Abstract
In the current study, the conjugate of 3-oxo-lithocholic acid with N-methylpiperazine and paraform was synthesized using the Mannich reaction and evaluated for antiviral activity. This modification resulted in a dramatic increase of antiviral activity combined with a two-fold decrease of toxicity. Together, these effects led to a strong increase of selectivity of compound (SI = 40 vs. 3 for 3 and 2, correspondingly).
1. Introduction
Seasonal influenza is the most common human infectious disease, resulting in approximately 3–5 million cases of severe acute lower respiratory tract infection and about 250,000 to 500,000 deaths worldwide annually, thus posing a serious public health impact [1,2]. The most effective strategy for controlling influenza is the use of vaccines. Due to insufficient coverage of population of vaccination and significant rate of people with contraindications to vaccination, chemotherapy is also an important method for the prevention and treatment of viral diseases. The current anti-influenza remedies are presented by drugs from three classes: (i) amantadine and rimantadine, which are M2-ion channel blockers; (ii) oseltamivir, zanamivir, laninamivir, and peramivir targeting influenza neuraminidase; and (iii) baloxavir marboxil cap-dependent endonuclease (CEN) inhibitor [3]. However, due to globally spread viral amantadine resistance, the adamantanes are no longer recommended for clinical use [4]. Moreover, the influenza virus can also develop resistance to any type of drugs, including neuraminidase inhibitors [5]. Despite the short presence of baloxavir marboxil on the market, the isolation of resistant strains from patients has already been detected [6]. The drug resistance can be prevented by using a combination of two or more agents with different targets and mechanisms of action [7]. Hence, it is necessary to search for novel anti-influenza agents belonging to new structural types, including those whose targets and mechanisms of action differ from the currently used ones.
The steroid system has been extensively used as a privileged scaffold gifted with significantly diversified medicinal properties in the drug discovery and development process. Steroidal molecules are preferred for their rigidness and good ability to penetrate biological membranes. A slight alteration in the basic ring structure results in the formation of steroidal derivatives with a wide range of therapeutic activities. Steroids are not only active as such; conjugating them with various biologically active moieties results in increased lipophilicity, stability, and target specificity with decreased adverse effects [8]. In the process, steroidal bioconjugates have emerged as potential molecules with improved pharmacological and pharmaceutical properties [9,10,11]. Being important biomolecules, steroids have always played a vital role in the area of drug discovery. They are among the preferred molecules because of their fascinating rigid framework and amazing pattern of pharmacological properties [12]. They have an excellent ability to penetrate the biological cell membrane and bind to the specific receptor due to their favorable lipid solubility [12]. The antiviral properties of steroidal bioconjugates have also been explored and reported. Thus, Qingyu Yang et al. [13] developed some dehydroepiandrosterone-based bioconjugates having potent efficacy against DNA and RNA viruses. Among the several synthesized compounds, DHEA-conjugate with 2-OH-Ph moiety exhibited the best inhibitory effects against H1N1 and H3N2 influenza virus type A (IAV) in a dose-dependent manner. Treatment with conjugate 110 decreased progeny virus yield, viral RNA synthesis, and protein expression. The conjugate on further evaluation for cytotoxicity against MDCK cells using MTT assay exhibited CC50 111 ± 7.1 μg/mL.
2. Results and Discussion
2.1. Chemistry
The compounds were synthesized, as outlined in Scheme 1. As starting materials, we used 3-oxo-lithocholic acid 1 [14]. Through the reaction of 1 with propargylamine in CH2Cl2 via the intermediate acyl chloride, the corresponding propargylamide 2 with a yield of 89% was obtained. Then compound 2 was conjugated with N-methylpiperazine and paraform using the Mannich reaction in the presence of NaOAc and CuI in 1,4-dioxane. As a result, we obtained the propargylaminoalkyl derivatives 3 with 75% yield after purification. The structure of the compounds was confirmed by NMR, IR, and HRMS spectroscopy (see Supplementary Material Figures S1–S8). Thus, for compounds 2, the signals of the amide bond at δ 173.19 ppm (13C NMR) and δ 5.92 ppm (1H NMR) and those of the acetylene fragment at δ 71.42 and 79.74 ppm (13C NMR) and δ 2.65 ppm (1H NMR) were characteristic. The 1H NMR spectra of Mannich base 3 showed typical signals of the N-methylpiperazine fragment: the methyl group as singlet at δ 2.35 ppm and the methylene groups as multiplet at δ 2.55–2.70 ppm.
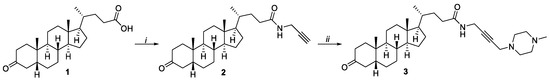
Scheme 1.
