Abstract
When 1-benzoylthiosemicarbazide (2) or thiosemicarbazide (1) were treated with benzoyl chloride in a basic medium, a mixture of two compounds was obtained: 1,2-dibenzoylthiosemicarbazide (3) and 1,4-dibenzoylthiosemicarbazide (4). To determine the structure of the novel compounds, 2D NMR spectroscopy techniques such as 1H-13C and 1H-15N were employed.
1. Introduction
The great interest in the last years for 3-substituted 4H-5-mercapto-1,2,4-triazoles (6) and their derivatives is due to their various uses, especially as biologically active compounds [1,2,3,4,5].
Within our studies on obtaining S-glycosides [6], we have synthesized 1H-3-phenyl-5-mercapto-1,2,4-triazoles (6) by the benzoylation of thiosemicarbazide with benzoyl chlorides in the presence of pyridine in organic solvent, followed by the cyclization of 1-benzoylthiosemicarbazides with bases in aqueous-alcoholic medium [7].
During monobenzoylation of thiosemicarbazide, the formation of several secondary compounds was observed, but they were easily removed during the cyclization of crude 1-benzoylthiosemicarbazides (2) to 5-mercapto-1,2,4-triazoles (6).
1-Acylthiosemicarbazides are used in the preparation of heterocyclic compounds [8,9], but the literature reports few details on the acylation reaction as follows:
- -
- During monobenzoylation of thiosemicarbazide with benzoyl chloride in pyridine, 1-benzoylthiosemicarbazide (2) is formed as the main product, along with small amounts of 1,4-dibenzoylthiosemicarbazide (4) [10];
- -
- After treatment of 1-benzoylthiosemicarbazide (2) with benzoyl chloride at 0 °C, 1,4-dibenzoylthiosemicarbazide (4) and 1,2-dibenzoylhydrazine (5) are formed in ~15% and ~25% yield, respectively [11];
- -
- Upon treatment of benzoyl chloride with excess thiosemicarbazide, 1-benzoylthiosemicarbazide (2) is formed with a high yield [12].
As a result, we were motivated to conduct experiments with the aim of separating and characterizing the compounds generated when thiosemicarbazide (1) reacted with two equivalents of benzoyl chloride, and when 1-benzoylthiosemicarbazide (2) reacted with one equivalent of benzoyl chloride in the presence of pyridine, using DMF as the solvent.
2. Results and Discussion
Following some preliminary experiments [13,14], we found that heating the crude products formed in the two reactions in an alkaline medium led to 1H-5-mercapto-3-phenyl-1,2,4-triazole (6) (η = 20%), originating from the cyclization and hydrolysis of 1,4-dibenzoylthiosemicarbazide (4) or from the cyclization of 1-benzoylthiosemicarbazide (2), and to 1,2-dibenzoylhydrazine (5) (η = 75%), which can originate only from the hydrolysis of 1,2-dibenzoylthiosemicarbazide (3). The reaction scheme is shown in Scheme 1:
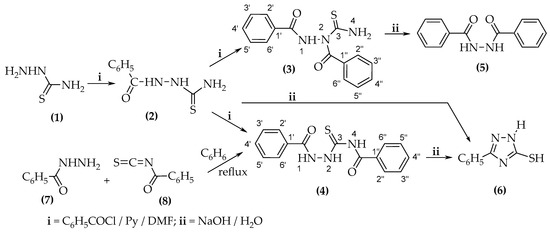
Scheme 1.
Synthesis of 1,2-dibenzoylthiosemicarbazide (3) and 1,4-dibenzoylthiosemicarbazide (4).
In this study, we provide an analysis of the isolation and identification process of 1,2-dibenzoylthiosemicarbazide (3). Furthermore, we present the outcomes obtained from the benzoylation reactions of thiosemicarbazide (1) and 1-benzoylthiosemicarbazide (2) with different amounts of benzoyl chloride in the presence of pyridine and NaH, using DMF as the solvent.
Using HPLC, we monitored the progress of the benzoylation process involving thiosemicarbazide (1) and 1-benzoylthiosemicarbazide (2) in the presence of pyridine and benzoyl chloride. The results revealed the development of a diverse mixture containing benzoylthiosemicarbazides (2), (3), and (4). Table 1 and Table 2 provide the molar percentages of these compounds at different stages of the reaction.

Table 1.
Benzoylation of thiosemicarbazide (1) conditions and products’ molar percentages.

Table 2.
Benzoylation of 1-benzoylthiosemicarbazide (2) conditions and products’ molar percentages.
