dis. 2019, 68, 895–902. [Google Scholar] [CrossRef]
- Samson, M.; Pizzorno, A.; Abed, Y.; Boivin, G. Influenza virus resistance to neuraminidase inhibitors. Antivir. Res. 2013, 98, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Govorkova, E.A.; Takashita, E.; Daniels, R.S.; Fujisaki, S.; Presser, L.D.; Patel, M.C.; Huang, W.; Lackenby, A.; Nguyen, H.T.; Pereyaslov, D.; et al. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2018–2020. Antivir. Res. 2022, 200, 105281. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Bowman, C.S.; Rost, G.; Wu, J. Population-wide emergence of antiviral resistance during pandemic influenza. PLoS ONE 2008, 3, 1839. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Suryan, A. A comprehensive review on steroidal bioconjugates as promising leads in drug discovery. ACS Bio Med Chem Au 2022, 2, 340–369. [Google Scholar] [CrossRef]
- Niemeyer, C.M. Bioconjugation Protocols: Strategies and Methods; Springer Science, Business Media: Secaucus, NJ, USA, 2004; Volume 283, pp. 5–100. [Google Scholar]
- Singh, Y.; Spinelli, N.; Defrancq, E.; Dumy, P. A novel heterobifunctional linker for facile access to bioconjugates. Org. Biomol. Chem. 2006, 4, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Salunke, D.B.; Hazra, B.G.; Pore, V.S. Steroidal conjugates and their pharmacological applications. Curr. Med. Chem. 2006, 13, 813–847. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Schmidt, A.; Freedman, L.P. Steroid hormone receptors and drug discovery: Therapeutic opportunities and assay designs. Assay Drug Dev. Technol. 2003, 1, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Mao, Q.; Liu, M.; Wang, K.; Wu, Z.; Fang, W.; Yang, Z.; Luo, P.; Ke, S.; Shi, L. The inhibitory effect of dehydroepiandrosterone and its derivatives against influenza A virus in vitro and in vivo. Arch. Virol. 2016, 161, 3061–3072. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Sarker, S.D.; Turner, A.B. A facile synthesis of ethyl (3-oxo-5β-cholan)-24-yl oxalate. Acta Chim. Slov. 2006, 53, 512–516. [Google Scholar]

| Compound | CC50, μM a | IC50, μM b | SI c |
|---|---|---|---|
| 2 | 24 ± 1 | 9 ± 2 | 3 |
| 3 | 46 ± 3 | 1 ± 0.2 | 40 |
| Rimantadine | 344 ± 19 | 61 ± 8 | >6 |
| Oseltamivir | >200 | 0.3 ± 0.01 | >667 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, A.V.; Smirnova, I.E.; Fedij, S.V.; Pavlyukova, Y.N.; Zarubaev, V.V.; Tran Thi Phuong, T.; Myint Myint, K.; Kazakova, O.B. Synthesis and Inhibition of Influenza H1N1 Virus by Propargylaminoalkyl Derivative of Lithocholic Acid. Molbank 2023, 2023, M1626. https://doi.org/10.3390/M1626
Petrova AV, Smirnova IE, Fedij SV, Pavlyukova YN, Zarubaev VV, Tran Thi Phuong T, Myint Myint K, Kazakova OB. Synthesis and Inhibition of Influenza H1N1 Virus by Propargylaminoalkyl Derivative of Lithocholic Acid. Molbank. 2023; 2023(2):M1626. https://doi.org/10.3390/M1626
Chicago/Turabian StylePetrova, Anastasiya V., Irina E. Smirnova, Sergey V. Fedij, Yulia N. Pavlyukova, Vladimir V. Zarubaev, Thao Tran Thi Phuong, Khine Myint Myint, and Oxana B. Kazakova. 2023. "Synthesis and Inhibition of Influenza H1N1 Virus by Propargylaminoalkyl Derivative of Lithocholic Acid" Molbank 2023, no. 2: M1626. https://doi.org/10.3390/M1626
APA StylePetrova, A. V., Smirnova, I. E., Fedij, S. V., Pavlyukova, Y. N., Zarubaev, V. V., Tran Thi Phuong, T., Myint Myint, K., & Kazakova, O. B. (2023). Synthesis and Inhibition of Influenza H1N1 Virus by Propargylaminoalkyl Derivative of Lithocholic Acid. Molbank, 2023(2), M1626. https://doi.org/10.3390/M1626