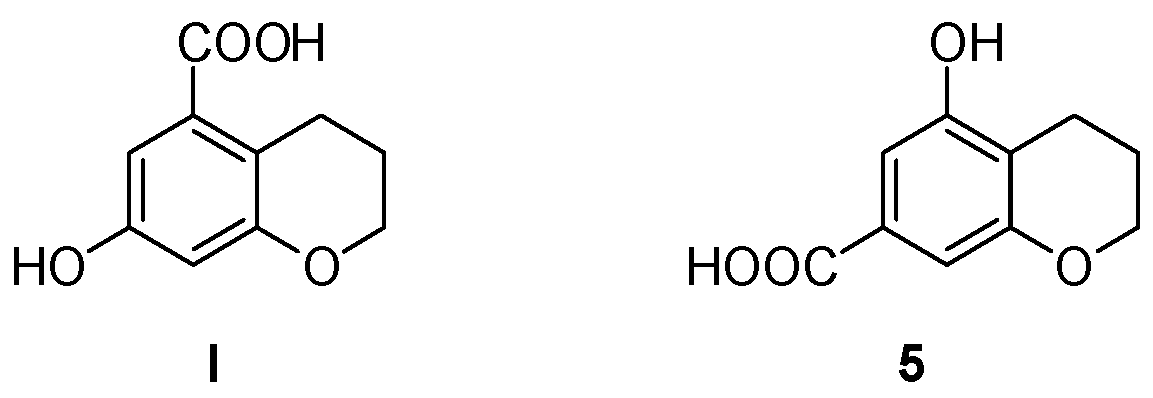Abstract
In the context of our ongoing studies on chromane derivatives as inhibitors of the salicylate synthase from M. tuberculosis, we isolated a new, unexpected compound from the cyclization of 3-(propargyloxy)-5-benzyloxy-benzoic acid methyl ester. Its molecular structure was elucidated by means of 1D and 2D NMR analyses, FT-IR, ESI-MS, and HRMS.
1. Introduction
Chromane is a bicyclic scaffold, ubiquitous in a wide variety of bioactive natural products and synthetic compounds exhibiting antitumor, anti-inflammatory, antiviral, antiprotozoal, and antimicrobial effects [1,2,3,4,5,6,7,8,9,10]. Among them, some have also shown moderate-to-good antitubercular activities [11,12,13,14,15,16,17,18].
As part of a project focusing on the design and synthesis of new inhibitors of the salicylate synthase MbtI from M. tuberculosis [19,20,21,22,23,24,25,26], we investigated several heterocyclic cores [27,28,29,30], including the chroman-4-one and chromane scaffolds [31,32]. Our studies led to the synthesis of a pool of derivatives, which were tested for their inhibitory effect towards this target, demonstrating promising activities [31,32].
With the aim of synthesizing 7-hydroxychroman-5-carboxylic acid I (Figure 1), we attempted the reduction of the corresponding 4-chromanone, following the approach used in our previous work [31]. However, this hydrogenation reaction, catalyzed by palladium on barium sulphate, was unsuccessful. The same outcome was obtained using different catalysts, including palladium on carbon (10%), or other reducing agents, such as zinc/acetic acid, hydrazine, and tert-butylamine–borane complex. Therefore, we developed a different strategy, which is discussed in the following paragraphs. This new approach led to the obtainment of an unexpected byproduct, which was isolated, characterized, and then used in the following steps to yield a new product (5).
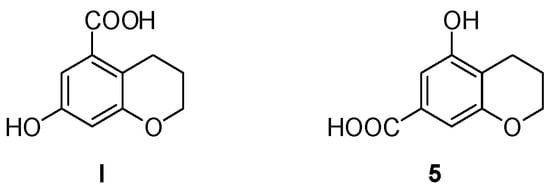
Figure 1.
The desired 7-hydroxychroman-5-carboxylic acid (I) and the unexpected 5-hydroxychroman-7-carboxylic acid (5), obtained from a byproduct of the new synthetic approach.
