Abstract
Viologens, i.e., quaternary 4,4′-bipyridinum salts, are a well-known class of functional organic compounds that have attracted in the past few decades a great deal of attention for their peculiar chemical and electrochemical properties and have therefore found numerous applications ranging from herbicides to electrochromic devices. In this paper, the synthesis and characterization of a novel viologen derivative are described. In the reported compound, the pyridinium nitrogen atoms have been quaternarized with the benzyl group and an additional unsaturated moiety, namely a 9,10-diethynylanthracene core, has been inserted between the charged pyridinium rings to extend the conjugation. Characterization by means of absorbance and diffuse reflectance UV–visible spectroscopy suggested intriguing optical and electronic properties, making this extended viologen a potential candidate for different optoelectronic applications.
1. Introduction
Viologens are an important class of conjugated compounds featuring a diquaternized-4,4′-bipyridinium salt system. Despite the well-known biological herbicide activity of their prototypical compound, that is, paraquat (N,N′-dimethyl-4,4′-bipyridinium dichloride) [1,2,3], viologens have been showing to possess a variety of interesting features [4], ranging from their redox behavior, owing to the existence of three stable and reversible redox states, leading to electrochromism [5,6], and to the ability of the formation of charge–transfer complexes [7,8]. For these reasons, they have found general applications in electrochromic and memory devices [9,10], as well as in light emitting devices [11], solar cells [12] and energy storage systems [13], among others.
In recent decades, several approaches aimed at successfully modulating such properties through the extension of the conjugated part placed between the two positively charged pyridinium rings were reported. Multiple carbon–carbon bonds [14,15], as well as carbon–heteroatom bonds [16] or diazo groups [17], in addition to aromatic and heteroaromatic rings [18,19,20], were used for this purpose. Moreover, a variety of multiarmed quaternary pyridinium salts have also been reported, since they were found to retain the peculiar properties of the systems originally defined as viologens [21,22].
In the last few years, our group focused on the synthesis of extended dimethyl viologens, bearing 1,4-diethynylaryl or 1,3,5-triethynylaryl moieties as the additional conjugated core [23,24,25]. Such compounds were tailored to be used as organic cations in the making of hybrid perovskites with potential applications in solar cells and light-emitting devices and, consistently with our expectations, they showed very interesting optoelectronic properties. With the same purpose, we also tested as a comparison an extended methylviologen bearing a 9,10-diethynylanthracene central subunit, already reported in the literature [26]. The performances achieved by this compound in the optoelectronic studies performed were excellent [27,28], and this encouraged our group to prepare a viologen with an analogous structure containing the benzyl groups in lieu of the methyl ones. Our aim was to verify if the change in the polarity caused by the introduction of the benzylic functions involved significant variations in the procedures for the attainment and purification of the product. Furthermore, the introduction of the benzyl group onto the pyridyl rings could be considered as the preliminary approach to a wider class of viologen derivatives, due to the high availability of many differently substituted benzyl halides, thus offering new possibilities of functionalization. Finally, we were interested in the characterization of the optical properties of this new extended viologen.
2. Results and Discussion
The synthetic route followed for the preparation of the target compound 4, shown in Scheme 1, was very similar to that already employed for the methylated analogous described in [27]. In particular, two of its four steps were constituted by palladium–copper-catalyzed Sonogashira cross-coupling reactions, necessary to link the aromatic moieties making up the extended viologen, namely the anthracene core and the pyridyl rings, to the acetylenic spacers. Differently from the compounds described in [23,24,25], the already reported introduction of the triple bonds on the pyridine rings as the first step [29], rather than on the central subunit, confirmed the former as a better yielding approach.
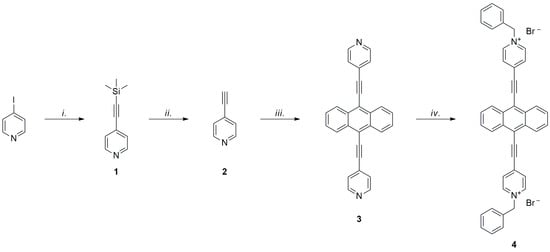
Scheme 1.