Synthesis of propargylaminoalkylated lithocholic acid. Reagent and conditions: (i). 1 (COCl)2, Et3N, CH2Cl2, 25 °C, 2 h; 2 propargyl amine, Et3N, CH2Cl2, 40 °C, 2 h; (ii). N-methylpiperazine, paraform, NaOAc, CuI, 1,4-dioxane, 60 °C, 8 h.
2.2. Biological Assay
Cytotoxicity and anti-influenza properties of the derivatives 2 and 3 were studied in MDCK cell culture against influenza virus A/PuertoRico/8/34 (H1N1). Oseltamivir carboxylate was used as a reference compound. As can be seen in Table 1, modification of 2 by addition of methylpiperazine moiety resulted in dramatic increase of antiviral activity combined with two-fold decrease of toxicity. Together, these effects led to strong increase of selectivity of compound (SI = 40 vs. 3 for 3 and 2, correspondingly).

Table 1.
Anti-influenza data of the derivatives 2 and 3.
The virus used in the study was resistant to adamantane derivatives targeting virus-specific proton pump M2. This is illustrated by low activity of reference compound Rimantadine (Table 1). Similar to the majority of currently circulating influenza viruses, this is due to S31N substitution in M2. High activity of triterpene-based compound 3 present here, therefore, suggests that its activity is based on an alternative mechanism.
3. Materials and Methods
The spectra were recorded at the Center for the Collective Use “Chemistry” of the Ufa Institute of Chemistry of the UFRC RAS and RCCU “Agidel” of the UFRC RAS. 1H and 13C NMR spectra were recorded on a “Bruker Avance-III” (Bruker, Billerica, MA, USA, 500 and 125.5 MHz respectively, δ, ppm, Hz) in CDCl3, internal standard—tetramethylsilane. Mass spectra were obtained on a liquid high-resolution chromatograph–mass spectrometer Agilent LC/Q-TOF 6530 (Santa Clara, CA, USA). Melting points were detected on a microtable «Rapido PHMK05» (Nagema, Dresden, Germany). Optical rotations were measured on a polarimeter Perkin-Elmer 241 MC (PerkinElmer, Waltham, MA, USA) in a tube length of 1 dm. Elemental analysis was performed on a Euro EA-3000 CHNS analyzer (Eurovector, Milan, Italy), the main standard is acetanilide. Thin-layer chromatography analyses were performed on Sorbfil plates (Sorbpolimer, Krasnodar, Russia), using the solvent system chloroform–ethyl acetate, 40:1. Substances were detected by a 10% solution of sulfuric acid solution with subsequent heating at 100–120 °C for 2–3 min. All chemicals were of reagent grade (Sigma-Aldrich, St. Louis, MO, USA). 3-Oxo-lithocholic acid 1 was synthesized as described in [14].
3.1. N-(Prop-2-yn-1-yl)-5β-Cholan-24-Amide (2)
To a solution of compound 1 (0.37 g, 0.5 mmol) in dry CH2Cl2 (15 mL), (COCl)2 (0.04 mL, 0.5 mmol), and Et3N (1 drop) were added. The reaction mixture was stirred at room temperature for 2 h, then CH2Cl2 was evaporated to give an intermediate acyl chloride. This residue was dissolved in CH2Cl2 (5 mL) and a CH2Cl2 solution of propargylamine (0.03 mL, 0.5 mmol) and Et3N (0.07 mL, 0.5 mmol) were added. The reaction mixture was refluxed for 2 h, the organic layer was diluted with cold water (20 mL) and separated. The aqueous layer was extracted with CH2Cl2 (2 × 15 mL), the combined extracts were washed with 5% HCl (3 × 15 mL), H2O (2 × 15 mL), dried over CaCl2, and the solvent was evaporated in a water jet vacuum. Purification of the crude product was performed using column chromatography on Al2O3 by elution with a mixture of petroleum ether—chloroform (1:0→1:1). Yield 89%, yellow powder. mp: 227–229 °C, IR (ύ max, cm−1) 3309 (υ C≡C–H), 1645 (υ C=O in CONH), 1539 (δ NH), 1714 (υ C=O) [α]20 D +12 (c 0.75, CH2Cl2). 1H NMR (δ, ppm, CDCl3, 500 MHz): 5.92 (1H, br. s, NH), 4.35 (2H, dd, J 1.5, J 7.1, H-25), 2.65 (1H, t, J 14.1, C≡CH), 2.35–1.05 (28H, m, CH, CH2), 0.98, 0.91, 0.89 (9H, s, 3CH3); 13C NMR (δ, ppm, CDCl3, 125.5 MHz): 213.4 (C-3), 173.1 (C-24), 79.7 (C-26), 71.4 (C-27), 56.4, 55.9, 44.3, 42.7, 42.3, 40.6, 40.0, 37.2, 36.9, 35.4, 35.3, 34.8, 33.2, 31.5, 29.1, 28.1, 26.5, 25.7, 24.1, 22.6, 21.7, 18.2, 12.0. Anal. Calcd for C27H41NO2: C, 78.78; H, 10.04; N, 3.40. Found: C, 78.85; H, 10.15; N, 3.37. HRMS [M + H]+ 411.3218, calcd for C27H41NO2: 411.3137.
