Synthesis and Spectroscopic Characterization of Furan-2-Carbaldehyde-d
Abstract
:1. Introduction
2. Results and Discussion
2.1. NMR Analysis Details
2.2. Comprehensive EI-MS Spectra Analysis
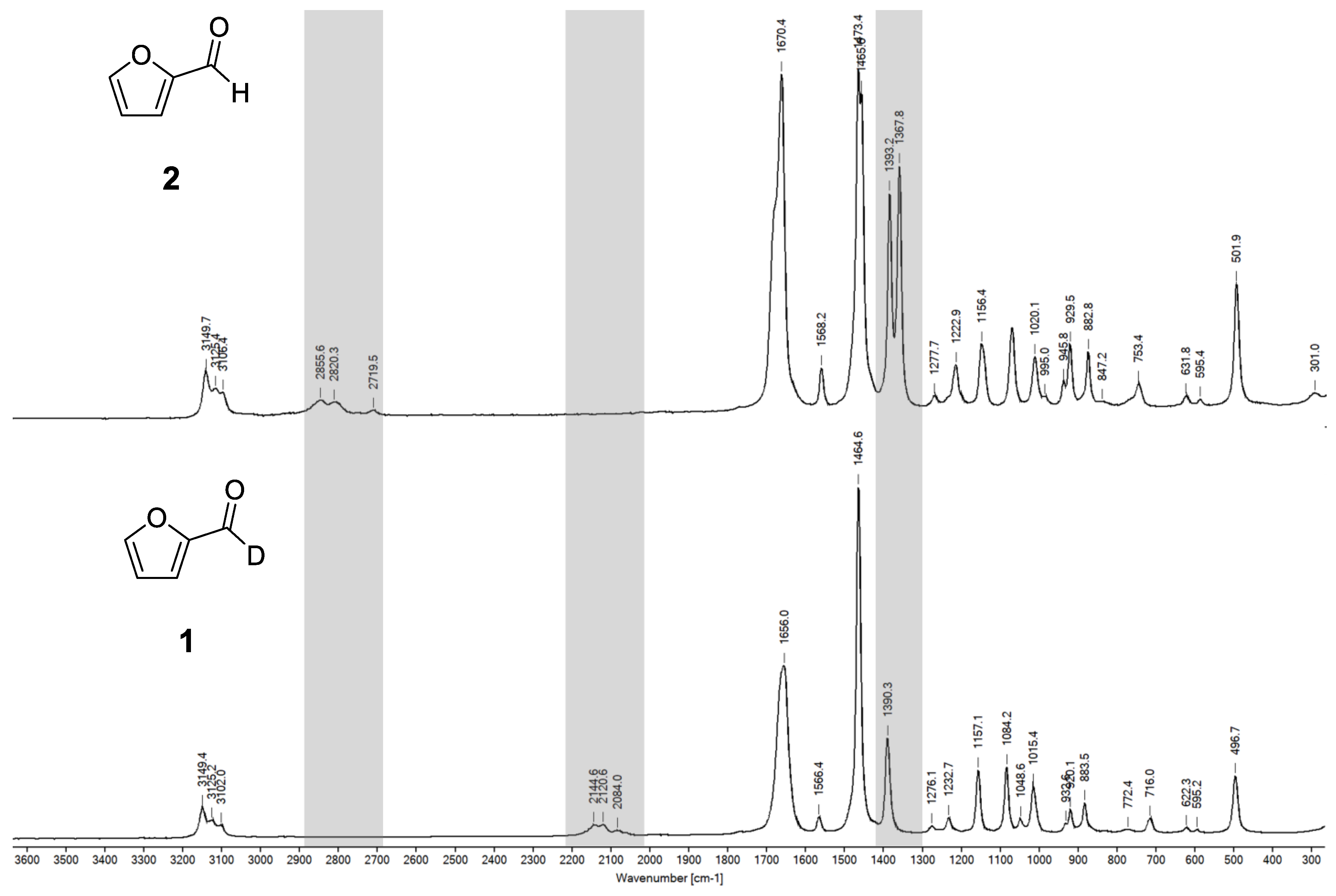
2.3. Comprehensive IR Spectra Analysis
2.4. Comprehensive Raman Spectra Analysis
3. Materials and Methods
3.1. Materials
3.2. Method of Synthesis
3.3. Instrumental Analytics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoydonckx, H.; Van Rhijn, W.; Van Rhijn, W.; De Vos, D.; Jacobs, P. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Cioc, R.C.; Lutz, M.; Pidko, E.A.; Crockatt, M.; Van Der Waal, J.C.; Bruijnincx, P.C. Direct Diels–Alder reactions of furfural derivatives with maleimides. Green Chem. 2021, 23, 367–373. [Google Scholar] [CrossRef]
- Hao, Z.; Hu, L.; Wang, X.; Liu, Y.; Mo, S. Synthesis of Heptamethine Cyanines from Furfural Derivatives. Org. Lett. 2023, 25, 1078–1082. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.P.; Van Der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Jaswal, A.; Singh, P.P.; Mondal, T. Furfural—A versatile, biomass-derived platform chemical for the production of renewable chemicals. Green Chem. 2022, 24, 510–551. [Google Scholar] [CrossRef]
- Simmons, E.M.; Hartwig, J.F. On the Interpretation of Deuterium Kinetic Isotope Effects in C-H Bond Functionalizations by Transition-Metal Complexes. Angew. Chem. Int. Ed. 2012, 51, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Yang, J. Deuterium: Discovery and Applications in Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Atzrodt, J.; Derdau, V.; Kerr, W.J.; Reid, M. Deuterium- and Tritium-Labelled Compounds: Applications in the Life Sciences. Angew. Chem. Int. Ed. 2018, 57, 1758–1784. [Google Scholar] [CrossRef] [PubMed]
- Duclos, R.I., Jr.; Hoener, B.-A. Syntheses of 2-(diacetoxymethyl-14C)-5-nitrofuran and carbon-14 labeled nitrofurazone. J. Label. Compd. Radiopharm. 1986, 23, 103–108. [Google Scholar] [CrossRef]
- Rao, N.; Kini, R.; Kad, P. Deuterated Drugs. Pharm. Chem. J. 2022, 55, 1372–1377. [Google Scholar] [CrossRef]