Synthesis of compound 4; i. PdCl2(PPh3)2 (10 mol%), CuI (5 mol%), TMSA, THF/i-Pr2NH, 80 °C, 24 h (89%); ii. K2CO3, MeOH, r.t., 3 h (quantitative); iii. 9,10-dibromoanthracene (0.5 eq.), PdCl2(PPh3)2 (20 mol%), CuI (10 mol%), THF/i-Pr2NH, 80 °C, 24 h (35%); iv. benzyl bromide, CH2Cl2, 40 °C, 48 h (90%).
Thus, the synthesis of the target extended viologen started with the coupling between 4-iodopyridine and trimethylsilylacetylene in a 3:1 mixture of tetrahydrofuran and diisopropylamine, using PdCl2(PPh3)2 and CuI as catalysts. This Sonogashira reaction afforded the desired product 1 as a light-brown oil in an 89% yield after chromatographic purification. Compound 1 was then converted to the corresponding terminal alkyne by the removal of the trimethylsilyl protecting group in basic conditions; therefore, it was treated with K2CO3 in methanol at room temperature, yielding 4-ethynylpyridine 2, a light-sensitive and volatile white solid, which was used without further purification for the subsequent palladium–copper-catalyzed coupling with the anthracene moiety. This was accomplished in non-optimized conditions, similar to those already described in the first step, by combining two equivalents of compound 2 with 9,10-dibromoanthracene, which led to the formation of the target bipyridyl derivative 3, obtained in a 35% yield as an orange solid. Finally, the nitrogen atoms of product 3 were quaternized to yield the desired extended viologen by stirring this compound in refluxing CH2Cl2 with an excess of benzyl bromide. Since the protocol was the same as already described for the structurally analogous dimethyl derivative, we were able to compare the results in terms of the yield and ease of purification of products. The bipyridinium salt 4 was obtained as a purple solid with a 90% yield. The decrease in polarity did not afford significant changes in the scarce solubility of salt 4 in dichloromethane, making once again its purification possible by filtration. The identification of the product and purity check were carried out by a 1H- and 13C-NMR analysis in DMSO-d6.
Owing to the symmetric structure of the dication of compound 4, the signal assignment of the monodimensional NMR spectra could be readily accomplished. In the 1H-NMR spectrum (Figure S7), a singlet, attributable to the four methylene protons of the two benzyl groups, deshielded due to the proximity of the charged nitrogen atoms, is visible at δ 5.90, while the aromatic protons of these substituents result in two distinct multiplets at around δ 7.48 and 7.59. As regards the protons on the anthracene core, giving rise to a second-order AA’XX’ spin system, their signals are visible as two centrosymmetric multiplets at δ 7.91 and 8.81 for the hydrogen atoms in position 1 and 2, respectively. Finally, the two multiplets at δ 8.70 and 9.35 are ascribed to the protons on the pyridinium rings, with the most deshielded signals attributed to those adjacent to the positive nitrogen. Notably, also these nuclei originate in an AA’XX’ spin system being chemically but not magnetically equivalent.
As far as the 13C-NMR spectrum (Figure S8) of compound 4 is concerned, the methylene carbons of the benzyl substituents are responsible for the signal at δ 63.3, the quaternary carbons of the benzene rings give a signal at δ 134.3, while the peaks at δ 127.0, 128.9 and 129.4 can be attributed to the remaining positions on these rings. The carbon atoms of the acetylene spacers can be readily recognized as signals at δ 97.9 and 99.1, while the peak at δ 117.8 can be ascribed to the quaternary anthracene carbons directly linked to the sp ones, and the other signals attributable to the anthracene carbons are those at δ 132.2, 129.5 and 129.3. Finally, concerning the signals of the pyridinium rings, the quaternary carbons produce a peak at δ 138.6, while those in position 2 and 3, respectively, give rise to peaks at δ 144.9 and 130.2.
In addition to the spectroscopic characterization necessary for the confirmation of its structure, the optical and electronic properties of the extended viologen 4 were investigated by means of UV–visible spectra, which were performed both in solution and in the solid state, therefore by diffuse reflectance spectroscopy.