2. Results and Discussion
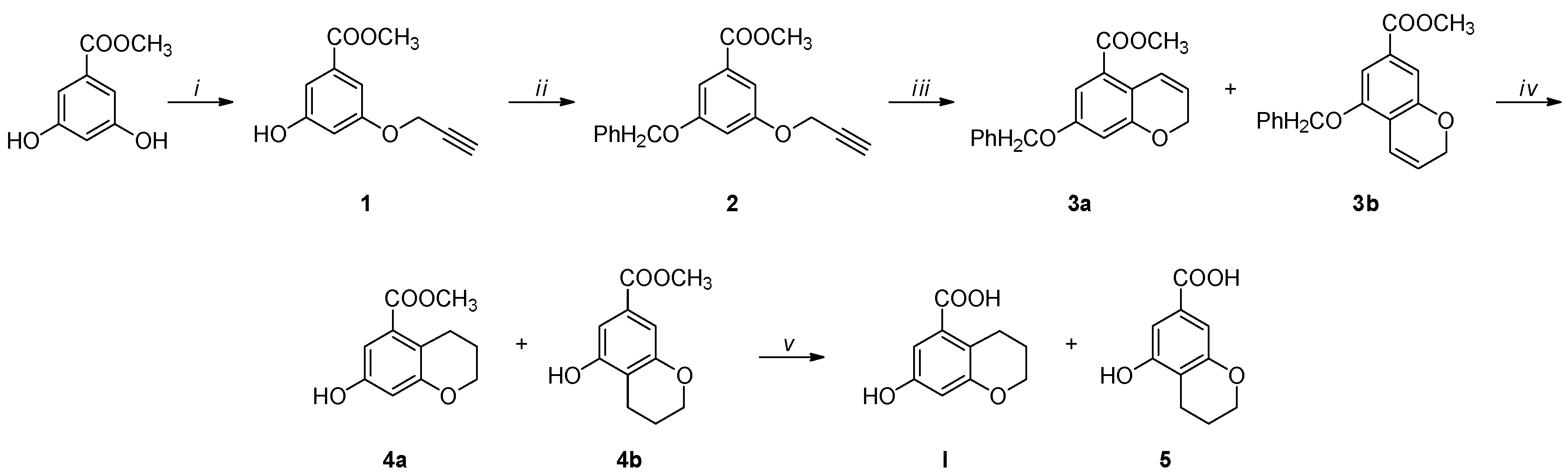
Considering our difficulties in obtaining the desired compound I by the same method developed for the previous derivatives [31], we implemented a new synthetic pathway, shown in Scheme 1.
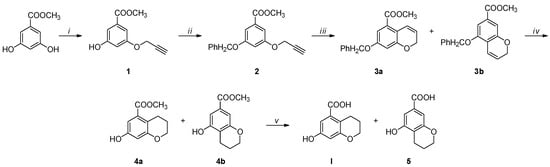
Scheme 1.
Reagents and conditions: (i) CH≡CCH2Br, K2CO3, 18-Crown-6, N2, DMF, 48 h, reflux; (ii) CH3OH, PPh3, DIAD, N2, 0 °C → RT, 24 h or PhCH2Br, K2CO3, N2, 4 h, reflux; (iii) DEA, N2, 24 h, 210 °C; (iv) 10% H2/Pd-C, MeOH, RT, 6 h; (v) NaOH, H2O/MeOH, 3 h, 58 °C.
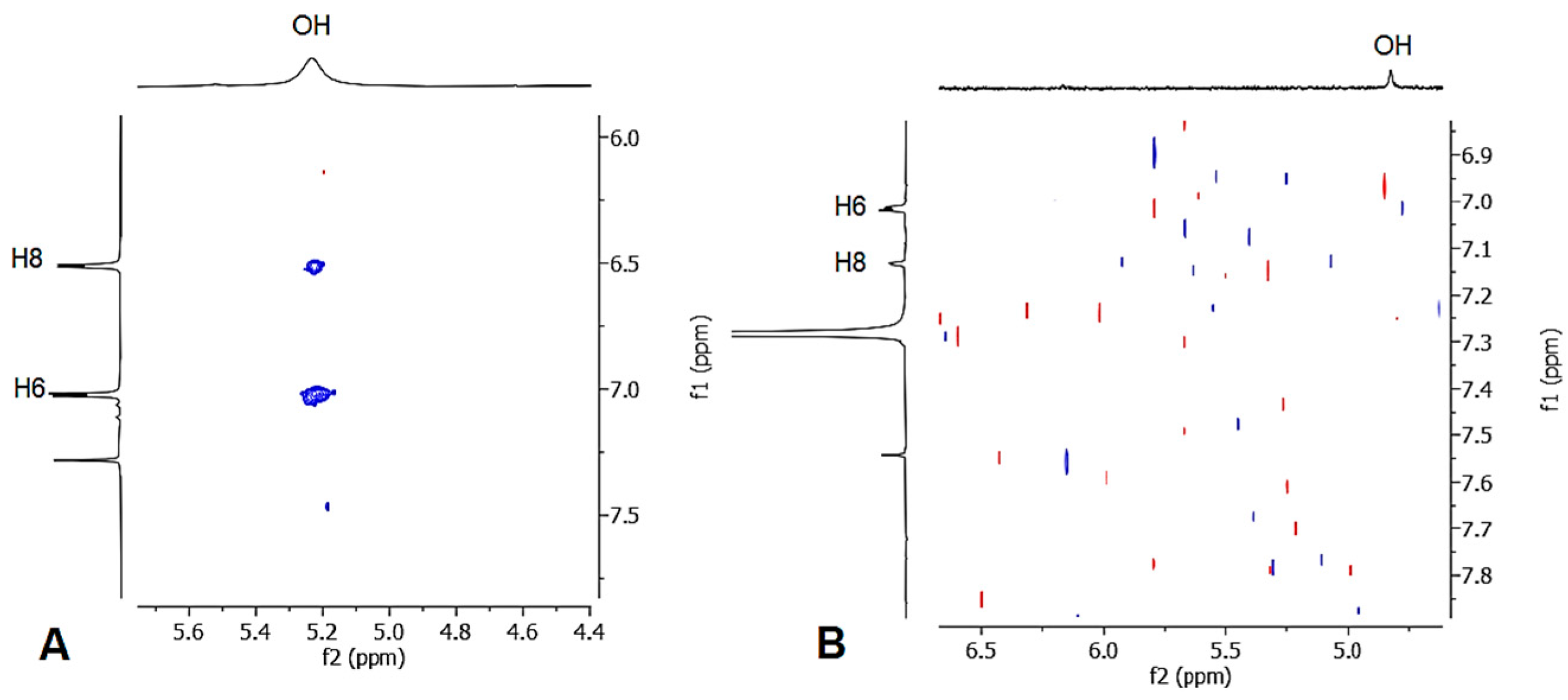
The mono-alkyl methyl benzoate 1 was obtained from the OH alkylation of 3,5-dihydroxymethylbenzoate using propargyl bromide, 18-crown-6, and anhydrous potassium carbonate in anhydrous dimethylformamide at 80 °C for 48 h. Compound 1 was purified from the 3,5 dialkyl derivative by column chromatography. Subsequently, the hydroxyl group was protected upon treatment with benzyl bromide, before being cyclized in N,N-diethyl aniline at 210 °C for 24 h, giving a mixture of compounds 3a and 3b. The hydrogenation of the mixture of the O-benzyl derivatives using 10% palladium on carbon afforded the simultaneous reduction of the double bond and the O-deprotection, giving a mixture of compounds 4a and 4b, which were easily separated by column chromatography. The obtained methyl esters were separately hydrolyzed to the corresponding carboxylic acids under basic conditions, using sodium hydroxide in a water–methanol mixture. The structure of compounds 4a and 4b was studied by mono- and bidimensional NMR techniques, ESI-MS, and FT-IR. NOESY experiments were carried out to unequivocally determine the hydroxyl chromane structures (see Supplementary Materials). The spectrum of compound 4a revealed a distinct correlation between the OH singlet and the two doublets of the aromatic