- Yang, Y.; Han, B.; Dong, F.; Lv, J.; Lu, H.; Sun, Y.; Lei, Z.; Yang, Z.; Ma, H. A Cost-Effective Way to Produce Gram-Scale 18O-Labeled Aromatic Aldehydes. Org. Lett. 2022, 24, 4409–4414. [Google Scholar] [CrossRef]
- Chadwick, D.J.; Chambers, J.; Hargraves, H.E.; Meakins, G.D.; Snowden, R.L. Preparation of substituted furan- and thiophen-2-carbaldehydes and -2-[2H]carbaldehydes, and of 2-furyl ketones. J. Chem. Soc. Perkin Trans. 1973, 2327–2332. [Google Scholar] [CrossRef]
- Chadwick, D.J.; Chambers, J.; Hodgson, P.K.G.; Meakins, G.D.; Snowden, R.L. Preparation of deuteriated furan- and thiophen-2-carbaldehydes and -2-carboxylic esters. J. Chem. Soc. Perkin Trans. 1974, 141–1145. [Google Scholar] [CrossRef]
- Ramalingam, K.; Nanjappan, P.; Kalvin, D.M.; Woodward, R.W. A practical large scale chemical synthesis of chiral glycines. Tetrahedron 1988, 44, 5597–5604. [Google Scholar] [CrossRef]
- Vasiliou, A.K.; Kim, J.H.; Ormond, T.K.; Piech, K.M.; Urness, K.N.; Scheer, A.M.; Robichaud, D.J.; Mukarakate, C.; Nimlos, M.R.; Daily, J.W.; et al. Biomass pyrolysis: Thermal decomposition mechanisms of furfural and benzaldehyde. J. Chem. Phys. 2013, 139, 104310. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.H.; Huang, P.R.; Chuang, G.J. Triphenylphosphine/Triethylamine-Mediated Decarboxylation of α-Oxocarboxylic Acids and Application in a One-Pot Synthesis of Deuterated Aldehydes. Asian J. Org. Chem. 2016, 5, 57–61. [Google Scholar] [CrossRef]
- Hu, C.H.; Li, Y. Visible-Light Photoredox-Catalyzed Decarboxylation of α-Oxo Carboxylic Acids to C1-Deuterated Aldehydes and Aldehydes. J. Org. Chem. 2023, 88, 6401–6406. [Google Scholar] [CrossRef]
- Sawama, Y.; Miki, Y.; Sajiki, H. N-Heterocyclic Carbene Catalyzed Deuteration of Aldehydes in D2O. Synlett 2020, 31, 699–702. [Google Scholar] [CrossRef]
- Shinada, T.; Nakayama, A.; Okamura, H.; Yasuno, Y. Recent Progress in the Synthesis of Deuterated Aldehyde. Bull. Chem. Soc. Jpn. 2022, 95, 1461–1473. [Google Scholar] [CrossRef]