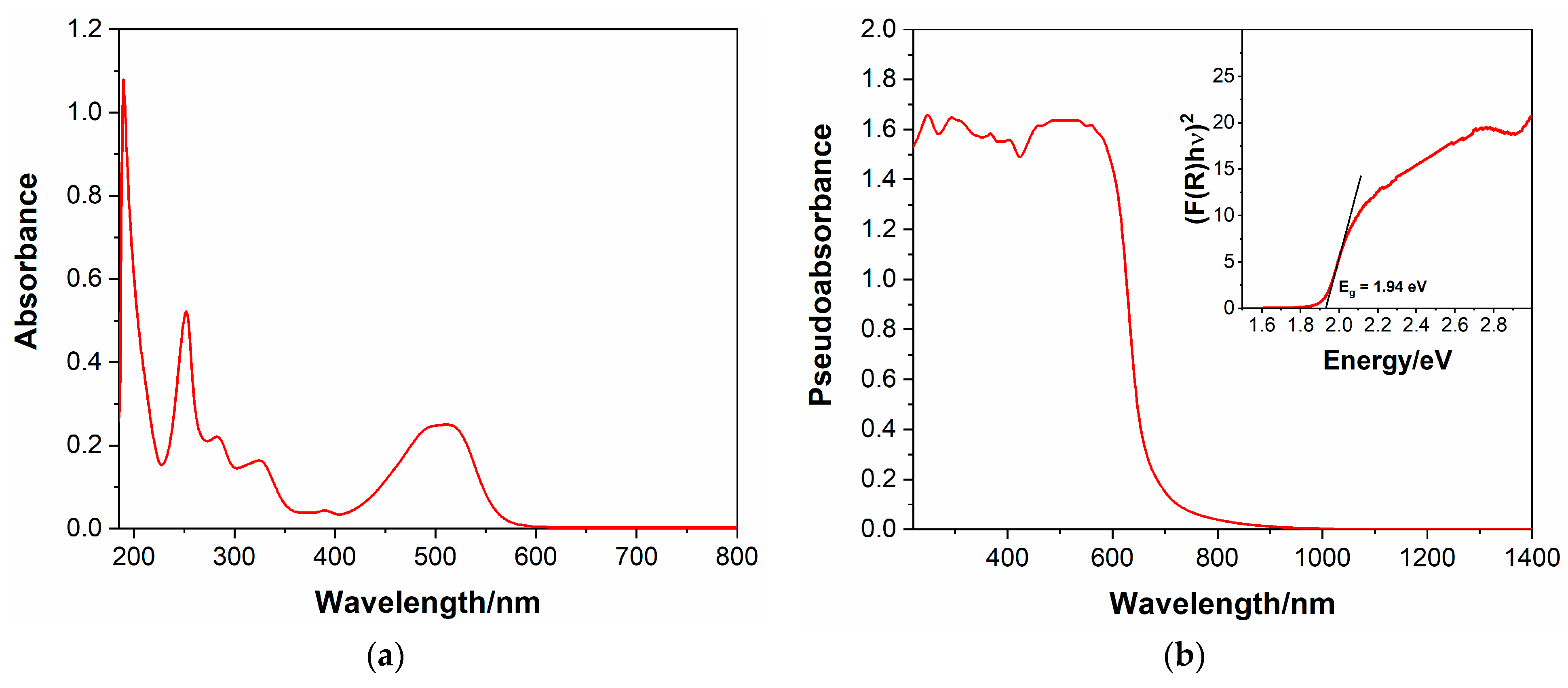
Similar to its methyl-substituted counterpart, compound 4 was found to be moderately soluble in H2O, allowing for the measurement of its UV–visible absorption spectrum in an H2O solution. A concentration of 7.5 × 10−6 M was used, given the high absorbance of the compound, especially in the UV region.
The UV–vis spectrum of dibromide salt 4 in an H2O solution is shown in Figure 1a; similar to other viologens and extended viologens, the compound presents several intense absorption bands in the UV and visible region, given the presence of multiple aromatic subunits. In particular, as regards the UV region, the first and most intense absorption maximum, characterized by an εmax of 134,900 M−1∙cm−1, can be found at λ = 190 nm, followed by another band with λmax = 252 nm and εmax = 69,600 M−1∙cm−1 and two weaker bands with maxima at λ = 283 nm and λ = 325 nm, respectively. The visible region is also characterized by a strong light absorption due to the HOMO-LUMO transition, with a broad band extended up to about 550 nm and characterized by an εmax = 33,300 M−1∙cm−1 at λmax = 510 nm.