During the process of the dibenzoylation of thiosemicarbazide (1) and the benzoylation of 1-benzoylthiosemicarbazide (2), we observed an increase over time of the percentage of 1,2-dibenzoylthiosemicarbazide (3) over the percentage of 1,4-dibenzoylthiosemicarbazide (4) and also the formation of the monobenzoylation product when starting from thiosemicarbazide (1).
For the benzoylation reaction of thiosemicarbazide (1), when using NaH as the base, only 1-benzoylthiosemicarbazide (2) is formed, while using the same base in the benzoylation reaction of 1-benzoylthiosemicarbazide (2) besides the unreacted starting material, a majority formation of 1,2-dibenzoylthiosemicarbazide (3) is observed.
Using the 2D 1H-13C and 1H-15N NMR spectra (HMBC and HSQC), we were able to accurately assign the corresponding proton and carbon signals. The chemical shifts of nitrogen atoms in the synthesized compounds were obtained from the inverse correlation spectra of 1H-15N type of direct coupling (HSQC or HMQC) or long-range coupling over two or three bonds (HMBC). In the case of 1,2-dibenzoylthiosemicarbazide, the chemical shifts for the nitrogen atoms 1-N and 4-N, respectively, were determined from the direct coupling 1H-15N HMQC spectrum and for the nitrogen atom 2-N from the long-range coupling 1H-15N HMBC spectrum, when long-range couplings over two bonds with the hydrogen atom 1-N-H and over three bonds with the hydrogen atoms 4-N-H, respectively. For 1,4-dibenzoylthiosemicarbazide, the chemical shifts for all the nitrogen atoms could be determined only from the direct coupling 1H-15N HMQC spectrum.
It can be observed that the protons bound to the nitrogen atom 4-N both in the case of 1,2-dibenzoylthiosemicarbazide and 1,4-benzoylthiosemicarbazide are diastereotopic, exhibiting different chemical shifts. Thus, in the 1H-15N HMQC spectra of these two compounds, two cross-peaks can be observed for the nitrogen atom 4-N, corresponding to the couplings with the two diastereotopic hydrogen atoms, Ha and Hb, respectively.
3. Materials and Methods
All of the compounds were purified and kept under argon; the reactions were also performed under an argon atmosphere. The solvents were purchased from commercial sources (Chimopar, Bucharest, Romania, Acros Organics, Geel, Belgium) and used after distillation and drying. Commercial thiosemicarbazide was recrystallized from water.
TLC analysis was performed on 60 F254 silica gel plates from Merck, using 1:1 hexane: ethyl acetate or 7:3 (v/v) as eluent.
Melting points were measured on a Böetius PHMK apparatus (Veb Analytik, Dresden, Germany) and were uncorrected.
IR spectra were recorded on a Jasco FT/IR-410 spectrophotometer (Jasco Corporation, Tokyo, Japan) in KBr pellets.
HPLC determinations were carried out on a Jasco HPLC system (quaternary pump and UV-VIS detector) with a C18 Phenomenex or Synergi 4u Hydro-RP 80 A column, with acetonitrile:water = 86:14 (v/v) flow 0.4 mL/min (25 °C) and a UV-2070 Plus detector.
GC-MS analysis was performed by using an ion trap mass spectrometer ITQ 1100 coupled with Gas Chromatograph Trace 1310 (Thermo Scientific, Waltham, MA, USA). MS parameters were set as follows: transfer line temperature at 300 °C, source temperature at 170 °C, a scan range between 50 and 700 amu, and a 70 eV electron was used for ionization. Separation was achieved on a capillary column of 30 m × 0.25 mm ID, 0.25 µm (TG–5MS, Thermo Scientific); the injection port temperature was set at 310 °C. An automated sample delivery system (TriPlus RSH, Thermo Scientific, Waltham, MA, USA) with a split ratio of 1/30 for 1.5 min followed by a splitless mode was used for sample injection. The oven program was set as follows: 100 °C (held 1 min) to 300 °C (held 3 min) with 10 °C/min.
NMR spectra were recorded on a Bruker DRX 400 MHz, Bruker AVANCE III 400 MHz and 500 MHz spectrometers (Bruker, Karlsruhe, Germany), in DMSO-d6 using TMS as an internal standard for protons and carbons. Chemical shifts are reported in ppm units and the coupling constants are given in Hz.
