Abstract
The paper records the rediscovery of the rare Urodontidius enigmaticus Louw, 1993 in South Africa, based on specimens reared from galls in the succulent leaves of Ruschia versicolor. The original account of some of the morphological characters of the species is corrected, and its habitus, antennae, pygidium and genitalia are illustrated. Its life history and galling habit on its host plant are described and illustrated, and its larva is compared with those of the genera Urodontellus Louw and Urodontus Louw, which represent different larval types with different life histories. The silk-spinning habits of the Urodontellus larva are briefly described. A tribute to the late Schalk Louw is presented, together with a list of his publications on weevils.
1. Introduction
The anthribid subfamily Urodontinae is remarkable in many ways. It is morphologically quite different from Anthribinae (including Choraginae), i.a., by not having a bracteate pronotal carina, the mandibles without a mycetangial pocket, the hind wings with only three veins, the hind gut with a rectal ring, tergite VIII exposed in the males and the gonocoxites of the ovipositor not apically sclerotised and dentate [1]. Biologically, it differs from Anthribinae in that its larvae do not feed on fungus-infected dead wood but on living plant tissues, developing in soft stems and seeds of particular plant families. The subfamily is also unusual in its distribution, being restricted to the Afrotropical and western Palaearctic regions but with a distinct centre of diversity in southern Africa and another in the western Palaearctic, the former though with a much higher generic diversity [2,3]. In contrast to the Palaearctic fauna, the southern African one remained very poorly known until Schalk Louw thoroughly revised it, describing several new genera and many new species, summarising the biological information available for it and attempting a first analysis of its phylogenetic relationships [2]. Little work has been done on this fauna since then, and many aspects of the taxonomy and biology of particular genera remain unknown.
During 2015, one of us (CHS) reared a species of Urodontinae from galls in the succulent leaves of Ruschia versicolor L. Bolus (Aizoaceae) in west-coastal Namaqualand, South Africa, and recorded its life history. He handed the specimens to Schalk Louw for identification, who surmised it to be a new species of Urodontus Louw and was busy studying it at the time of his sudden and untimely death, in April 2018. The study would have formed part of a larger treatment by him and CHS of gall formation in Urodontinae and its phylogenetic and evolutionary implications. Unfortunately this did not come to fruition. After Schalk’s death, the first author of this paper, also an old friend and colleague of Schalk, joined CHS to complete the study of this species, which, on closer inspection and comparison with type specimens, turned out to be the arcane Urodontidius enigmaticus Louw.
Urodontidius enigmaticus is the sole known species of the genus Urodontidius, which was described by Schalk Louw in his revision of the Urodontinae of southern Africa [2]. The species is remarkable for its extraordinary antennal structure in the male. Its description was based on only four specimens, one pair collected in 1985 in the Namaqualand region of the western Northern Cape province of South Africa and two males taken much earlier at Willowmore in the Eastern Cape province of the same country. No further specimens appear to have been collected since. The hosts and life history of the species also remained unknown, aside from the fact that the pair from Namaqualand had been found on flowers of a species of Eberlanzia (Aizoaceae) [2].
In this paper, we report the rediscovery of this rare species, describe and illustrate additional morphological aspects of it and record the extraordinary galling habit and life history of its larva. We also take this opportunity to pay tribute to the late Schalk Louw and his contributions to the study of weevils, particularly in southern Africa.
2. Materials and Methods
2.1. Specimens
The study is based on 1 male and 28 adult females and 4 larvae of Urodontidius enigmaticus, as sent to RGO from the University of the Free State in Bloemfontein, South Africa, where they were retrieved from Schalk Louw’s office by his colleague Charles Haddad. All these specimens had been reared from succulent leaf galls on Ruschia versicolor at Kommandokraal (31.50°S, 18.21°E) in the Western Cape province of South Africa in November 2015 by CHS. We also studied photographs of the holotype and only female paratype of U. enigmaticus, housed in the National Museum in Bloemfontein, kindly provided to us by Burgert Muller. Specimens of other genera and species of Urodontinae in the Australian National Insect Collection (ANIC) were studied for comparison.
2.2. Illustrations
Photographs of specimens and structures were taken using a Leica DFC500 camera mounted on a Leica M205C microscope. Photographs taken at different focus levels were combined into single images using the software program Leica Application Suite V4.9, and these images were enhanced as necessary using the Adobe Photoshop CS6 software.
3. Diagnosis, Distribution and Life History
Genus Urodontidius Louw, 1993Urodontidius Louw, 1993: 11 [2].Type species, by original designation: Urodontidius enigmaticus Louw, 1993.
Urodontidius enigmaticus Louw, 1993Urodontidius enigmaticus Louw, 1993: 12, Figures 7, 40 and 52 [2].
Diagnosis.Urodontidius enigmaticus is readily distinguishable from all other Urodontinae by its antennae, especially the extraordinary clubs of the male (Figure 1e). Its large size, dark-brown colour, very sparse vestiture and shape of the elytra and pygidium (Figure 1a–d) also set it apart from all other urodontines. Louw’s description of the genus and the species [2] is accurate except for that of the antennae. He described these as being 10-segmented, but this is incorrect. The antennae of the female comprise 11 segments: a short, medially constricted scape, a 7-segmented funicle (the 1st segment enlarged, the 7th broadened) and a 3-segmented club (Figure 1f). The segmentation of the club is astonishingly variable, ranging from two loose segments and one fused (Figure 1g) to three fused ones (Figure 1f, h) to two fused ones (Figure 1i) to a large single one (Figure 1j). The fusion line between the last two segments is fairly distinct in some specimens (in lateral outline as well as on the surface) but almost obsolete in others. The apical edge of the terminal (third) club segment also varies, from symmetrically subtruncate to slightly emarginate to asymmetrically excised (Figure 1g–j). In the male, the club is modified into a single, short but very broad, asymmetrical segment (Figure 1e). Louw misinterpreted the funicle as being 6-segmented and the 7th, broader funicle segment as belonging to the club, but the club of the female is distinctly 3-segmented in most specimens (including in the female paratype), without the 7th funicle segment. Louw’s drawing of the club of the male as being 2-segmented, consisting of a smaller penultimate segment in addition to the large terminal one (Figure 7 [2]), is also incorrect, as we could ascertain from a photo of the antenna of the holotype.