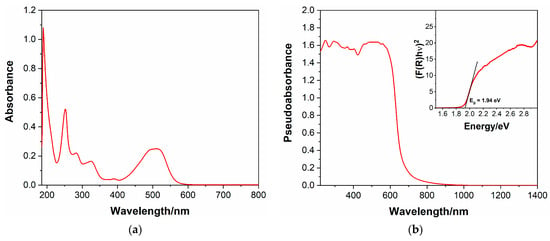
Figure 1.
(a) UV–visible absorption spectrum of compound 4 in an H2O solution (c = 7.5 × 10−6 M); (b) UV–visible diffuse reflectance spectrum of compound 4 and a Tauc plot (inset) assuming a direct band gap.
In Figure 1b, the UV–vis spectrum of the title compound in solid form (polycristalline powder) is presented. The primary diffuse reflectance data were transformed by applying the Kubelka-Munk function, represented by Equation (1), where R∞ is the reflectance into the pseudoabsorbance F(R), allowing for a qualitative comparison with the spectrum in solution.
F(R) = (1 − R∞)2/2R∞
Similar to the spectrum measured in the water solution, the viologen presents broad absorption bands in the UV and visible spectral region, extending up to about 700 nm, that is, to longer wavelengths than the spectrum presented in Figure 1a. This may suggest the presence of charge–transfer interactions taking place from the bromide anion to the electron-poor viologen dication in the solid form, which cannot be observed in solution.
Additionally, the measure of the diffuse reflectance UV–vis spectrum allowed for the determination of the band gap of compound 4, an extremely important characteristic by which to evaluate the semiconducting properties and the potential optoelectronic applications of this viologen. This was achieved by the use of the Tauc plot [30], shown in the inset of Figure 1b, in which (F(R)hν)1/α is plotted against photon energy. A direct band gap was assumed, hence the exponent α = ½ (see figure), since this led to the best approximation of the wavelength (and therefore, energy) of the absorption edge in the pseudoabsorbance spectrum. By this procedure, an optical band gap of 1.94 eV was found, revealing semiconducting properties for the synthesized compound, which could be conveniently exploited in a wide range of fields, from light emitting devices to photocatalysis, just to mention a couple.
3. Materials and Methods
All the reagents were purchased from Merck (Merck KGaA, Darmstadt, Germany), Fluorochem (Fluorochem Ltd., Glossop, UK), and TCI (TCI EUROPE N.V., Zwijndrecht, Belgium) and used as received unless otherwise stated. Dry solvents were distilled according to standard procedures: tetrahydrofuran was distilled over Na/benzophenone, diisopropylamine was distilled over KOH, methanol was distilled over Na and dichloromethane was distilled over P2O5. All the reactions were carried out under an inert Ar atmosphere, in flame-dried glassware. The reactions and chromatographic separations were monitored by thin layer chromatography (TLC) on 0.25 mm silica gel plates Merck Kieselgel 60 F254, Merck KGaA, Darmstadt, Germany) and revealed under a UV lamp (λ = 254 nm). Column chromatography was carried out on silica gel Merck-Kieselgel 60 (Merck KGaA, Darmstadt, Germany), 0.063–0.20 mm, 70–230 mesh as a stationary phase.
1H and 13C-NMR spectra were recorded on a Bruker Avance 400 (Bruker Corporation, Billerica, Massachusetts, USA) spectrometer (400 MHz) using 5 mm tubes, and chloroform-d (CDCl3) and dimethyl sulfoxide d6 (DMSO-d6) as solvents. Spectral data of compounds 1–3 are in agreement with those reported in the literature [27,31].
UV–vis spectra were acquired with a Shimadzu (Shimadzu Corporation, Kyoto, Japan) UV2600 UV–vis spectrophotometer, equipped with an ISR-2600 Plus integrating sphere, by using BaSO4 as a reference for the diffuse reflectance mode. The same instrument was also employed for the measurement of the UV–visible spectrum in solution using SUPRASIL quartz cells (10 mm optical path). The C, H, N and S elemental analysis was performed with an EA 1110 (Carlo Erba reagents, Cornaredo, Italy) CHNS-O elemental analyzer. The melting point was measured with a MEL-TEMP II apparatus using open capillary tubes and was uncorrected. The ESI-MS spectrum was acquired with a Thermo Fisher Scientific MSQ Plus (Thermo Fisher Scientific, Waltham, MA, USA) mass spectrometer, with an ESI source operating in the positive ion mode and single-quadrupole mass detector. The FT-IR spectrum was measured in attenuated total reflectance with a Bruker LUMOS II (Bruker Corporation, Billerica, MA, USA) FT-IR microscope in the range 4000–250 cm−1.
