Benzo[d][1,2,3]oxadithiole 2-Oxide
Abstract
:1. Introduction
2. Results
3. Experimental
3.1. General Experimental Details
3.2. Synthesis of Benzo[d][1,2,3]oxadithiole 2-Oxide 3
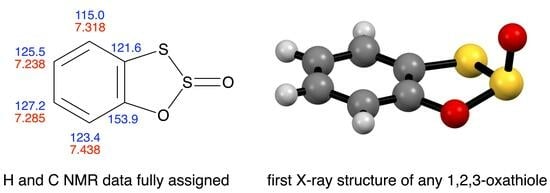
3.3. X-ray Structure Determination of 3
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Anschütz, R.; Posth, W. Über zwei cyclische ester des brenzcatechins. Ber. Dtsch. Chem. Ges. 1894, 27, 2751–2753. [Google Scholar] [CrossRef]
- De Jongh, D.C.; Van Fossen, R.Y. Mass spectra and pyrolysis of o-phenylene sulfite and related compounds. J. Org. Chem. 1972, 37, 1129–1135. [Google Scholar] [CrossRef]
- Aitken, R.A.; Horsburgh, C.E.R. Flash vacuum pyrolysis of o-phenylene sulfite: Formation and purification of cyclopentadienone dimer. In Comprehensive Organic Chemistry Experiments for the Laboratory Classroom; Afonso, C.A.M., Candeias, N.R., Pereira Simão, D., Trinidade, A.F., Coelho, J.A.S., Tan, B., Franzén, R., Eds.; RSC: Cambridge, UK, 2016; Chapter 10.9; pp. 690–693. [Google Scholar] [CrossRef]
- Schulz, R.; Schweig, A. Cyclopentadienthion. Angew. Chem. Int. Ed. Engl. 1981, 20, 570–571. [Google Scholar] [CrossRef]
- Wilson, G.E., Jr.; Belkind, B.A. Teraoxysulfuranes from phenols. Synthesis and the dehydration of alcohols. J. Am. Chem. Soc. 1978, 100, 8124–8130. [Google Scholar] [CrossRef]
- Hansen, P.E. Carbon-hydrogen spin-spin coupling constants. Prog. NMR Spectrosc. 1981, 14, 175–295. [Google Scholar] [CrossRef]
- Schulz, G.; Serke, I.; Kapovits, I. Molecular structure of o-phenylene sulfite, an electron diffraction study. J. Chem. Soc. Faraday Trans. 2 1979, 75, 1612–1619. [Google Scholar] [CrossRef]
- Boer, F.P.; Flynn, J.J. Structural studies of strained cyclic esters. Catechol sulfate. J. Am. Chem. Soc. 1969, 91, 6604–6609. [Google Scholar] [CrossRef]
- Gembicky, M. Experimental Crystal Structure Determination. CCDC 1851728. 2018. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/ccdc.csd.cc204w57&sid=DataCite (accessed on 3 April 2024). [CrossRef]
- Kuzmich, A.S.; Khomenko, T.M.; Fedorov, S.N.; Makarieva, T.N.; Shubina, L.K.; Komarova, N.I.; Korchagina, D.V.; Rybalova, T.V.; Volcho, K.P.; Salakhutdinov, N.F. Cytotoxic and cancer preventive activity of benzotrithioles and benzotrithiole oxides, synthetic analogues of varacins. Med. Chem. Res. 2017, 26, 397–404. [Google Scholar] [CrossRef]
- Kimura, T.; Hanzawa, M.; Horn, E.; Kawai, Y.; Ogawa, S.; Sato, R. Preparation and conformational analysis of 6,10-disubstituted[1,2,3]trithiolo[h]benzopentathiepin monoxides. Tetrahedron Lett. 1997, 38, 1607–1610. [Google Scholar] [CrossRef]