Figure 1.
Morphological aspects of Urodontidius enigmaticus Louw: (a) habitus of male, dorsal view; (b) habitus of female, dorsal view; (c) habitus of male, lateral view (antennal club missing); (d) habitus of female, lateral view; (e) right antenna of male, lateral view; (f) left antenna of female, lateral view; (g–j) variation in shape of antennal club of female, from (g) 3-segmented to (j) 1-segmented.
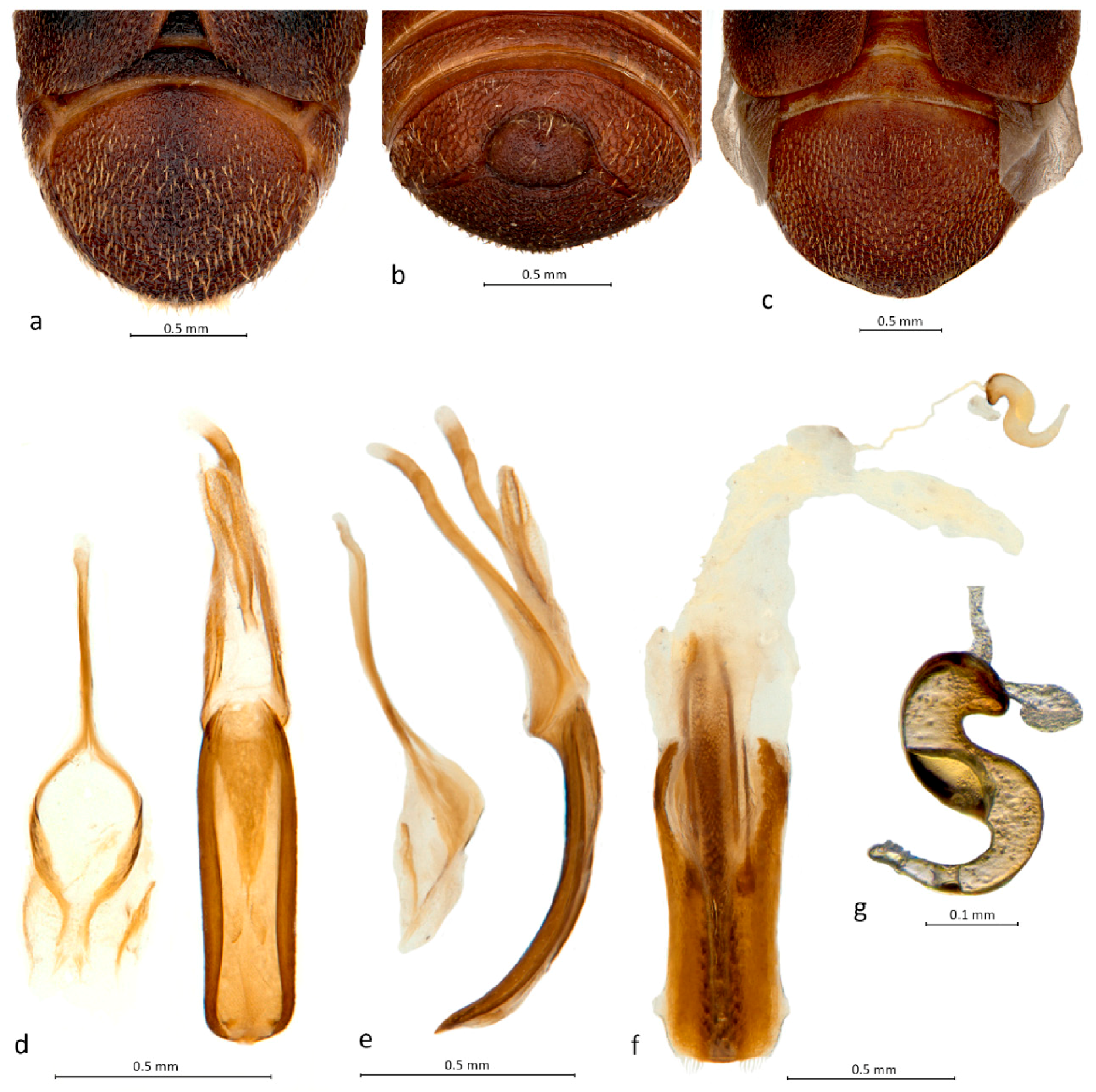
As in other urodontines, the pygidium is sexually dimorphic in Urodontidius, in the female formed by tergite VII (Figure 2c) and in the male by tergite VII as well as a small tergite VIII (Figure 2a,b). The latter was referred to as a supplementary sclerite by Louw [2]. In the female, this last tergite is concealed beneath tergite VII.

Figure 2.
Abdomen and genitalia of Urodontidius enigmaticus Louw: (a) pygidium of male, caudal view; (b) pygidium of male, ventral view; (c) pygidium of female, caudal view; (d) male genitalia, dorsal view; (e) male genitalia, lateral view; (f) female genitalia, dorsal view; (g) spermatheca.