Compounds 1–3 were prepared by slight modifications of the procedures reported in [31].
Synthesis of 4-((trimethylsilyl)ethynyl)pyridine (1): In a Schlenk tube, 20 mL of degassed and anhydrous 3:1 THF/i-Pr2NH mixture, followed by 2.00 g (9.76 mmol) of 4-iodopyridine were introduced under magnetic stirring. When the 4-iodopyridine was dissolved in the solvent mixture, 342 mg (0.488 mmol) of PdCl2(PPh3)2 and 186 mg (0.976 mmol) of CuI were added, followed by 1.81 mL (12.69 mmol) of trimethylsilylacetylene, and the mixture was kept 24 h at 80 °C under stirring. After that, the reaction mixture was allowed to cool down and filtered on a Buchner funnel to remove the solid phase, which was washed with 100 mL of CH2Cl2. The liquid organic phase was then washed with H2O (3 × 20 mL), dried over anhydrous Na2SO4, filtered and evaporated in vacuo. The crude product was purified by column chromatography (9:1 petroleum ether/ethyl acetate), to afford 1.53 g (8.73 mmol) of 1 (89% yield), as an amber oil. 1H-NMR (CDCl3, 400 MHz) δ 8.55 (m, 2H), 7.30 (m, 2H) 0.26 (s, 9H); 13C-NMR (CDCl3, 100 MHz) δ 149.8, 131.4, 126.0, 102.1, 100.1, −0.2.
Synthesis of 4-ethynylpyridine (2): In a round-bottomed flask, 0.970 g (5.53 mmol) of 1 and 0.863 g (6.25 mmol) of K2CO3 were added to 10 mL of anhydrous methanol. The resulting suspension was stirred for 3 h at room temperature, protected from light, and its completion was monitored by TLC (85:15 petroleum ether/ethyl acetate). Once the reaction was complete, 10 mL of H2O was added and the mixture was transferred to a separatory funnel and extracted with CH2Cl2 (3 × 10 mL), then the combined organic phase was dried over anhydrous Na2SO4, filtered and evaporated in vacuo at room temperature. The crude product 2, thus obtained as a light-sensitive and volatile white solid, was sufficiently pure to be used without further purification. 1H-NMR (CDCl3, 400 MHz) δ 8.59 (m, 2H), 7.34 (m, 2H) 3.29 (s, 1H); 13C-NMR (CDCl3, 100 MHz) δ 149.9, 130.4, 126.2, 82.0, 81.0.
Synthesis of 9,10-bis(pyridin-4-ylethynyl)anthracene (3): In a Schlenk tube, 0.212 g (0.63 mmol) of 9,10-dibromoanthracene was added under magnetic stirring to 10 mL of the degassed and anhydrous 3:1 THF/i-Pr2NH mixture, followed by 29 mg (0.032 mmol) of PdCl2(PPh3)2, 12 mg (0.063 mmol) of CuI and 133 mg (1.29 mmol) of 2. The mixture was kept for 48 h at 80 °C under stirring, then allowed to cool down and filtered on a Buchner funnel to remove the solid phase, which was washed with 100 mL of CH2Cl2. The organic phase was washed with H2O (3 × 20 mL), dried over anhydrous Na2SO4, filtered and evaporated in vacuo, then the crude product was purified by chromatographic column (7:3 petroleum ether/ethyl acetate → ethyl acetate). This afforded 84 mg (0.22 mmol) of product 3 (35% yield), as an orange solid, along with 34 mg (0.095 mmol) of the monosubstituted product (15% yield), as a yellow solid. 1H-NMR (CDCl3, 400 MHz) δ 8.70 (m, 4H), 8.55 (m, 4H), 7.62 (m, 4H), 7.58 (m, 4H); 13C-NMR (CDCl3, 100 MHz) δ 149.9, 133.2, 130.1, 127.6, 126.9, 125.5, 116.7, 98.8, 90.6.