hydrogens, whereas the spectrum of compound 4b displayed a weak correlation between the OH and only one of aromatic hydrogens (Figure 2). Finally, high-resolution mass spectrometry (HRMS) was employed to support the NMR and FT-IR analyses, unequivocally confirming the obtainment of the byproduct 5, hydrolyzed in basic conditions from 4b.
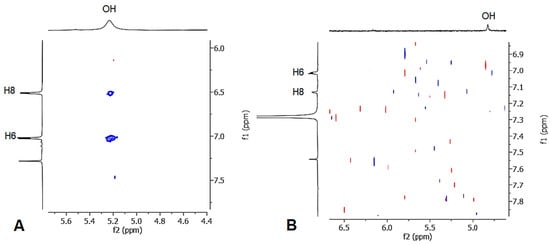
Figure 2.
NOESY spectra of compounds 4a (A) and 4b (B).
3. Materials and Methods
All reagents and solvents were purchased from Sigma-Aldrich/Merck (Merck KGaA, Darmstadt, Germany). Reactions involving air-sensitive reagents were carried out using anhydrous solvents, in oven-dried glassware, and under nitrogen atmosphere. The reactions were monitored by TLC analysis on Silica Gel Matrix plates (0.25 nm; Merck), which were visualized under a UV lamp operating at a wavelength of 254 or 365 nm. When necessary, the spots were evidenced using an ethanolic KMnO4 solution. Melting points were recorded on a Büchi apparatus (Büchi, Flawil, Switzerland) and are uncorrected.
Mono- and bidimensional NMR spectra were recorded at room temperature on a Varian-Mercury Oxford 300 cryomagnet (Oxford Instruments, Abingdon, UK), operating at 300 MHz for 1H and 75 MHz for 13C, or on a Bruker Avance 500 (Billerica, MA, USA) instrument, operating at 500 MHz for 1H and 125 MHz for 13C. Depending on the solubility of the compound, CDCl3 or DMSO-d6 were used as deuterated solvents for all spectra run. Chemicals shifts are expressed in ppm (δ) from tetramethylsilane resonance in the indicated solvents; coupling constants (J-values) are given in Hertz (Hz). 1H signals are reported in the following order: ppm, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet), number of protons, and assignments. The APT sequence was used to distinguish methyl and methine signals from those due to methylene and quaternary carbons.