- Kimura, T.; Hanzawa, M.; Tsujimura, K.; Takahashi, T.; Kawai, Y.; Horn, E.; Fujii, T.; Ogawa, S.; Sato, R. Preparation and conformational analysis of 6,10-diethyl[1,2,3]trithiolo[4,5-h]benzopentathiepin monoxides: Isolation and optical properties of chiral benzopentathiepin derivatives. Bull. Chem. Soc. Jpn. 2002, 75, 817–824. [Google Scholar] [CrossRef]
- CrysAlisPro v1. 171.42.96a Rigaku Oxford Diffraction; Rigaku Co.: Tokyo, Japan, 2023. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]






| Position | 3 | 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| δC | δH | 1JCH | 2JCH | 3JCH | δC | δH | 1JCH | 2JCH | |
| 3a | 121.6 | – | – | – | – | 142.7 | – | – | – |
| 4 | 115.0 | 7.318 | 165 | 8.0 | – | 124.4 | 7.132 | 163 | 7.6 |
| 5 | 125.5 | 7.238 | 162 | 7.2 | – | 112.4 | 7.192 | 165 | – |
| 6 | 127.2 | 7.285 | 163 | 8.9 | – | 112.4 | 7.192 | 165 | – |
| 7 | 123.4 | 7.438 | 164 | 8.1 | 3.4 | 124.4 | 7.132 | 163 | 7.6 |
| 7a | 153.9 | – | – | – | – | 142.7 | – | – | – |
| Compound | 3JH4–H5 | 4JH4–H6 | 5JH4–H7 | 3JH5–H6 | 4JH5–H7 | 3JH6–H7 |
|---|---|---|---|---|---|---|
| 3 | 8.0 | 1.2 | 0.4 | 7.2 | 1.6 | 7.2 |
| 1 | 7.9 | 1.5 | 0.4 | 7.9 | 1.5 | 7.9 |
| Compound | 3 | 1 [7] | 6 [8] | 6 [9] |
|---|---|---|---|---|
| O(1)–S(2) | 1.6524(9) | 1.670(4) | 1.591 | 1.6047(14) |
| S(2)–S/O(3) | 2.1426(4) | 1.670(4) | 1.609 | 1.6093(15) |
| S(2)=O | 1.4549(10 | 1.423(6) | 1.392, 1.380 | 1.4150(15), 1.4131(14) |
| S/O(3)–C(3a) | 1.7565(13) | 1.401(9) | 1.404 | 1.401(2) |
| C(3a)–C(7a) | 1.3879(18) | 1.391(20) | 1.363 | 1.378(3) |
| C(7a)–O(1) | 1.3931(15) | 1.401(9) | 1.393 | 1.408(2) |
| C(7a)–O(1)–S(2) | 116.09(8) | 109.6(4) | 108.63 | 108.47(12) |
| O(1)–S(2)–S/O(3) | 93.01(3) | 92.9(3) | 96.97 | 97.19(7) |
| S(2)–S/O(3)–C(3a) | 91.66(4) | 109.6(4) | 108.76 | 108.63(12) |
| S/O(3)–C(3a)–C(7a) | 114.74(10) | 111.6(4) | 110.75 | 111.61(15) |
| C(3a)–C(7a)–(O1) | 116.79(11) | 111.6(4) | 112.58 | 111.53(15) |
| Flap angle * | 25.76 | 19.8(18) | 14.22 | 15.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aitken, R.A.; Cordes, D.B.; Goyal, A.; McKay, A.P. Benzo[d][1,2,3]oxadithiole 2-Oxide. Molbank 2024, 2024, M1803. https://doi.org/10.3390/M1803
Aitken RA, Cordes DB, Goyal A, McKay AP. Benzo[d][1,2,3]oxadithiole 2-Oxide. Molbank. 2024; 2024(2):M1803. https://doi.org/10.3390/M1803
Chicago/Turabian StyleAitken, R. Alan, David B. Cordes, Arun Goyal, and Aidan P. McKay. 2024. "Benzo[d][1,2,3]oxadithiole 2-Oxide" Molbank 2024, no. 2: M1803. https://doi.org/10.3390/M1803