Synthesis of 4,4′-(Anthracene-9,10-diylbis(ethyne-2,1-diyl))bis(1-benzyl-1-pyridinium) bromide (4): In a Schlenk tube, 26 mg (0.068 mmol) of 3 were dissolved in 2 mL of anhydrous CH2Cl2 and 0.38 mL (2.72 mmol) of benzyl bromide was added. The reaction mixture was heated at 40 °C for 48 h under vigorous magnetic stirring, and its completion was verified by TLC (ethyl acetate). The mixture was then allowed to cool and the product, obtained as a red-purple precipitate, was filtered at atmospheric pressure and washed with 5 mL of cold CH2Cl2 and then 5 mL of cold diethyl ether. Additional product dissolved in the supernatant was recovered by crystallization from a methanol/diethyl ether mixture. The solid was then dried in air for 24 h, yielding 44 mg (0.061 mmol) of 4 (90% yield). 1H-NMR (DMSO-d6, 400 MHz) δ 9.34 (m, 4H), 8.81 (m, 4H), 8.70 (m, 4H), 7.91 (m, 4H), 7.59 (m, 4H), 7.48 (m, 6H), 5.90 (s, 4H); 13C-NMR (DMSO-d6, 100 MHz) δ 144.9, 138.6, 134.3, 132.2, 130.2, 129.5, 129.4, 129.3, 128.9, 127.0, 117.8, 99.1, 97.9, 63.3. Melting point: 205–208 °C (dec.). MS (+ESI), m/z: [M]2+: 281.5. Elemental analysis, calculated: C 69.82; H 4.19; N 3.88. Found: C: 69.07; H: 4,34; N: 3.74.
4. Conclusions
In this work, the synthesis of a new extended viologen, featuring a 9,10-diethynylanthracenyl core and the benzyl groups on quaternary pyridinium nitrogen atoms, was described. The compound was obtained through a four-step route using well-established reactions, with an overall 28% yield on the four passages, and was easily isolated in pure form, similar to its methyl-substituted diiodide counterpart. The optical properties of this dibromide salt were investigated by means of absorption and diffuse reflectance UV–vis spectroscopy, showing intense light absorption both in the UV and the visible spectral range. Additionally, a value of 1.94 eV for the optical band gap was determined from the Tauc plot, revealing the semiconducting nature of this viologen and thus its potential interest for different applications in optoelectronics.
Supplementary Materials
The following supporting information are available online. Figure S1: 1H-NMR spectrum of compound 1; Figure S2: 13C-NMR spectrum of compound 1; Figure S3: 1H-NMR spectrum of compound 2; Figure S4: 13C-NMR spectrum of compound 2; Figure S5: 1H-NMR spectrum of compound 3; Figure S6: 13C-NMR spectrum of compound 3; Figure S7: 1H-NMR spectrum of compound 4; Figure S8: 13C-NMR spectrum of compound 4; Figure S9: ATR-FTIR spectrum of compound 4; Figure S10: ESI-MS spectrum of compound 4.
Author Contributions
Conceptualization, A.D. and L.R.; methodology, A.D., A.L., V.N. and L.R.; formal analysis, A.D.; investigation, V.N., A.D., F.C. and L.R.; data curation, A.D., F.C., A.L. and L.R.; writing—original draft preparation, A.D. and L.R.; writing—review and editing, A.D., A.L. and L.R.; visualization, L.R.; supervision, A.D. and A.L.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
The authors wish to thank Sapienza Università di Roma for its financial support (project no. RM120172B9EE80D0).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors wish to thank Danilo Dini for the fruitful discussion.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tsai, W.-T. A review on environmental exposure and health risks of herbicide paraquat. Toxicol. Environ. Chem. 2013, 95, 197–206. [Google Scholar] [CrossRef]