FT-IR spectra were recorded on a SPECTRUM ONE (PerkinElmer, Waltham, MA, USA) instrument, using the DATA MANAGER v.2 software (Perkin Elmer), between 4000 and 600 cm−1 (liquid samples) or 450 cm−1 (solid samples) performing 8 scans at a resolution of 4 cm−1. Liquid samples were deposited on NaCl plates, while solids were mixed in a 1:100 w/w ratio with KBr and pressed through a hydraulic press (14 tons) to small tablets.
Mass spectrometry analyses were carried out on a LCQ Advantage (ThermoFisher Scientific, Waltham, MA, USA), equipped with an ESI electrospray ionization source and an Ion Trap mass analyzer; ionization: ESI positive or ESI negative; capillary temperature: 250 °C; source voltage: 5.50 kV; source current: 4.00 μA; multipole 1 and 2 offset, −5.50 V and −7.50 V, respectively; intermultipole lens voltage: −16.00 V; trap DC offset voltage: −10.00 V. The high-resolution mass spectrometry (HRMS) analysis was carried out on a Q-ToF Synapt G2-Si HDMS system (Waters, Milford, MA, USA).
- Synthesis of 3-propargyloxy-5-hydroxy benzoic acid methyl ester (1)
Under a nitrogen flow, 3,5-dihydroxybenzoate (3 g, 17.86 mmol), propargyl bromide (1.70 g, 14.29 mmol), and 18-crown-6 (0.38 g, 1.43 mmol) were dissolved in dry DMF (94 mL). Anhydrous K2CO3 (5.43 g, 39.29 mmol) was added, and the reaction was refluxed at 80 °C for 48 h. The mixture was then cooled to room temperature, filtered, and the filtrate was evaporated under vacuum. The crude product was purified by column chromatography using a 4:1 mixture of petroleum ether/EtOAc as the eluent. Yield: 30%. TLC (petroleum ether/EtOAc 8:2): Rf = 0.28. Ivory-colored solid. m.p.: 123–127 °C. FT-IR (KBr): ν 3425, 3294, 3282, 2986, 2929, 2876, 2850, 1714, 1605, 1626, 1600, 1496, 1453, 1434, 1376, 1154 cm−1. 1H NMR (300 MHz, DMSO): δ 9.91 (s exch D2O, 1H, OH), 6.99 (dd, J = 2.1, 1.3 Hz, 1H, H2), 6.97 (d, J = 2.2, J = 1.3 Hz, 1H, H6), 6.62 (t, J = 2.2 Hz, 1H, H4), 4.77 (d, J = 2.4 Hz, 2 H, CH2), 3.80 (s, 3H, CH3), 3.57 (t, J = 2.4 Hz, 1H, CH) ppm. 13C NMR (75 MHz, DMSO): δ 166.4, 159.0, 158.8, 131.9, 109.8, 107.5, 106.6, 79.5, 78.9, 56.1, 52.6 ppm. MS (ESI): m/z calcd for C11H10O4 206.06, found 205.07 [M − H]−.
- Synthesis of 3-propargyloxy-5-benzyloxy-benzoic acid methyl ester (2)
To a solution of compound 1 (300 mg, 1.46 mmol) in anhydrous acetone (6 mL), K2CO3 (500 mg, 3.64 mmol) was added under a nitrogen flow. Benzyl bromide (0.27 mg, 1.6 mmol) was dripped, and the reaction mixture heated at 55 °C for 4 h. After cooling to room temperature, the mixture was filtered and evaporated in vacuum, and the crude residue was purified by column chromatography using hexane/EtOAc 8:2. Yield: 42%. TLC (hexane/EtOAc 8:2): Rf = 0.31. Brown solid. m.p.: 123–127 °C. FT-IR (KBr): ν 3294, 3278, 3066, 3009, 2949, 2922, 2871, 2843, 1716, 1605, 1475, 1453, 1442, 1384, 1324, 1236, 1161, 1057 cm−1. 1H NMR (300 MHz, DMSO): δ 7.45–7.24 (m, 7H, Harom, H2, H6), 6.80 (t, J = 2.4 Hz, 1H, H4), 5.08 (s, 2 H, CH2Ph), 4.70 (d, J = 2.4 Hz, 2H, H2), 3.57 (t, J = 2.4 Hz, 1H, CH) ppm. 13C NMR (75 MHz, DMSO): δ 166.6, 159.8, 158.6, 136.4, 132.1, 128.6, 128.1, 127.5, 109.0, 108.4, 107.4, 75.8, 70.4, 56.1, 52.3 ppm. MS (ESI): m/z calcd for C18H16O4 296.32, found 295.56 [M − H]−.