4. Experimental
Synthesis of Benzoylthiosemicarbazides
- (a)
- Benzoylation of thiosemicarbazide (I)
To a solution of 0.01 mol thiosemicarbazide (1) in 5 mL DMF, 0.012 mol or 0.022 mol pyridine was added, at room temperature, and 0.01 and 0.02 mol benzoyl chloride, respectively.
- (b)
- Benzoylation of 1-benzoylthiosemicarbazide (II)
To a solution of 0.01 mol of 1-benzoylthiosemicarbazide (2) in 5 mL of DMF, 0.012 mol of pyridine was added, at room temperature, and 0.01 mol of benzoyl chloride.
The benzoylated thiosemicarbazide mixtures I and II were used to monitor the benzoylation of thiosemicarbazide (1) and 1-benzoylthiosemicarbazide (2) by HPLC.
1-benzoylthiosemicarbazide (2) was obtained by the reaction of thiosemicarbazide with benzoyl chloride in DMF in the presence of pyridine at 50 °C and precipitation in water. White crystalline powder (η = 62%), m.p.= 191–194 °C (MeOH) (lit. 196–198 °C) [14]. IR (ν/cm−1): νasNH2 = 3542; νsNH2 = 3418; νNH = 3240; νC=O = 1684, νskC6H5 = 1605; νC=S = 1086; γskC6H5 = 704; 1H-NMR δH (DMSO-d6, 500 MHz): 10.40 (s, 1H, 1-N-H), 9.36 (s, 1H, 2-N-H), 7.90 (d, 2H, J = 7.4 Hz, 2′-H, 6′-H), 7.89 (br. s, 1H, 4-N-Ha), 7.65 (br. s, 1H, 4-N-Ha), 7.56 (t, 1H, J = 7.4 Hz, 4′-H), 7,47 (t, 2H, J = 7.6 Hz, 3′-H, 5′-H); 13C-NMR δC (DMSO-d6, 125 MHz): 181.9 (C=S), 165.7 (C=O), 132.3 (1′-C), 131.6 (4′-C), 128.0 (3′-C, 5′-C), 127.7 (2′-C, 6′-C); 15N-NMR δN (DMSO-d6, 50.6 MHz): 132.1 (1-N), 121.8 (2-N), 108.6 (4-N). All related spectra can be found in Supplementary Materials section.
1,4-Dibenzoylthiosemicarbazide (4) was obtained from benzoylhydrazine (7) and benzoyl isothiocyanate (8), according to a method from the literature [15]. White crystalline powder (η = 67%), m.p. = 179–180 °C (EtOH) (lit. 178 °C) [16]; IR (ν/cm−1): νNH = 3298, 3270; νC=O =1666, 1655; νskC6H5 = 1598, 1579; νC=S = 1076; γskC6H5 = 711, 698; 1H-NMR δH (DMSO-d6, 400 MHz): 12.44 (s, 1H, 1-N-H), 11.79 (s, 1H, 4-N-H), 11.16 (s, 1H, 2-N-H), 8.00 (d, 2H, J = 7.6 Hz, 2″-H, 6″-H), 7.96 (d, 2H, J = 7.6 Hz, 2′-H, 6′-H), 7.66 (t, 1H, J = 7.4 Hz, 4″-H), 7,61 (t, 1H, J = 7.4 Hz, 4′-H), 7.54 (t, 4H, J = 7.6 Hz, 3′-H, 5′-H, 3″-H, 5″-H); 13C-NMR δC (DMSO-d6, 100 MHz): 180.4 (C=S), 167.7 (4-N-C=O), 164.4 (1-N-C=O), 133.1 (4″-C); 132.0 (1′-C, 1″-C), 131.8 (4′-C), 128.6 (2″-C, 6″-C), 128.4 (3″-C, 5″-C ), 128.3 (3′-C, 5′-C), 127.6 (2′-C, 6′-C); 15N-NMR δN (DMSO-d6, 40 MHz): 155.1 (4-N), 146.1 (1-N), 138.3 (2-N); MS (70 eV) m/z: 299 (M+, 1%), 281 (M+-H2O, 7%), 222 (M+-·C6H5, 25%), 105 (M+- C8H8N3OS, 100%) Calcd. for C15H13N3O2S: C, 60.19; H, 4.38; N, 14.04; S, 10.71. Found (%): C, 59.99; H, 4.18; N, 13.87; S, 10.49. All related spectra can be found in Supplementary Materials section.