In the male genitalia, the penis consists of a flat, slightly curved, elongate, parallel-sided pedon and a narrowly triangular tectum extending only over the basal half of the pedon (Figure 2d,e). The temones (apodemes) are as long as the pedon, very broad (deep) at the base (with the tectal and pedal arms discernible but connected) but narrower in the distal half, and they are only membranously connected to the pedon and tectum (Figure 2e). The endophallus is equipped with two long, narrow, parallel, medially connected sclerites, possibly a flattened flagellum, of about half the length of the pedon (Figure 2d,e). The tegmen is quite reduced, the sides of the ring broad but weakly sclerotised, not articulated in the middle (Figure 2e), and the parameral sector narrow, very weakly sclerotised, with a pair of apical clusters of few strong, long setae (Figure 2d). In the female, the ovipositor is a strong, flat, apically truncate tube with large, broad, flattened lateral rods, without a median tranverse bar and without styli and apical teeth, and the median rod is long (protruding beyond the apices of the lateral rods) and thick, covered with large, sharp denticles directed caudad (especially in the apical third) and anteriorly flanked by two pairs of narrow lateral rods (Figure 2f). The spermatheca (Figure 2g) is strongly S-shaped, weakly sclerotised and undifferentiated, with an apparently small gland and the duct entering the bursa copulatrix in an apical position. The densely, coarsely dentate internal rod of the ovipositor appears to function like a round grater or coarse file that can be pushed in and out of plant tissues to create a hole for oviposition.
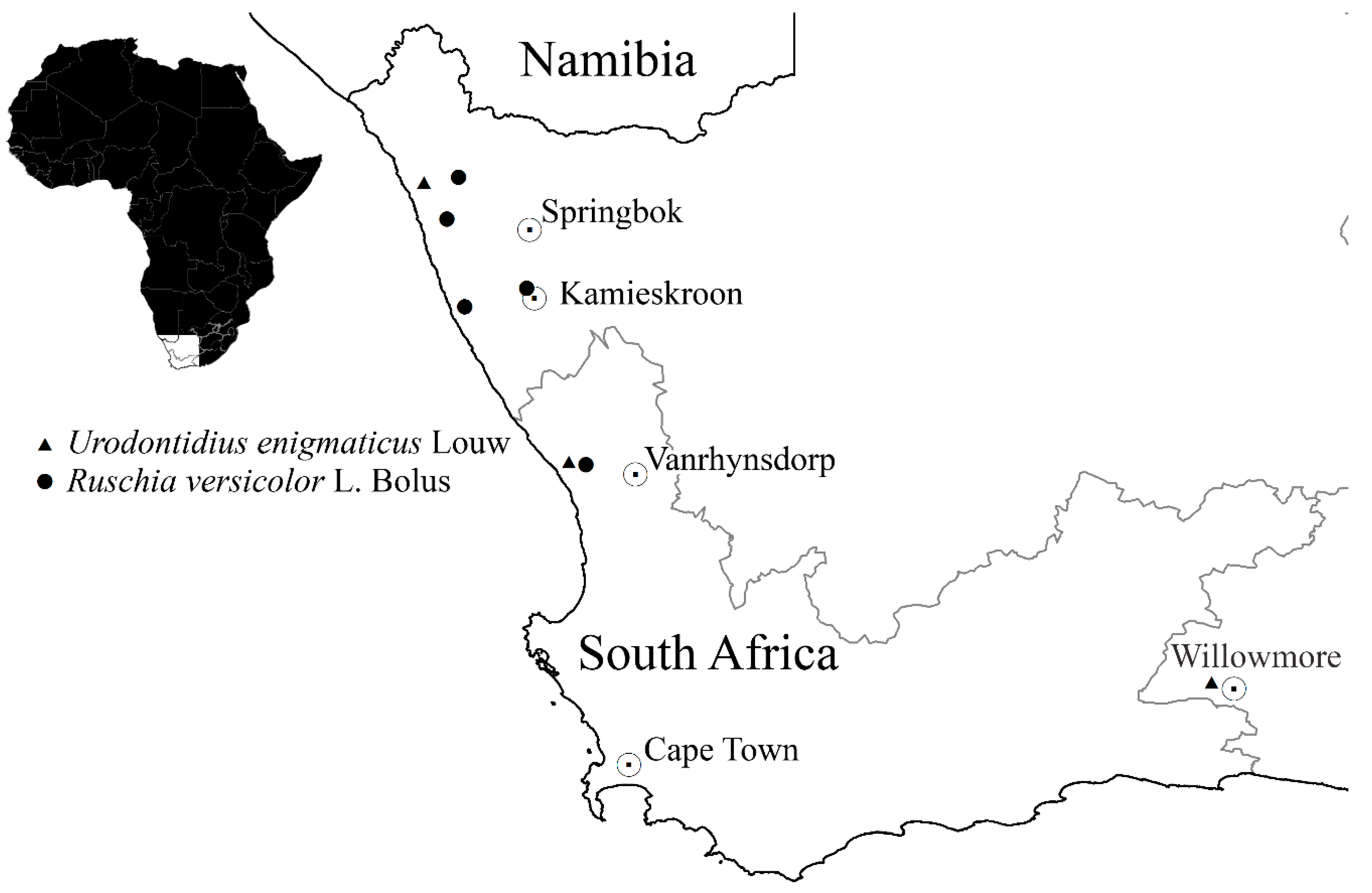
Distribution.Urodontidius enigmaticus occurs in the Namaqualand area of the Northern and Western Cape provinces of South Africa, less certainly also in the Eastern Cape (Figure 3). It was previously only known from the four specimens comprising its type series [2], a pair collected at Steenbok in the Northern Cape province (close to the Atlantic coast, between Port Nolloth and Nigramoep) and two males from Willowmore in the Eastern Cape province, collected by Dr. J. H. J. C. Brauns (1857–1929) about a century ago. The locality of our specimens, Kommandokraal, is about 100 km south of Steenbok. The occurrence of the species at or near Willowmore, very distant from the other two known localities, requires confirmation.