- Synthesis of methyl 7-(benzyloxy)-2H-chromene-5-carboxylate (3a) and methyl 5-(benzyloxy)-2H-chromene-7-carboxylate (3b)
Under a nitrogen flow, a solution of compound 2 (180 mg, 0.618 mmol) in N,N-diethylaniline (2.5 mL, 16.83 mmol) was heated at 210 °C for 24 h. After cooling, the mixture was diluted with diethyl ether (5 mL) and washed (4 × 10 mL) with aq. HCl (5%) and brine. The organic phase was dried over Na2SO4 and filtered. The crude residue obtained by evaporation in vacuo was purified by column chromatography using hexane/EtOAc 9:1, affording a mixture of compounds 3a and 3b. Light-yellow oil. Yield: 56%. TLC (hexane/EtOAc 8:2): Rf= 0.35. FT-IR (KBr): ν 3090, 3066, 3033, 2952, 2918, 2849, 1721, 1609, 1585, 1497, 1454, 1435, 1375, 1302, 1238, 1150, 1029 cm−1. 1H NMR (300 MHz, CDCl3): δ 7.45–7.32 (m, 11H, Harom, H4-3b), 7.22 (m, 2H, H6-3b), 7.13 (d, J = 1.3 Hz, 1H, H6-3a), 6.84 (d, J = 6.7 Hz, 1H, H4-3a), 6.62 (d, J = 1.3 Hz, 1H, H8-3a), 5.86–5.76 (m, 2H, H3-3a, H3-3b), 5.11 (s, 2H, CH2Ph-3b), 5.05 (s, 2H, CH2Ph-3a), 4.82 (m, 2H, H2-3b), 4.82 (m, 2H, H2-3a), 3.88 (s, 6H, CH3) ppm. MS (ESI): m/z calcd for C18H16O4 296.10, found 327.80 [M + CH3OH − H]−.
- Synthesis of methyl 7-hydroxychromane-5-carboxylate (4a) and methyl 5-hydroxychromane-7-carboxylate (4b)
A solution of the mixture of 3a and 3b (100 mg, 0.339 mmol) in dry methanol (2.8 mL) was reduced with hydrogen under atmospheric pressure and room temperature over 10% Pd/C (18 mg, 0.0017) for 6 h. The catalyst was filtered off on a celite pad, and the solvent was evaporated under vacuum. The crude residue was purified by column chromatography using cyclohexane/isopropanol 9:1, affording 4a as a pale-yellow oil, yield 30%, and 4b as a pale-yellow oil, yield 40%. TLC (cyclohexane/isopropanol 9:1): Rf for 4a = 0.30 and Rf for 4b = 0.24. FT-IR for 4a (KBr): ν 3395, 2952, 2877, 2843, 1716, 1699, 1615, 1589, 1470, 1453, 1436, 1385, 1313, 1271, 1229, 1142, 1073 cm−1. 1H NMR (300 MHz, CDCl3) for 4a: δ 7.00 (d, J = 2.7 Hz, 1H, H6), 6.49 (d, J = 2.7 Hz, 1H, H8), 5.09 (broad s exch D2O, 1H, OH), 4.20–4.15 (m, 2H, H2), 3.87 (s, 3H, CH3), 2.71 (t, J = 6.6 Hz, 2H, H4), 2.05–1.84 (m, 2H, H3) ppm. 13C NMR (75 MHz, CDCl3) 4a: δ 167.5, 156.4, 154.0, 131.1, 116.8, 110.3, 107.8, 66.2, 51.9, 23.4, 22.3 ppm. FT-IR for 4b (KBr): ν 3433, 3353, 2947, 2870, 2845, 1693, 1615, 1586, 1467, 1435, 1423, 1383, 1308, 1269, 1228, 1142, 1073 cm−1. 1H NMR (300 MHz, CDCl3) for 4b: δ 7.09 (d, J = 1.8 Hz, 1H, H8), 7.08 (d, J = 1.8 Hz, 1H, H6), 5.45 (broad s exch D2O, 1H, OH), 4.28–3.99 (m, 2H, H2), 3.87 (s, 3H, CH3), 3.00 (t, J = 6.6 Hz, 2H, H4), 2.05–1.97 (m, 2H, H3) ppm. 13C NMR (75 MHz, CDCl3) for 4b: δ 166.8, 156,4, 154.1, 128.5, 115.3, 110.8, 107.3, 66.2, 52.1, 21.4, 19.4 ppm.