3.2. N-(4-(4-Methylpiperazin-1-yl)but-2-yn-1-yl)-5β-Cholan-24-Amide (3)
To a solution of compound 2 (0.25 g, 0.6 mmol) in 12 mL of dry dioxane was added paraformaldehyde (0.18 g, 6 mmol), N-methylpiperazine (80 µL, 0.72 mmol), NaOAc (0.25 g, 3 mmol), and CuI (6 mg, 0.03 mmol). The reaction mixture was stirred under argon for 8 h at 60 °C. After the reaction was complete by TLC, the mixture was diluted with water, extracted with CHCl3 (3 × 20 mL), the combined organic layer was washed with water, dried over CaCl2, and evaporated under reduced pressure. The residue was purified by SiO2 column chromatography (eluent CHCl3-MeOH 100:0→70:1). Yield 75%, yellow powder. mp: 65–67 °C, IR (ύ max, cm−1) 3296 (υ C≡C), 1645 (υ C=O in CONH), 1539 (δ NH), 1714 (υ C=O), 754 (υ CN) [α]20 D +45 (c 0.75, CH2Cl2). 1H NMR (δ, ppm, CDCl3, 500 MHz): 6.20 (1H, br. s, NH), 4.00 (2H, t, J 2.3, H-25), 3.25 (2H, d, J 3.8, H-28), 2.70–2.55 (8H, m, 4CH2), 2.35 (3H, s, H-33), 2.30–1.00 (28H, m, CH, CH2), 0.98, 0.91, 0.89 (9H, s, 3CH3); 13C NMR (δ, ppm, CDCl3, 125.5 MHz): 213.4 (C-3), 173.2 (C-24), 81.5 (C-26), 77.6 (C-27), 56.3, 56.0, 54.5, 50.8, 46.9, 45.2, 44.2, 42.7, 42.3, 40.6, 40.0, 37.1, 36.9, 35.4, 34.8, 33.1, 31.5, 29.6, 29.3, 29.3, 28.1, 26.5, 25.7, 24.1, 22.6, 21.1, 18.3, 14.0, 12.0. Anal. Calcd for C33H53N3O4: C, 75.67; H, 10.20; N, 8.02. Found: C, 75.67; H, 10.20; N, 8.02. HRMS [M + H]+ 524.4228, calcd for C33H53N3O4: 523.4138.
3.3. Biological Activity
Viruses and cells. Influenza virus A/Puerto Rico/8/34 (H1N1) was obtained from the collection of viruses of the St. Petersburg Pasteur Institute. Before the experiment, virus was propagated in the allantoic cavity of 10- to 12-day-old chicken embryos for 48 h at 36 °C. The infectious titer of the virus was determined in Madin-Darby Canine Kidney (MDCK) cells (ATCC-CCL-34) grown in 96-well plates in alpha-MEM medium with 10% fetal bovine serum.
3.3.1. Cytotoxicity Assay
MDCK cells were seeded onto 96-well culture plates (104 cells per well) and incubated at 36 °C in 5% CO2 until a continuous monolayer formation. To assess the toxicity of compounds, a series of their 3-fold dilutions at concentrations of 300 to 3.7 μg/mL in Eagle’s Minimal Essential Medium (MEM) were prepared. The dilutions were added to the wells of the plates. Cells were incubated for 72 h at 36 °C in a CO2 incubator under 5% CO2. Further, a microtetrazolium (MTT) assay was performed on 96-well plates. The cells were washed 2 times with saline (0.9% NaCl), and 100 μL/well of MTT solution [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] at a concentration of 0.5 μg/mL in MEM was added. The plates were incubated for 1 h at 36 °C, the liquid was removed, and dimethylsulfoxide (DMSO) (0.1 mL per well) was added. The optical density (OD) of the cells was measured on a Thermo Multiskan FC spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at a wavelength of 540 nm. Based on the obtained data, the CC50, the concentration of the compound that destroys 50% of the cells in the culture, was calculated for each specimen.