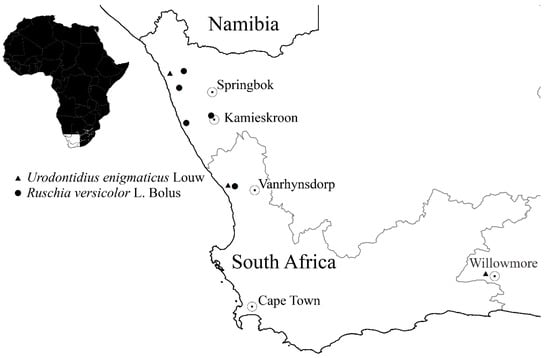
Figure 3.
Distribution of Urodontidius enigmaticus and Ruschia versicolor.
Host plants. Two hosts are recorded for U. enigmaticus, a species of Eberlanzia, on whose flowers Schalk Louw found a pair of specimens in 1985 [2], and Ruschia versicolor (Figure 4a,b), from whose leaf galls our specimens were reared. Both genera belong to the family Aizoaceae and are some of the many “mesembs” for which Namaqualand is famous. Ruschia versicolor is a perennial prostrate shrub that can grow up to 400 mm high. Its fleshy, succulent leaves are cylindrical and may grow to 60 mm in length. Some leaves form clusters. Leaves are initially green but turn pink when they get older. According to the Botanical Database of Southern Africa (BODATSA), the species has a fairly limited distribution (Figure 3) in coastal Namaqualand Sandveld [4], but it is apparently not threatened. Eberlanzia is closely related to Ruschia (also placed in the tribe Ruschiae of the subfamily Ruschioideae) but much smaller, comprising only eight species in southern Namibia and south-western South Africa [5]. Its leaves are also succulent but much shorter, sometimes spinose. While the identification underlying this host record cannot be verified, it seems likely that U. enigmaticus may also form galls in the succulent leaves of Eberlanzia.

Figure 4.
Biological aspects of Urodontidius enigmaticus Louw: (a) host plant (Ruschia versicolor, Aizoaceae) in habitat; (b) leaf gall on Ruschia versicolor incited by larva; (c) female emerged from gall; (d) female next to larval feeding chamber in gall; (e) larva in feeding chamber in gall; (f) larva, ventral view; (g) larva, head with mandibles; (h) larvae of U. enigmaticus, Urodontellus lilii (Fåhraeus) and Urodontus scholtzi Louw in comparison, lateral view. (Photographs c, d, e by Hennie de Klerk).
Life history and gall development. The larva of U. enigmaticus is a fairly flaccid white grub without appendages (Figure 4e,f,h) and totally lacking in areas of sclerotisation except for the sharp mandibles (Figure 4g). It incites a gall in the fleshy leaves of its host, in which it evidently feeds on the jelly-like cells lining the inside of the galls. The small quantity of frass present in the gall suggests that it feeds on low-fibre, highly nutritious food. Soon after the larva has established in the soft, succulent tissue, an elongate, hard woody capsule starts to develop around it (Figure 4e), while the soft tissue surrounding the capsule swells noticeably to two to three times its size. The gall is often bilobed (Figure 4b) as a result of swollen development of two deformed leaves growing from the galled leaf bud. The internal capsule quickly develops to its final size and initially dwarfs the young larva, but as the larva grows it fills more and more of the available space until eventually the enclosed adult fills it almost completely (Figure 4d). The outer cell layers of the capsule are woody from the beginning, whereas initially it is lined internally by layers of spongy tissue. As these are consumed by the larva and it grows, the capsule sides become smoother. Duration of the larval development is about five months, and the pupal stage lasts about two weeks. Urodontidius enigmaticus has two generations per year on Ruschia versicolor at Kommandokraal. First-generation adults (Figure 4c,d) emerge in winter (June), mate and lay eggs on developing leaf buds; those of the second generation emerge during November. The relatively short development period of the larva supports the assumption of two generations existing per year, as compared to the much longer larval development and single generation per year of Urodontus scholtzi Louw, which induces woody stem galls in Galenia (also Aizoaceae) [2,6].
4. Discussion
In his phylogenetic reconstruction of the genera of Urodontinae, Louw [2] related Urodontidius most closely to Breviurodon Strejcek, based on the fusion of the club segments in both. However, this fusion as well as the segmentation of the antennae are different in the two genera. In Urodontidius the antennae are 11-segmented, comprising a 7-segmented funicle and a variably fused club (3-segmented in the female, 1-segmented enlarged in the male), whereas in Breviurodon the antennae are 10-segmented, the funicle being 6-segmented and the club spindle-shaped with three fused but distinct segments in both sexes [7,8]. Louw ([8], Figure 4) drew the funicle of Breviurodon decellei Louw as being 7-segmented, but this is evidently an error. Urodontidius is thus unlikely to be as closely related to Breviurodon as Louw concluded from his phylogenetic analysis [2]. Although its 11-segmented antennae may instead suggest a closer relationship to Bruchela Dejean and Urodontellus Louw, its larval development in aizoaceous hosts indicates that it may indeed belong in the clade of genera with 10-segmented antennae. Further phylogenetic study is necessary to resolve its relationships and elucidate the origin of its unusual galling habit.