- Dhaouadi, A.; Adhoum, N. Degradation of paraquat herbicide by electrochemical advanced oxidation methods. J. Electroanal. Chem. 2009, 637, 33–42. [Google Scholar] [CrossRef]
- Donaher, S.E.; Van den Hurk, P. Ecotoxicology of the herbicide paraquat: Effects on wildlife and knowledge gaps. Ecotoxicology 2023, 32, 1187–1199. [Google Scholar] [CrossRef]
- Ding, J.; Zheng, C.; Wang, L.; Lu, C.; Zhang, B.; Chen, Y.; Li, M.; Zhai, G.; Zhuang, X. Viologen-inspired functional materials: Synthetic strategies and applications. J. Mater. Chem. A 2019, 7, 23337–23360. [Google Scholar] [CrossRef]
- Sagara, T.; Tahara, H. Redox of Viologen for Powering and Coloring. Chem. Rec. 2021, 21, 2375–2388. [Google Scholar] [CrossRef]
- Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.-C. Viologen-based electrochromic materials and devices. J. Mater. Chem. C 2019, 7, 4622–4637. [Google Scholar] [CrossRef]
- Monk, P.M.S.; Hodgkinson, N.M. Charge-transfer complexes of the viologens: Effects of complexation and the rate of electron transfer to methyl viologen. Electrochim. Acta 1998, 43, 245–255. [Google Scholar] [CrossRef]
- Ponnu, A.; Sung, J.; Spears, K.G. Ultrafast Electron-Transfer and Solvent Adiabaticity Effects in Viologen Charge-Transfer Complexes. J. Phys. Chem. A 2006, 110, 12372–12384. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Guo, S.; Bian, J.; He, X.; Li, H.; Li, J. Viologen-based flexible electrochromic devices. J. Energy Chem. 2024, 93, 453–470. [Google Scholar] [CrossRef]
- Parashar, R.K.; Kandpal, S.; Pal, N.; Manna, D.; Pal, B.N.; Kumar, R.; Mondal, P.C. Coexistence of Electrochromism and Bipolar Nonvolatile Memory in a Single Viologen. ACS Appl. Mater. Interfaces 2023, 15, 51527–51537. [Google Scholar] [CrossRef]
- Yang, D.-D.; Xiao, T.; Yang, Y.-Y.; Xue, J.-H.; Shi, Y.-S.; Ma, Q.; Zheng, X.-J. Two viologen-based complexes as persistent luminescent materials and their applications in inkless print and anticounterfeiting. Chem. Eng. J. 2024, 488, 151047. [Google Scholar] [CrossRef]
- Lu, F.; Fu, S.; Wang, L.; Du, S.; Dou, Z.; Yang, X.; Li, J. Enhanced performance of inverted polymer solar cells by adding benzyl viologen dichloride into ZnO electron transport layer. Opt. Mater. 2023, 139, 113782. [Google Scholar] [CrossRef]
- Kathiresan, M.; Ambrose, B.; Angulakshmi, N.; Mathew, D.E.; Sujatha, D.; Stephan, A.M. Viologens: A versatile organic molecule for energy storage applications. J. Mater. Chem. A 2021, 9, 27215–27233. [Google Scholar] [CrossRef]
- Ashton, P.R.; Ballardini, R.; Balzani, V.; Gandolfi, M.T.; Marquis, D.J.-F.; Pérez-Garcia, L.; Prodi, L.; Stoddart, J.F.; Venturi, M. The self assembly of controllable [2]catenanes. J. Chem. Soc. Chem. Commun. 1994, 177–180. [Google Scholar] [CrossRef]
- Yamashita, Y.; Hanaoka, T.; Takeda, Y.; Mukai, T.; Miyashi, T. Syntheses and Properties of Dipyridylnorbornadienes. Bull. Chem. Soc. Jpn. 1988, 61, 2451–2458. [Google Scholar] [CrossRef]
- Rockley, J.; Summers, L. Chemical Constitution and Activity of Bipyridinium Herbicides. XIII. Synthesis, Reduction Potential and Biological Activity of 1-Methyl-4-[[(methylpyridinium-4-yl)Imino]methyl]pyridinium Diiodide. Aust. J. Chem. 1980, 33, 1397–1400. [Google Scholar] [CrossRef]
- Kniep, F.; Walter, S.M.; Herdtweck, E.; Huber, S.M. 4,4′-Azobis(halopyridinium) Derivatives: Strong Multidentate Halogen-Bond Donors with a Redox-Active Core. Chem. Eur. J. 2012, 18, 1306–1310. [Google Scholar] [CrossRef]