- Synthesis of 7-hydroxychromane-5-carboxylic acid (I)
A solution of powdered NaOH (6 mg, 0.142 mmol) in a mixture of water (1 mL) and methanol (0.4 mL) was added to compound 4a (10 mg, 0.048 mmol) and stirred at 55 °C for 3 h. After the evaporation of methanol under reduced pressure, the pH of the solution was adjusted to pH 3–4, by the addiction of 1 M HCl, and the precipitate was recovered by filtration. Yield: 65%. Light-brown solid. TLC (hexane/EtOAc 1:1): Rf = 0.13. FT-IR (KBr): ν 3362, 3070, 2918, 2849, 1688, 1615, 1585, 1489, 1457, 1427, 1384, 1354, 1306, 1275, 1241, 1138 cm−1. 1H NMR (300 MHz, DMSO): δ 12.70 (broad s exch D2O, 1H, COOH), 9.42 (broad s exch D2O, 1H, OH), 6.79 (d, J = 2.6 Hz, 1H, H6), 6.30 (d, J = 2.6 Hz, 1H, H8), 4.04 (t, J = 4.08 Hz, 2H, H2), 2.83 (t, J = 6.5 Hz, 2H, H4), 1.86–1.78 (m, 2H, H3) ppm. 13C NMR (125.75 MHz, DMSO): δ 168.8, 156.3, 156.0, 114.5, 110. 3, 108.8, 107.1, 66.0, 23.3, 22.4 ppm. MS (ESI): m/z calcd for C10H10O4 194.06, found 193.19 [M − H]−.
- Synthesis of 5-hydroxychromane-7-carboxylic acid (5)
A solution of powdered NaOH (12 mg, 0.284 mmol) in a mixture of water (1 mL) and methanol (0.4 mL) was added to compound 4b (20 mg, 0.096 mmol) and stirred at 55 °C for 3 h. After the evaporation of methanol under reduced pressure, the pH of the solution was adjusted to pH 3–4 by the addition of 1 M HCl, and the precipitate was recovered by filtration. Yield: 77%. Light-brown solid. TLC (hexane/EtOAc 1:1): Rf = 0.14. FT-IR (KBr): ν 3396, 3206, 3077, 2949, 2927, 2871, 2855, 1682, 1614, 1584, 1512, 1424, 1387, 1348, 1307, 1269, 1144, 1072, 988 cm−1. 1H NMR (300 MHz, DMSO): δ 12.62 (broad s exch D2O, 1H, COOH), 9.72 (broad s exch D2O, 1H, OH), 6.89 (s, 1H, H6), 6.72 (s, 1H, H8), 4.04 (t, J = 4.09 Hz, 2H, H2), 2.54 (t, J = 6.4 Hz, 2H, H4), 1.90–1.82 (m, 2H, H3) ppm. 13C NMR (75 MHz, DMSO-d6): δ 167.7, 156.2, 155.8, 129.7, 115.5, 108.8, 107.3, 66.0, 21.5, 19.7 ppm. HRMS (ESI/Q-ToF): m/z calcd for [C10H10O4 − H]− 193.0501, found 193.0502.
4. Conclusions
Methyl 5-(benzyloxy)-2H-chromene-7-carboxylate was obtained as a side product from the cyclization of 3-(propargyloxy)-5-benzyloxy-benzoic acid methyl ester. After simultaneous deprotection of the hydroxyl group and double-bond reduction, the purified compounds were characterized by spectroscopic methods (FT-IR, 1H and 13C NMR, NOESY, and HSQC). HRMS analysis of the corresponding carboxylic acid was also performed to definitively confirm its identity.
Supplementary Materials
The following are available online, 1H NMR, 13C NMR, FT-IR, ESI-MS spectra of all compounds, H-H NOESY NMR spectra of compounds 4a and 4b, and HRMS of compound 5.