3.3.2. CPE Reduction Assay
The compounds in appropriate concentrations were added to MDCK cells (0.1 mL per well). MDCK cells were further infected with A/Puerto Rico/8/34 (H1N1) influenza virus (m.o.i 0.01). Plates were incubated for 72 h at 36 °C at 5% CO2. After that, cell viability was assessed by the MTT test, as described above. The cytoprotective activity of compounds was considered as their ability to increase the values of the OD compared to the control wells (with virus only; no drugs). Based on the obtained results, the IC50 values, i.e., the concentration of compounds that results in 50% cell protection, were calculated using GraphPad Prism 6.01 software. IC50 values in μg/mL were then calculated into micromoles. For each compound, the value of the selectivity index (SI) was calculated as a ratio of CC50 to IC50.
4. Conclusions
Conjugate of 3-oxo-lithocholic acid with N-methylpiperazine was prepared using Mannich reaction and showed significant antiviral activity with a value of SI 40 and IC50 1 μM.
Supplementary Materials
The following supporting information can be downloaded online. Figures S1–S8: 1H and 13C NMR data of compounds 2 and 3; Biological assay.
Author Contributions
O.B.K. brought the idea, A.V.P. conducted the experiment, did structure elucidation and prepared the manuscript; V.V.Z., S.V.F. and Y.N.P. made biological experiments; T.T.T.P. and K.M.M. made biological experiments. I.E.S. prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The reported study was performed under the e-Asia_Health and funded by RFBR project number 21-53-70201 (Russian Federation), MOST (Vietnam), and MOE project number HE-098 (Myanmar).
Data Availability Statement
Not available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Global Influenza Strategy 2019–2030; World Health Organization: Geneva, Switzerland, 2019. Available online: https://apps.who.int/iris/handle/10665/311184 (accessed on 20 March 2019).
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, T. Baloxavir Marboxil: The First Cap-dependent Endonuclease inhibitor for the treatment of influenza. Ann. Pharmacother. 2019, 53, 754–759. [Google Scholar] [CrossRef]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin. Infect. Dis. 2019, 68, 895–902. [Google Scholar] [CrossRef]
- Samson, M.; Pizzorno, A.; Abed, Y.; Boivin, G. Influenza virus resistance to neuraminidase inhibitors. Antivir. Res. 2013, 98, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Govorkova, E.A.; Takashita, E.; Daniels, R.S.; Fujisaki, S.; Presser, L.D.; Patel, M.C.; Huang, W.; Lackenby, A.; Nguyen, H.T.; Pereyaslov, D.; et al. Global update on the susceptibilities of human influenza viruses to neuraminidase inhibitors and the cap-dependent endonuclease inhibitor baloxavir, 2018–2020. Antivir. Res. 2022, 200, 105281. [Google Scholar] [CrossRef] [PubMed]
- Moghadas, S.M.; Bowman, C.S.; Rost, G.; Wu, J. Population-wide emergence of antiviral resistance during pandemic influenza. PLoS ONE 2008, 3, 1839. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Suryan, A. A comprehensive review on steroidal bioconjugates as promising leads in drug discovery. ACS Bio Med Chem Au 2022, 2, 340–369. [Google Scholar] [CrossRef]
- Niemeyer, C.M. Bioconjugation Protocols: Strategies and Methods; Springer Science, Business Media: Secaucus, NJ, USA, 2004; Volume 283, pp. 5–100. [Google Scholar]
- Singh, Y.; Spinelli, N.; Defrancq, E.; Dumy, P. A novel heterobifunctional linker for facile access to bioconjugates. Org. Biomol. Chem. 2006, 4, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Salunke, D.B.; Hazra, B.G.; Pore, V.S. Steroidal conjugates and their pharmacological applications. Curr. Med. Chem. 2006, 13, 813–847. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Schmidt, A.; Freedman, L.P. Steroid hormone receptors and drug discovery: Therapeutic opportunities and assay designs. Assay Drug Dev. Technol. 2003, 1, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Mao, Q.; Liu, M.; Wang, K.; Wu, Z.; Fang, W.; Yang, Z.; Luo, P.; Ke, S.; Shi, L. The inhibitory effect of dehydroepiandrosterone and its derivatives against influenza A virus in vitro and in vivo. Arch. Virol. 2016, 161, 3061–3072. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Sarker, S.D.; Turner, A.B. A facile synthesis of ethyl (3-oxo-5β-cholan)-24-yl oxalate. Acta Chim. Slov. 2006, 53, 512–516. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).