- Porter III, W.W.; Vaid, T.P.; Rheingold, A.L. Synthesis and Characterization of a Highly Reducing Neutral “Extended Viologen” and the Isostructural Hydrocarbon 4,4′′′′-Di-n-octyl-p-quaterphenyl. J. Am. Chem. Soc. 2005, 127, 16559–16566. [Google Scholar] [CrossRef]
- Dessì, A.; Calamante, M.; Mordini, A.; Zani, L.; Taddei, M.; Reginato, G. Microwave-activated synthesis of thiazolo[5,4-d]thiazoles by a condensation/oxidation sequence. RSC Adv. 2014, 4, 1322–1328. [Google Scholar] [CrossRef]
- Alberto, M.E.; De Simone, B.C.; Cospito, S.; Imbardelli, D.; Veltri, L.; Chidichimo, G.; Russo, N. Experimental and theoretical characterization of a new synthesized extended viologen. Chem. Phys. Lett. 2012, 552, 141–145. [Google Scholar] [CrossRef]
- Natali, M.; Luisa, A.; Iengo, E.; Scandola, F. Efficient photocatalytic hydrogen generation from water by a cationic cobalt(ii) porphyrin. Chem. Commun. 2014, 50, 1842–1844. [Google Scholar] [CrossRef] [PubMed]
- Bar, G.; Larina, N.; Grinis, L.; Lokshin, V.; Gvishi, R.; Kiryuschev, I.; Zaban, A.; Khodorkovsky, V. RGB organic electrochromic cells. Sol. Energy Mater. Sol. Cells 2012, 99, 123–128. [Google Scholar] [CrossRef]
- Romagnoli, L.; D’Annibale, A.; Latini, A. 4,4′-([2,20-Bithiophene]-5,5′-diylbis(ethyne-2,1-diyl))bis(1-methylpyridin-1-ium) Iodide. Molbank 2023, 2023, M1733. [Google Scholar] [CrossRef]
- Romagnoli, L.; D’Annibale, A.; Latini, A. 4,40,400-(Benzene-1,3,5-triyltris(ethyne-2,1-diyl))tris(1-methylpyridin-1-ium) Iodide. Molbank 2023, 2023, M1742. [Google Scholar] [CrossRef]
- Romagnoli, L.; Latini, A.; D’Annibale, A. 4,4′-(Thiophene-2,5-diylbis(ethyne-2,1-diyl))bis(1-methyl-1-pyridinium) Iodide. Molbank 2024, 2024, M1817. [Google Scholar] [CrossRef]
- Fudickar, W.; Bauch, M.; Ihmels, H.; Linker, T. DNA-Triggered Enhancement of Singlet Oxygen Production by Pyridinium Alkynylanthracenes. Chem. Eur. J. 2021, 27, 13591–13604. [Google Scholar] [CrossRef]
- Romagnoli, L.; D’Annibale, A.; Blundo, E.; Polimeni, A.; Cassetta, A.; Chita, G.; Panetta, R.; Ciccioli, A.; Latini, A. Synthesis, Structure, and Characterization of 4,4′-(Anthracene-9,10-diylbis(ethyne-2,1-diyl))bis(1-methyl-1-pyridinium) Bismuth Iodide (C30H22N2)3Bi4I18, an Air, Water, and Thermally Stable 0D Hybrid Perovskite with High Photoluminescence Efficiency. Cryst. Growth Des. 2022, 22, 7426–7433. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, L.; D’Annibale, A.; Blundo, E.; Patra, A.; Polimeni, A.; Meggiolaro, D.; Andrusenko, I.; Marchetti, D.; Gemmi, M.; Latini, A. 4,4′-(Anthracene-9,10-diylbis(ethyne-2,1-diyl))bis(1-methyl-1-pyridinium) Lead Iodide C30H22N2Pb2I6: A Highly Luminescent, Chemically and Thermally Stable One-Dimensional Hybrid Iodoplumbate. Chem. Mater. 2023, 35, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Kole, G.K.; Košćak, M.; Amar, A.; Majhen, D.; Božinović, K.; Brkljaca, Z.; Ferger, M.; Michail, E.; Lorenzen, S.; Friedrich, A.; et al. Methyl Viologens of Bis-(4′-Pyridylethynyl)Arenes–Structures, Photophysical and Electrochemical Studies, and their Potential Application in Biology. Chem. Eur. J. 2022, 28, e202200753. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV−Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Friščić, T.; MacGillivray, R. Enforced Face-to-Face Stacking of Organic Semiconductor Building Blocks within Hydrogen-Bonded Molecular Cocrystals. J. Am. Chem. Soc. 2006, 128, 2806–2807. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).