1,2-Dibenzoylthiosemicarbazide (3) was obtained from the reaction mixture from the benzoylation of thiosemicarbazide (1) with two equivalents of benzoyl chloride in DMF in the presence of pyridine or from the benzoylation of 1-benzoylthiosemicarbazide (2) with one equivalent of benzoyl chloride in DMF in the presence of pyridine, precipitation in water, and two recrystallizations from acetonitrile, in 42% and 45% yield, respectively. White crystalline powder, m.p. = 159–161 °C. IR (ν/cm−1): νNH = 3386, 3125; νC=O = 1711, 1672; νskC6H5 = 1600, 1580; νC=S = 1073; γskC6H5 = 706, 735.
1H-NMR δH (DMSO-d6, 400 MHz): 11.37 (s, 1H, 1-N-H), 9.84 (s, 1H, 4-N-Ha), 9.52 (s, 1H, 4-N-Hb), 7.70–7.69 (m, 2H, 2′-H, 6′-H), 7.69–7.68 (m, 2H, 2″-H, 6″-H), 7.53 (tt, 1H, Jo = 7.3 Hz, Jm = 1.3 Hz, 4″-H), 7.47–7.38 (m, 5-H, 3′-H, 4′-H, 5′-H, 3″-H, 5″-H); 13C-NMR δC (DMSO-d6, 100 MHz): 183.7 (C=S), 172.1 (2-N-C=O), 165.1 (1-N-C=O), 134.8 (1″-C); 132.0 (4″-C), 131.6 (1′-C), 131.1 (4′-C), 128.3 (3′-C, 5′-C), 127.6 (3″-C, 5″-C), 127.4 (2′-C, 6′-C); 127.3 (2″-C, 6″-C); 15N-NMR δN (DMSO-d6, 40 MHz): 168.4 (2-N), 145.9 (1-N); 130.8 (4-N)); MS (70 eV) m/z: 299 (M+, 1%), 281(M+-H2O,18%), 222 (M+-·C6H5, 14%), 105 (M+- C8H8N3OS, 100%); Calcd. (%) for C15H13N3O2S: C, 60.19; H, 4.38; N, 14.04; S, 10.71. Found (%): C, 59.98; H, 4.18; N, 13.78; S, 10.51. All related spectra can be found in Supplementary Materials section.
5. Conclusions
The benzoylation and dibenzoylation of thiosemicarbazide (1), as well as the benzoylation of 1-benzoylthiosemicarbazide (2) with benzoyl chloride in DMF in the presence of pyridine or NaH, lead to mixtures of compounds, from which the two isomers, 1,2-dibenzoylthiosemicarbazide (3) and 1,4-dibenzoylthiosemicarbazide (4), have been identified, isolated, and characterized.
1,2-Dibenzoylthiosemicarbazide (3) is, surprisingly, a compound that has not been isolated to date, which is formed preferentially over its isomer, 1,4-dibenzoylthiosemicarbazide (4).
Supplementary Materials
The following supporting information can be downloaded. Figure S1. 1H NMR spectrum of the compound (2), Figure S2. 13C NMR spectrum of the compound (2), Figure S3. 13C DEPT135 spectrum of the compound (2), Figure S4. COSY 1H-1H spectrum of the compound (2), Figure S5. HSQC 1H-13C spectrum of the compound (2), Figure S6. HMBC 1H-13C spectrum of the compound (2), Figure S7. HMBC 1H-15N spectrum of the compound (2), Figure S8. HSQC 1H-15N spectrum of the compound (2), Figure S9. FT-IR spectrum of the compound (2), Figure S10. 1H NMR spectrum of the compound (3), Figure S11. 13C NMR spectrum of the compound (3), Figure S12. 13C DEPT135 spectrum of the compound (3), Figure S13. COSY 1H-1H spectrum of the compound (3), Figure S14. HMQC 1H-13C spectrum of the compound (3), Figure S15. HMBC 1H-15N spectrum of the compound (3), Figure S16. HMQC 1H-15N spectrum of the compound (3), Figure S17. FT-IR spectrum of the compound (3), Figure S18. MS spectrum of the compound (3), Figure S19. 1H NMR spectrum of the compound (4), Figure S20. 13C NMR spectrum of the compound (4), Figure S21. 13C DEPT135 spectrum of the compound (4), Figure S22. COSY 1H-1H spectrum of the compound (4), Figure S23. HMQC 1H-13C spectrum of the compound (4), Figure S24. HMBC 1H-13C spectrum of the compound (4), Figure S25. HMQC 1H-15N spectrum of the compound (4), Figure S26. FT-IR spectrum of the compound (4), Figure S27. MS spectrum of the compound (4).