In contrast to other Anthribidae, all species of Urodontinae as known develop in living plant tissues. The life styles and hosts of the subfamily fall in two groups: the larvae of the Palaearctic genus Bruchela and of the southern African genus Urodontellus develop in seed capsules of, respectively, the brassicalean families Resedaceae and Brassicaceae and the monocotyledonous families Iridaceae and Asphodelaceae, whereas the larvae of the southern African genera Urodontidius and Urodontus Louw (and probably of Urodoplatus Motschulsky as well) develop predominantly in the stems and flower heads of Aizoaceae [2]. The larvae of these two groups also differ markedly (Figure 4h). The larvae of Urodontellus (U. lilii (Fåhraeus)) have a prognathous, sclerotised head; large antennae; broad, bluntly bidentate mandibles; a small clypeus and labrum; a semilunar, anteriorly evenly thickly cylindrical body rapidly thinning posteriad from abdominal segment V (A-V), with A-VIII and A-IX small, weakly sclerotised and A-X with narrowly sclerotised lateral anal lobes; a dorsal pair of conical ambulatory ampullae on each of segments T-III and A-1 to A-VII; distinct clusters of long fine setae ventrally on all segments except A-IX and A-X. In contrast, the larvae of Urodontus (U. mesemoides Louw, U. scholtzi) have a hypognathous, well sclerotised head; small antennae; broad, sharply bidentate mandibles; a large clypeus and labrum; a crescentic, evenly thickly cylindrical body slightly thinning in the posterior third, with A-IX small and short and A-X very small with indistinct, unsclerotised anal lobes; all segments without ambulatory ampullae and very sparsely setose (a few long dorsal setae on A-VI to A-IX). The larva of Urodontidius (U. enigmaticus) differs significantly from both these forms, having the head hypognathous, unsclerotised except for the small, narrow, finely bidentate mandibles; the body only slightly curved, flattened (broader than high), in lateral view gradually thickening from T-1 to A-V, then thinning more rapidly posteriad, with A-VIII and A-IX small, short, and A-X with a weak transverse cleft, the anus possibly closed; segments A-I to A-VII laterally with thick, elongate ambulatory ampullae, the largest on A-V, dorsally without ampullae but the membranes between the abdominal segments medially eversible; the entire body without any macrosetae. This represents a distinct third body type in Urodontinae.
The life history of these larval types also differs. The larvae of Bruchela and Urodontellus are very mobile, moving in and between seed capsules [9] on their backs using the dorsal ampullae [10], and they are able to spin threads from the tip of their abdomen. The larvae of Bruchela use these threads to close the open Reseda seed capsule in which they feed, as a suspension when they drop to the ground for pupating and for constructing a cocoon in the soil [10]. The larvae of Urodontellus pupate in the closed seed capsules in which they feed, but they also spin a silken cocoon, inside the capsule. They can also drop from the capsule suspended by a silken thread secreted from the abdomen, and they can, in fact, roll up the thread again with the tip of their abdomen in a gyrating motion to ascend back into the capsule (Stefan Neser, pers. obs. 2007, 2018). This action is evidently performed by the peculiarly sclerotised anal lobes of the larva. The silk of Urodontellus is proteinacous in nature, with high percentages of amino acids and its infrared absorption spectrum showing strong amide peaks (Andrew Walker, pers. com. 2012), suggesting that the silk is derived from the Malpighian tubules, which are pink in colour [11]. The larvae of Urodontus instead are not or are only very weakly mobile, apparently spending their entire development inside their feeding cells in the soft vegetative tissues of the stems and flower heads of their hosts or, in the case of Urodontus scholtzi and U. tesserus Louw, in woody stem galls. The larvae of Urodontidius appear to be a more specialised version of the latter, feeding in hard cells in large succulent galls, in which they probably move around using their lateral segmental ampullae and the eversible dorsal intersegmental membranes. A detailed morphological comparison of all known urodontine larvae remains to be carried out.
Gall formation in succulents is unusual, possibly because of the high levels of fluids in tissues, although gall midges (Cecidomyiidae) have been recorded to induce them in various Aizoaceae in southern Africa [12]. As far as we could ascertain, however, this is the first record of a beetle galling a succulent plant. Besides this being an unusual habit for insects in general, it is atypical for Urodontinae in that gall formation in the subfamily is very rare (only known for three out of 91 species), and galling a succulent plant adds to the uniqueness of the phenomenon.
5. A Tribute to Schalk Louw
Schalk van der Merwe Louw was born on the 28th March 1952 in Windhoek, Namibia (then South West Africa). He attended school in Windhoek from 1959 to 1970 and subsequently enrolled in undergraduate studies at the University of Pretoria in South Africa, from 1972 to 1975, obtaining a B. Sc. degree in Zoology and Entomology in 1974 and a first-class B. Sc. Honours degree in Entomology in 1975. He then proceeded with studies at the same university for an M. Sc. degree, which he obtained in 1979 in arid-zone insect ecology, and for a Ph. D. degree in weevil systematics, which was bestowed on him in 1985. He started his research career in 1976 as Curator of Invertebrates at the State Museum in Windhoek, where he remained until 1981, when he moved to South Africa to become Head of the Entomology Department at the National Museum in Bloemfontein. In 1992 he took up a position as Senior Lecturer in the Department of Zoology and Entomology at the University of the Orange Free State in Bloemfontein, where he remained for the rest of his career, rising to Associate Professor in 1996 and to full Professor in 2002. Over his career he was a member of many learned societies, served on the executive committees and editorial boards of several scientific societies and associations and played an active role in the entomology scene in southern Africa.
He married Elizabeth Susanna van Niekerk on the 7th January 1978, and the couple had two children: a daughter, Sarita, born on the 27th September 1979, and a son, Schalk Merwe, born on the 16th June 1983.
Schalk’s love of beetles was kindled and nurtured by the eminent southern African coleopterists of the 1970s and 1980s, in particular Mary-Louise Penrith, with whom he worked at the State Museum in Windhoek, and the late Sebastian Endrödy-Younga of the former Transvaal Museum in Pretoria and Erik Holm, who played a leading role in invertebrate research at the then Namib Desert Research Station at Gobabeb in Namibia and later became Professor of Entomology at the University of Pretoria. Like Mary-Lou and Sebastian, Schalk initially also studied Tenebrionidae, but on the collecting expeditions he undertook with them into remote parts of the Namib and Kalahari deserts he soon discovered his love for weevils, which remained with him for the rest of his life. The cryptic, terricolous desert weevils held a particular fascination for him, which led to several research papers, among them his classic revisions of the genus Hyomora (Cyclominae) and the subfamily Microcerinae, the latter flowing from his Ph. D. research and becoming his magnum opus in weevil systematics.