Author Contributions
Conceptualization of the work: E.P.; synthesis: M.M. and G.C.; analysis and analytical study: E.P. and D.N.; writing—original draft preparation, review, and editing: M.M. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the University of Milan (Linea B).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
High-resolution mass spectrometry analyses were performed at the Mass Spectrometry facility of the Unitech COSPECT at the University of Milan (Italy).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ellis, G.P. (Ed.) Chemistry of Heterocyclic Compounds; Chemistry of Heterocyclic Compounds: A Series of Monographs; Wiley: Hoboken, NJ, USA, 1977; Volume 31, ISBN 9780471382126. [Google Scholar]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The combinatorial synthesis of bicyclic privileged structures or privileged substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef]
- Jadhav, S.R.; Karan Kumar, B.; Joshi, R.P.; Suryakant, C.K.; Chandu, A.; Muzaffar-Ur-Rehman, M.; Khetmalis, Y.M.; Nandikolla, A.; Murugesan, S.; Chandra Sekhar, K.V.G. Retrospective Review of Chromane Analogues as Anti-protozoal Leads: A Decade’s Worth of Evolution. Curr. Top. Med. Chem. 2023, 23, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Emami, S.; Ghanbarimasir, Z. Recent advances of chroman-4-one derivatives: Synthetic approaches and bioactivities. Eur. J. Med. Chem. 2015, 93, 539–563. [Google Scholar] [CrossRef] [PubMed]
- Ghatpande, N.G.; Jadhav, J.S.; Kaproormath, R.V.; Soliman, M.E.; Shaikh, M.M. A brief overview on recent advances in spiro[chromane-2,4′-piperidine]-4(3H)-one-functionalized compounds in medicinal chemistry research. Bioorg. Med. Chem. 2020, 28, 115813. [Google Scholar] [CrossRef]
- Otgon, O.; Nadmid, S.; Paetz, C.; Dahse, H.M.; Voigt, K.; Bartram, S.; Boland, W.; Dagvadorj, E. Chromane Derivatives from Underground Parts of Iris tenuifolia and Their In Vitro Antimicrobial, Cytotoxicity and Antiproliferative Evaluation. Molecules 2021, 26, 6705. [Google Scholar] [CrossRef]
- Pecio, Ł.; Pecio, S.; Mroczek, T.; Oleszek, W. Spiro-Flavonoids in Nature: A Critical Review of Structural Diversity and Bioactivity. Molecules 2023, 28, 5420. [Google Scholar] [CrossRef]
- Bridi, H.; de Carvalho Meirelles, G.; von Poser, G.L. Structural diversity and biological activities of phloroglucinol derivatives from Hypericum species. Phytochemistry 2018, 155, 203–232. [Google Scholar] [CrossRef] [PubMed]
- Fridén-Saxin, M.; Seifert, T.; Landergren, M.R.; Suuronen, T.; Lahtela-Kakkonen, M.; Jarho, E.M.; Luthman, K. Synthesis and evaluation of substituted chroman-4-one and chromone derivatives as sirtuin 2-selective inhibitors. J. Med. Chem. 2012, 55, 7104–7113. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Zhou, Y.; Wang, Z.; Wang, J.; Wang, M. Asymmetric synthesis of chiral (thio)chromanes and exploration on their structure–activity relationship in macrophages. RSC Adv. 2023, 13, 30391–30400. [Google Scholar] [CrossRef]
- Simelane, S.B.; Moshapo, P.T.; Masuka, R.W. Organic and Medicinal Chemistry Benzopyran-Core as an Antimycobacterial Agent. Org. Med. Chem. Int. J. 2020, 10, 56–72. [Google Scholar]
- Mujahid, M.; Gonnade, R.G.; Yogeeswari, P.; Sriram, D.; Muthukrishnan, M. Synthesis and antitubercular activity of amino alcohol fused spirochromone conjugates. Bioorg. Med. Chem. Lett. 2013, 23, 1416–1419. [Google Scholar] [CrossRef]
- Chitti, S.; Nandikolla, A.; Khetmalis, Y.M.; Van Calster, K.; Kumar, B.V.S.; Kumar, B.K.; Murugesan, S.; Cappoen, D.; Sekhar, K.V.G.C. Design, Synthesis and Biological Evaluation of Novel Spiro-[Chroman-2,4′-Piperidin]-4-One Analogs as Anti-Tubercular Agents. Chem. Biodivers. 2022, 19, e202200304. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Peng, C.F.; Chen, I.S.; Tsai, I.L. Antitubercular chromones and flavonoids from Pisonia aculeata. J. Nat. Prod. 2011, 74, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Dongamanti, A.; Aamate, V.K.; Devulapally, M.G.; Gundu, S.; Balabadra, S.; Manga, V.; Yogeeswari, P.; Sriram, D.; Balasubramanian, S. Bis-spirochromanones as potent inhibitors of Mycobacterium tuberculosis: Synthesis and biological evaluation. Mol. Divers. 2017, 21, 999–1010. [Google Scholar] [CrossRef]