Author Contributions
B.V. designed the experiments; B.I., T.A. and B.V.-N. performed the experiments; B.V. analyzed the spectral data; B.I. wrote the manuscript; B.V.-N provided supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Potts, K.T. The Chemistry of 1,2,4-Triazoles. Chem. Rev. 1961, 61, 87–127. [Google Scholar] [CrossRef]
- Shaker, R.M. The Chemistry of Mercapto- and Thione- Substituted 1,2,4-Triazoles and Their Utility in Heterocyclic Synthesis. Arkivoc 2006, 2006, 59–112. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Wang, Y. Recent Researches in Triazole Compounds as Medicinal Drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef] [PubMed]
- Küçükgüzel, Ş.G.; Çıkla-Süzgün, P. Recent Advances Bioactive 1,2,4-Triazole-3-Thiones. Eur. J. Med. Chem. 2015, 97, 830–870. [Google Scholar] [CrossRef] [PubMed]
- Ledeti, I.; Vlase, T.; Vlase, G.; Șuta, L.-M.; Fuliaș, A.; Belu, I. Synthesis and solid-state characterization of a cobalt(II) triazolic complex. Rev. Chim. 2014, 65, 896–902. [Google Scholar]
- Şişu, E.; Lascu, A.; Bercean, V.-N.; Căproiu, M.; Zamfir, A.; Şişu, I.; Neanu, C.; Csunderlik, C.; Rusu, V.; Katalinic, J.P. New glycosidic derivatives of 3-mercapto-5-p-tolyl-1,2,4-triazole. Rev. Chim. 2003, 54, 181–183. [Google Scholar]
- Bercean, V.-N.; Badea, V.; Șișu, E.; Bindilă, L.; Csunderlick, C. Simplified method for obtaining 3-aryl-5-mercapto-1,2,4-triazoles. Rev. Chim. 2003, 54, 368–369. [Google Scholar]
- Hassan, A.A.; El-Sheref, E.M.; Abou-Zied, A.H. Heterocyclization of Acylthiosemicarbazides. J. Heterocycl. Chem. 2012, 49, 38–58. [Google Scholar] [CrossRef]
- Mustafa, S.M.; Nair, V.A.; Chittoor, J.P.; Krishnapillai, S. Synthesis of 1, 2, 4-Triazoles and Thiazoles from Thiosemicarbazide and Its Derivatives. Mini-Rev. Org. 2004, 1, 375–385. [Google Scholar] [CrossRef]
- Hoggarth, E. 250. Compounds Related to Thiosemicarbazide. Part I. 3-Phenyl-1 : 2 : 4-Triazole Derivatives. J. Chem. Soc. 1949, 1160–1163. [Google Scholar] [CrossRef]
- Hoggarth, E. 251. Compounds Related to Thiosemicarbazide. Part II. 1-Benzoylthiosemicarbazides. J. Chem. Soc. 1949, 1163–1167. [Google Scholar] [CrossRef]
- Rivera, N.R.; Balsells, J.; Hansen, K.B. Synthesis of 2-Amino-5-Substituted-1,3,4-Oxadiazoles Using 1,3-Dibromo-5,5-Dimethylhydantoin as Oxidant. Tetrahedron Lett. 2006, 47, 4889–4891. [Google Scholar] [CrossRef]
- Creangă, A.-A. Synthesis, Study and Properties of Heterocyclic Mercaptans from Azole Class, Compounds with Potential Cytotoxic Effect. Ph.D. Thesis, University Politehnica of Timișoara, Timișoara, Romania, 2010. Available online: https://dspace.upt.ro/xmlui/handle/123456789/749 (accessed on 20 July 2023).
- Creangă, A.-A.; Bercean, V.-N.; Badea, V.; Ledeți, I.; Csunderlik, C. Benzoylation of Thiosemicarbazide. Chem. Bull. Politeh. Univ. Timis. 2008, 53, 45–48. [Google Scholar]
- Stollé, R.; Fehrenbach, K. Über Amino-Abkömmlinge von Thio- Und Furodiazolen-1,3,4. J. Prakt. Chem. 1929, 122, 289–318. [Google Scholar] [CrossRef]
- Badea, V.; Şofei, M.D.; Venter, M.M.; Bercean, V.N. Regioselective Alkylation of 1H-7-Ethoxycarbonyl-6-Methyl-3-Phenyl-Pyrazolo[5,1-c][1,2,4]Triazole and 1H-6-Methyl-3-Phenyl-Pyrazolo[5,1-c][1,2,4]Triazole. Tetrahedron 2007, 63, 1467–1473. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).