When Schalk moved to Bloemfontein, his interest in weevils widened to include their associations with plants, and he set up an experimental site at Glen, just outside the city, where several brachycerine and cyclomine species appeared annually to munch on the leaves of the numerous geophytic lilies that sprung to life after rains. His paper of the life history and immature stages of the huge Brachycerus ornatus remains a benchmark study on the biology of this iconic African weevil genus. From Bloemfontein he explored the weevil fauna of the Orange Free State, in particular at the museum’s research station at Florisbad and at Krugersdrift Dam but also on expeditions into the drier sandy areas of the north-western Orange Free State and the Northern Cape province.
In 1991 Schalk went small in weevil terms, embarking on a study of the enigmatic and taxonomically badly neglected southern African fauna of the anthribid subfamily Urodontinae, which is particularly species-rich in the Namaqualand and Richtersveld regions of South Africa and Namibia. His 1993 revision drastically increased the known diversity and host range of this group, with the description of four new genera and twenty new species and an analysis of their host associations. These weevils also ignited a new passion in Schalk, that of studying galls and the evolution of galling behavior in weevils, on which he published a number of papers and book chapters and delivered talks at international symposia in Russia and Hungary. While his employ at the university allowed him few opportunities to continue his studies of weevil systematics and forced him to engage in various other research fields, he managed to keep a connection to weevils in his research on new crops, in particular on pigweed (Amaranthus), which is loved by a lixine weevil (Hypolixus haerens) in South Africa, and this research also enabled Schalk to pursue an interest in tritrophic associations between plants, insects and parasites/pathogens.
Schalk retired from his university at the end of 2017 but maintained an association with it as a Research Fellow. He had plans to re-engage in weevil systematics and biology, first of which was to complete a global perspective on the diversity and pattern of gall induction in weevils, for which he had unpublished data from Horace Burke available and which he intended to contribute to this Special Issue on weevils. Alas, ill health prevented him from completing this project and finally even the smaller, present paper as he had conceived in its place.
Schalk was one of the “Young Turks” at the first international weevil symposium, held in 1988 during the XVIIIth International Congress of Entomology in Vancouver, Canada, where we gathered to learn of weevils from the previous generation of gurus such as Willy Kuschel, Katsura Morimoto, Horace Burke, Elbert Sleeper, Stephen Wood, Charlie O’Brien and Anne Howden (Figure 5d). In 1996 Schalk co-organised the weevil symposium at the XXth International Congress of Entomology in Florence, Italy, together with Enzo Colonnelli and Giuseppe Osella, and co-edited the proceedings from it, and at the XXIst International Congress of Entomology, held in 2000 in Foz de Iguassu, Brasil, he co-organised a symposium on biodiversity and biogeographical research in Africa with Wolfram Mey (Berlin). In 1999 he visited Australia to participate in the John Lawrence Beetle Symposium in Canberra, Australia, keen to meet up with old and new colleagues, discuss various weevil matters, and enjoy the company (Figure 5e).

Figure 5.
Schalk Louw: (a) collecting weevils at Port Alfred, November 1992; (b) collecting weevils at Lapalala, January 1987; (c) with colleagues at a rhino carcass, Lapalala, January 1987; (d) with Rolf Oberprieler (left) and Elbert Sleeper at the weevil symposium during the XVIIIth International Congress of Entomology, Vancouver, July 1988; (e) at dinner with colleagues during the John Lawrence Beetle Symposium, Canberra, December 1999 (f.l.t.r. Rolf Oberprieler, Jyrki Muona, John Lawrence, Schalk Louw, Willy Kuschel, Vladimir Zherikhin, Clarke Scholtz). (Photos RGO except d).
Apart from his numerous and wide-ranging contributions to the knowledge of weevils and other beetles (Appendix A), we also remember Schalk as a pleasant and valuable companion on numerous collecting trips in southern Africa (Figure 5a–c). He had a knack for finding cryptic beetles on the ground, a skill he had honed early during his many expeditions in Namibia, and he was always ready to show and share his daily haul with others. During Willy Kuschel’s visit to South Africa in 1992, Schalk took great pride in showing Willy his monster Brachycerus weevils at Glen and the multitude of terricolous genera at Krugersdrift Dam that were wholly unknown to Willy [13]. Another memorable episode was a joint 1993 expedition to the Richtersveld [14], where Schalk not only showed everyone how to really hunt for ground weevils in the desert but also enthusiastically joined in the smoking of thick cigars to keep the annoying blackflies at bay that descended onto our camp in the afternoon, just when we could sit down to sort and admire our daily catches and wash down the dust in our throats with a beer.
Schalk’s untimely passing leaves a large gap both in the entomological community in South Africa and in international weevil systematics and ecology.
Author Contributions
Both authors contributed equally to the design, analysis and writing of the paper.
Funding
This research received no external funding.
Acknowledgments
We thank Charles Haddad of the University of the Free State for tracing the specimens of Urodontidius enigmaticus in Schalk Louw’s office and sending them to RGO in Australia for study, and for forwarding the draft manuscript of this paper as Schalk was working on at the time of his death. Burgert Muller of the National Museum in Bloemfontein kindly provided photographs of the holotype and paratype of U. enigmaticus housed in his museum. Debbie Jennings (ANIC), Carmen Jacobs (University of Pretoria) and Hennie de Klerk are thanked for the photographs of the adults and larvae of U. enigmaticus, and we acknowledge Arthur V. Evans (Virginia, U.S.A.) for permission to publish the photograph in Figure 5d. We also like to acknowledge Stefan Neser (Pretoria) for sharing photographs and video clips of silk-climbing and cocoon-spinning Urodontellus larvae.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Publications on weevils by Schalk van der Merwe Louw
- Louw, S. v. d. M. (1976) The genus Brachycerus (Coleoptera: Curculionidae). State Museum Newsletter, 5, 4–6.