- Kapadiya, K.M.; Jadeja, Y.S.; Banik, A.; Khunt, R.C. In Silico and in Vitro Studies of Fluorinated Chroman-2-Carboxilic Acid Derivatives as an Anti-tubercular Agent. Folia Med. 2018, 60, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Sadorn, K.; Saepua, S.; Boonyuen, N.; Komwijit, S.; Rachtawee, P.; Pittayakhajonwut, P. Phenolic glucosides and chromane analogs from the insect fungus Conoideocrella krungchingensis BCC53666. Tetrahedron 2019, 75, 3463–3471. [Google Scholar] [CrossRef]
- Mujeeb, S.; Singh, K.; Yogi, B.; Kumar, P.; Kumar, P. Design, Synthesis and Anti-tubercular Evaluation of Some Novel Chroman-Hydrazone Derivatives. Asian J. Chem. 2023, 35, 483–492. [Google Scholar] [CrossRef]
- Gomes, A.C.; Moreira, A.C.; Mesquita, G.; Gomes, M.S. Modulation of Iron Metabolism in Response to Infection: Twists for All Tastes. Pharmaceuticals 2018, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hendrickson, R.C.; Meikle, V.; Lefkowitz, E.J.; Ioerger, T.R.; Niederweis, M. Comprehensive analysis of iron utilization by Mycobacterium tuberculosis. PLoS Pathog. 2020, 16, e1008337. [Google Scholar] [CrossRef]
- Hameed, S.; Pal, R.; Fatima, Z. Iron Acquisition Mechanisms: Promising Target Against Mycobacterium tuberculosis. Open Microbiol. J. 2015, 9, 91–97. [Google Scholar] [CrossRef]
- Chao, A.; Sieminski, P.J.; Owens, C.P.; Goulding, C.W. Iron acquisition in Mycobacterium tuberculosis. Chem. Rev. 2019, 119, 1193–1220. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, M.; Koduru, T.N.; Kumar, N.; Salimi, S.; Desai, K.; Prabhu, N.P.; Sritharan, M. Iron uptake and transport by the carboxymycobactin-mycobactin siderophore machinery of Mycobacterium tuberculosis is dependent on the iron-regulated protein HupB. BioMetals 2021, 34, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Shyam, M.; Shilkar, D.; Verma, H.; Dev, A.; Sinha, B.N.; Brucoli, F.; Bhakta, S.; Jayaprakash, V. The Mycobactin Biosynthesis Pathway: A Prospective Therapeutic Target in the Battle against Tuberculosis. J. Med. Chem. 2021, 64, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Shyam, M.; Shilkar, D.; Rakshit, G.; Jayaprakash, V. Approaches for targeting the mycobactin biosynthesis pathway for novel anti-tubercular drug discovery: Where we stand. Expert Opin. Drug Discov. 2022, 17, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Adhikrao, P.A. Targeting Mycobacterium tuberculosis iron-scavenging tools: A recent update on siderophores inhibitors. RSC Med. Chem. 2023, 14, 1885–1913. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Stelitano, G.; Gelain, A.; Pini, E.; Chiarelli, L.R.; Sammartino, J.C.; Poli, G.; Tuccinardi, T.; Beretta, G.; Porta, A.; et al. Shedding X-ray light on the role of magnesium in the activity of M. tuberculosis salicylate synthase (MbtI) for drug design. J. Med. Chem. 2020, 63, 7066–7080. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Stelitano, G.; Chiarelli, L.R.; Cazzaniga, G.; Gelain, A.; Barlocco, D.; Pini, E.; Meneghetti, F.; Villa, S. Synthesis, Characterization, and Biological Evaluation of New Derivatives Targeting MbtI as Antitubercular Agents. Pharmaceuticals 2021, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Stelitano, G.; Griego, A.; Chiarelli, L.R.; Cazzaniga, G.; Gelain, A.; Pini, E.; Camera, M.; Canzano, P.; Fumagalli, A.; et al. Synthesis and assessment of the in vitro and ex vivo activity of salicylate synthase (MbtI) inhibitors as new candidates for the treatment of mycobacterial infections. Pharmaceuticals 2022, 15, 992. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Villa, S.; Chiarelli, L.R.; Meneghetti, F.; Bellinzoni, M. Structural Study of a New MbtI-Inhibitor Complex: Towards an Optimized Model for Structure-Based Drug Discovery. Pharmaceuticals 2023, 16, 1559. [Google Scholar] [CrossRef]
- Pini, E.; Poli, G.; Tuccinardi, T.; Chiarelli, L.; Mori, M.; Gelain, A.; Costantino, L.; Villa, S.; Meneghetti, F.; Barlocco, D. New Chromane-Based Derivatives as Inhibitors of Mycobacterium tuberculosis Salicylate Synthase (MbtI): Preliminary Biological Evaluation and Molecular Modeling Studies. Molecules 2018, 23, 1506. [Google Scholar] [CrossRef]
- Mori, M.; Meneghetti, F.; Chiarelli, L.R.; Diego, A.; Nava, D.; Gelain, A.; Cazzaniga, G.; Villa, S.; Pini, E. 6-Hydroxy-2-methylbenzofuran-4-carboxylic Acid. Molbank 2020, 2020, M1143. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).