- Woodall, E.L.; Simanis, J.A.; Hamaker, C.G.; Goodell, J.R.; Mitchell, T.A. Unique Reactivity of anti- and syn-Acetoxypyranones en Route to Oxidopyrylium Intermediates Leading to a Cascade Process. Org. Lett. 2013, 15, 3270–3273. [Google Scholar] [CrossRef]
- Liu, M.; Xie, Y.; Li, J.; Pan, H.J.; Tian, H.; Shi, Y.A. Synthesis of Optically Active Deuterated Primary Amines via Reduction of N-tert-Butanesulfinyl Aldimines. J. Org. Chem. 2014, 79, 8417–8421. [Google Scholar] [CrossRef]
- Kaufman, R.H.; Law, C.M.; Simanis, J.A.; Woodall, E.L.; Zwick, C.R.; Wedler, H.B.; Wendelboe, P.; Hamaker, C.G.; Goodell, J.R.; Tantillo, D.J.; et al. Oxidopyrylium-Alkene [5 + 2] Cycloaddition Conjugate Addition Cascade (C3) Sequences: Scope, Limitation, and Computational Investigations. J. Org. Chem. 2018, 83, 9818–9838. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, C.; Wang, J. Enantioselective Intermolecular Enamide–Aldehyde Cross-Coupling Catalyzed by Chiral N-Heterocyclic Carbenes. J. Am. Chem. Soc. 2016, 138, 4706–4709. [Google Scholar] [CrossRef] [PubMed]
- Umar, Y.; Tijani, J. Density functional theory study of the rotational barriers, conformational preference, and vibrational spectra of 2-formylfuran and 3-formylfuran. J. Struct. Chem. 2016, 56, 1305–1312. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaster, J.; Dressler, E.; Geitner, R.; Groß, G.A. Synthesis and Spectroscopic Characterization of Furan-2-Carbaldehyde-d. Molbank 2023, 2023, M1654. https://doi.org/10.3390/M1654
Jaster J, Dressler E, Geitner R, Groß GA. Synthesis and Spectroscopic Characterization of Furan-2-Carbaldehyde-d. Molbank. 2023; 2023(2):M1654. https://doi.org/10.3390/M1654
Chicago/Turabian StyleJaster, Jonas, Elias Dressler, Robert Geitner, and Gregor Alexander Groß. 2023. "Synthesis and Spectroscopic Characterization of Furan-2-Carbaldehyde-d" Molbank 2023, no. 2: M1654. https://doi.org/10.3390/M1654
APA StyleJaster, J., Dressler, E., Geitner, R., & Groß, G. A. (2023). Synthesis and Spectroscopic Characterization of Furan-2-Carbaldehyde-d. Molbank, 2023(2), M1654. https://doi.org/10.3390/M1654