- Louw, S. v. d. M. (1981) Revision of the genus Hyomora Pascoe, 1865 (Coleoptera: Curculionidae: Rhytirrhininae). Cimbebasia (A), 5, 225–250.
- Louw, S. v. d. M. (1982) The occurrence of Microcerinae (Coleoptera: Curculionidae) in Botswana. Botswana Notes and Records, 14, 11–22.
- Louw, S. v. d. M. (1983) A new species of Hyomora Pascoe (Coleoptera: Curculionidae: Rhytirrhininae) with notes on the distribution of the genus. Navorsinge van die Nasionale Museum, Bloemfontein, 4 (6), 169–175.
- Louw, S. v. d. M. (1985a) African Curculionidae in collections in Europe and England. Curculio, 18, 7–8.
- Louw, S. v. d. M. (1985b) The status of Hyomora adversaria occidentalis Louw (Coleoptera: Curculionidae: Rhytirrhininae). Journal of the Entomological Society of Southern Africa, 48 (2), 342–343.
- Oberprieler, R. G., & Louw, S. v. d. M. (1985) Curculionoidea. Pp. 270–280. In: Scholtz, C. H., & Holm, E. (Eds.), Insects of Southern Africa. Butterworths, Durban, 502 pp.
- Louw, S. v. d. M. (1986a) Revision of the Microcerinae (Coleoptera: Curculionidae) with an analysis of their phylogeny and zoogeography. Memoirs van die Nasionale Museum, Bloemfontein, 21, 1–331.
- Louw, S. v. d. M. (1986b) Curculionidae collections in southern Africa. Curculio, 21, 3–4.
- Louw, S. v. d. M. (1987) In situ predation by ants (Hymenoptera: Formicidae) on the eggs of Brachycerus ornatus Drury (Coleoptera: Curculionidae: Brachycerinae). The Coleopterists Bulletin, 41 (2), 180.
- Louw, S. v. d. M. (1988a) Weevils in bird diets. Rostrum, 19, 4.
- Louw, S. v. d. M. (1988b) Snuitkewers in die maaginhoude van voëls. Nasionale Museum Nuus, 34, 24–25.
- Louw, S. v. d. M. (1988c) Notes on adult overwintering of Entiminae and Microcerinae (Coleoptera: Curculionidae) in southern Africa. The Coleopterists’ Bulletin, 42 (2), 155–156.
- Louw, S. v. d. M. (1988d) Arboreal Coleoptera associated with Leucosidea sericea (Rosaceae) at the Golden Gate Highlands National Park. Koedoe, 31, 53–70.
- Louw, S. v. d. M. (1988e) Taxonomic and nomenclatorial notes on Rhytirrhininae (Coleoptera: Curculionidae). The Coleopterists’ Bulletin, 42 (3), 217–218.
- Louw, S. v. d. M. (1990a) The life-history and immature stages of Brachycerus ornatus Drury (Coleoptera: Curculionidae). Journal of the Entomological Society of Southern Africa, 53 (1), 27–40.
- Louw, S. v. d. M. (1990b) General processing and storage of a Curculionidae (Coleoptera) larval collection. Pp. 49–52. In: Herholdt, E. M. (Ed.). Natural History Collections: their Management and Value. Transvaal Museum Special Publication No. 1, Transvaal Museum, Pretoria.
- Louw, S. v. d. M. (1991) A new species of Breviurodon Strejcek (Coleoptera: Urodontidae) from Zaire and its bearing on urodontid phylogeny. African Journal of Zoology, 105, 323–329.
- Louw, S. v. d. M. (1993a) Systematics of the Urodontidae (Coleoptera: Curculionoidea) of southern Africa. Entomology Memoirs of the Department of Agriculture, Republic of South Africa, 87, 1–92.
- Louw, S. v. d. M. (1993b) Breeding populations of Lixus carinerostris Boheman and Calodemas prolixus Faust (Coleoptera: Curculionidae) co-existing on Mesembryanthemum. The Coleopterists Bulletin, 47 (4), 335–339.
- Louw, S. v. d. M. (1993c) Seed-feeding Urodontidae weevils and the evolution of the galling habit. Pp. 186–193. In:Price, P. W., Mattson, W. J., & Baranchikov, Y. N. (Eds.). The Ecology and Evolution of Gall-forming Insects. United States Department of Agriculture, Forest Service, North Central Forest Experiment Station. General Technical Report NC-174.
- Kok, O. B., & Louw, S. v. d. M. (1994) Bird and mammal predators of curculionid and tenebrionid beetles in semi-arid regions of South Africa. Journal of African Zoology, 108, 555–563.
- Louw, S. v. d. M., Van Eeden, C. F., & Weeks, W. J. (1995) Weevil infestation on cultivated Amaranthus in South Africa. African Crops Science Journal, 3 (1), 93–98.
- Louw, S. v. d. M. (1995) Systematics and biogeography of the subfamily Microcerinae (Coleoptera: Curculionidae): A re-evaluation based on larval morphology. Memoirs of the Entomological Society of Washington, 14, 169–174.
- Van Eeden, C. F., Weeks, W. J., & Louw, S. v. d. M. (1996) Insects associated with wild and cultivated Amaranthus spp. (Amaranthaceae) in South Africa. Proceedings of 2nd Crop Science Conference for Eastern and Southern Africa, University of Malawi, Blantyre.
- Price, P. W., & Louw, S. v. d. M. (1996) Resource manipulation through resource modification of the host plant by a galling weevil, Urodontus scholtzi Louw (Coleoptera: Urodontidae). African Entomology, 4 (2), 103–110.
- Louw, S v. d. M. (1998a) Weevils systematics in the 21st Century. Atti Museo Regionale di Scienze Naturale Torino, pp. 7–17.
- Louw, S. v. d. M. (1998b) Solving the riddle: Combining life-history analysis and morphological comparison in weevil phylogenetics. Atti Museo Regionale di Scienze Naturale Torino, pp. 19–26.
- Louw, S. v. d. M. (1998c) The gall-inhabiting weevil (Coleoptera) community on Galenia africana (Aizoaceae): co-existence or competition? Pp. 122–126. In: Csóka, G., Mattson, W. J., Stone, G. N., & Price, P. W. (Eds.). The Biology of Gall-inducing Arthropods. United States Department of Agriculture, Forest Service, North Central Forest Experiment Station, General Technical Report NC-199.
- Blodgett, J. T., Swart, W. J., Kloppers, F. J., & Louw, S. v. d. M. (1998) Identification of fungi associated with Hypolixus haerens in Amaranthus hybridus stems. South African Journal of Science, 94 (11), xxv–xxvi.
- Louw, S. v. d. M., Swart, W. J., Honiball, S. J., & Chen, W. (2002) Weevil-fungus interaction on Amaranthus hybridus (Amaranthaceae) in South Africa. African Entomology, 10 (2), 361–364.
- Kiggundu, A., Gold, C. S., Labuschagne, M. T., Vuylsteke, D., & Louw, S. v. d. M. (2003) Levels of resistance to Banana Weevil (Cosmopolites sordidus) in Musa germplasm in Uganda. Euphytica, 133, 267–277.
- Louw, S. v. d. M. (2004) Microcerini Lacordaire, 1863 (Coleoptera, Curculionoidea). Pp. 905–935. In: Sforzi, A., & Bartolozzi, L. (Eds.). 2004. Brentidae of the World. Monographia di Museo Regionale di Scienzi Naturali, Torino, 38, 971 pp.
- Blodgett, J. T., Swart, W. J., & Louw, S. v. d. M. (2004) Identification of fungi and fungal pathogens associated with Hypolixus haerens and decayed and cankered stems of Amaranthus hybridus. Plant Disease, 88, 333–337.
- Kok, O. B., Louw, S. v. d. M., & Kok, A. C. (2005) Snuitkewers in die diëet van die Swart Korhaan: meer as net voedsel? Suid-Afrikaanse Tydskrif vir Natuurwetenskap en Tegnologie, 24 (4), 118–123.
References
- Kuschel, G. A phylogenetic classification of Curculionoidea to families and subfamilies. Mem. Entomol. Soc. Wash. 1995, 14, 5–33. [Google Scholar]
- Louw, S. Systematics of the Urodontidae (Coleoptera: Curculionoidea) of southern Africa. Entomol. Mem. Dept. Agric. 1993, 87, 1–92. [Google Scholar]
- Osella, G.; Colonnelli, E.; Zuppa, A.M. Mediterranean Curculionoidea with southern African affinities. In Taxonomy, Ecology and Distribution of Curculionoidea (Coleoptera: Polyphaga), Proceedings of the XX. International Congress of Entomology, Florenze, Italy, 28 August 1996; Colonnelli, E., Louw, S., Osella, G., Eds.; Museo Regionale de Scienze Naturali: Torino, Italy, 1998; pp. 221–265. [Google Scholar]
- Mucina, L.; Rutherford, M.C. The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 2006, 19, 1–807. [Google Scholar]
- Germishuizen, G.; Meyer, N.L. Plants of southern Africa: An annotated checklist. Strelitzia 2003, 14, 1–1231. [Google Scholar]
- Price, P.W.; Louw, S.v.d.M. Resource manipulation through architectural modifications of the host plant by a gall-forming weevil, Urodontus scholtzi (Coleoptera: Anthribidae). Afr. Entomol. 1996, 4, 103–110. [Google Scholar]
- Strejcek, J. Breviurodon africanus get. et sp. n. aus Congo-Brazzaville (Coleoptera, Urodonidae). Annls. Hist.-Nat. Mus. Nat. Hung. 1981, 73, 203–205. [Google Scholar]
- Louw, S. A new species of Breviurodon Stejcek (Coleoptera: Urodontidae) from Zaire and its bearing on urodontid phylogeny. J. Afr. Zool. 1991, 105, 323–329. [Google Scholar]
- Bailey, P.; Sagliocco, J.-L.; Vitou, J.; Cooke, D. Prospects for biological control of cutleaf mignonette, Reseda lutea (Resedaceae), by Baris picicornius and Bruchela spp. in Australia. Aust. J. Exp. Agric. 2002, 42, 185–194. [Google Scholar] [CrossRef]
- Buddeberg, K.D. Beobachtungen über Lebensweise und Entwicklungsgeschichte einiger bei Nassau vorkommender Käfer: Mecinus janthinus Germ., Baris morio Schh., Phlocosinus thujae Perris, Urodon conformis Suffr. Jahrb. Nassau. Ver. Naturkd. 1883, 36, 124–144. [Google Scholar]
- May, B.M. Larvae of Curculionoidea (Insecta: Coleoptera): A systematic overview. Fauna N. Z. 1993, 28, 1–223. [Google Scholar]
- Dorchin, N.; Harris, K.M.; Jaschhof, M. 22. Cecidomyiidae (gall midges). In Manual of Afrotropical Diptera. Volume 2. Nematocerous Diptera and Lower Brachycera. Suricata 5; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; South African National Biodiversity Institute: Pretoria, South Africa, 2017; pp. 581–599. [Google Scholar]
- Louw, S.; Oberprieler, R.G. Willy Kuschel visits South Africa. Curculio 1993, 34, 5–6. [Google Scholar]
- Oberprieler, R.G.; Mansell, M.W.; Louw, S. Richtersveld—An insectile paradise / Richtersveld se insekte. Custos 1993, 21, 30–35. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).