Plasticity in Three-Dimensional Geometry of Branching Corals Along a Cross-Shelf Gradient
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Size and Collection
2.2. Photogrammetry & Measurements of Complexity
2.3. Statistical Analysis
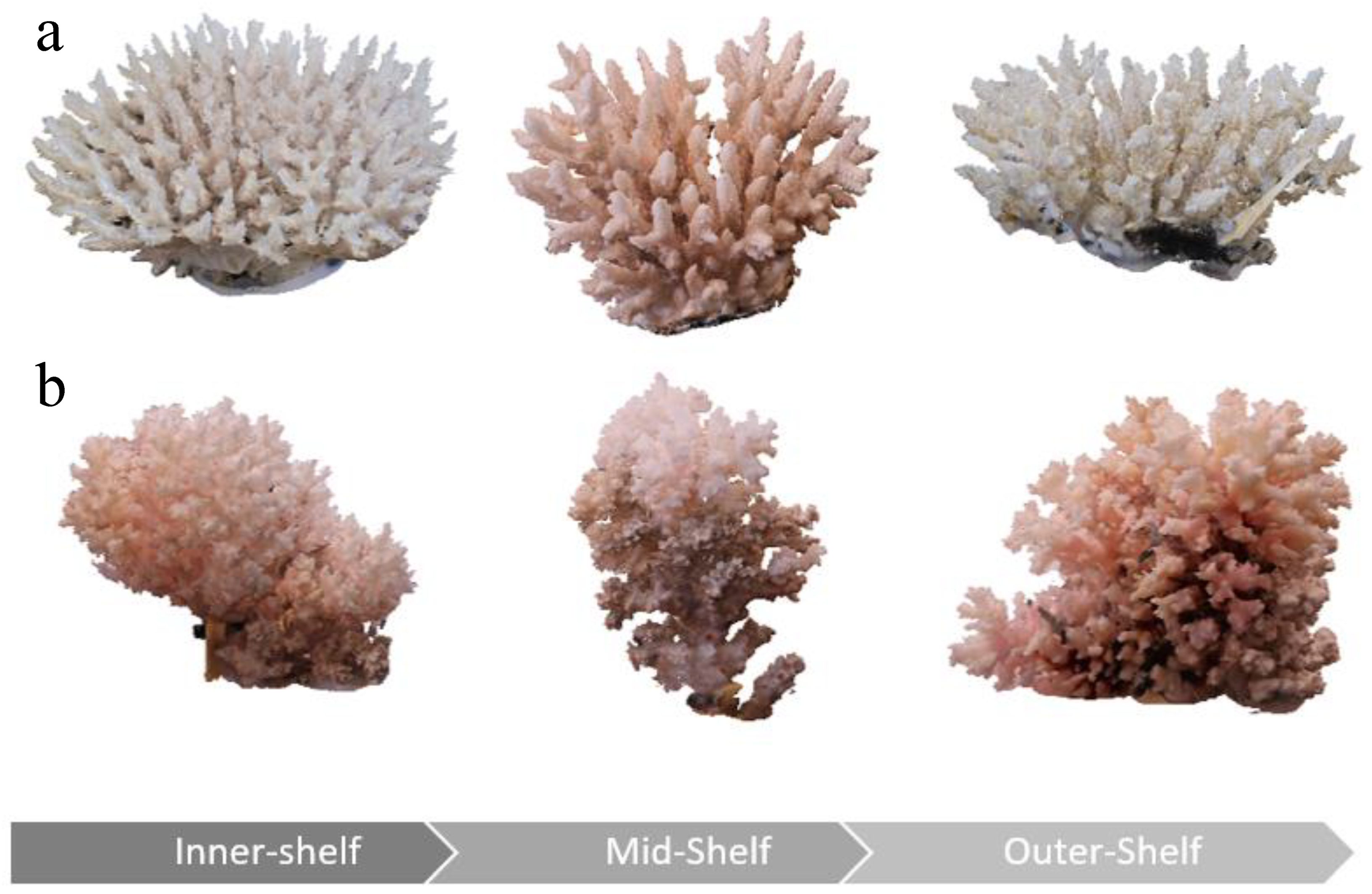
3. Results
3.1. Proportion Occupied (PrOcc)
3.2. Surface Area: Volume Ratio (SAV)
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hillebrand, H.; Matthiessen, B. Biodiversity in a complex world: Consolidation and progress in functional biodiversity research. Ecol. Lett. 2009, 12, 1405–1419. [Google Scholar] [CrossRef]
- Messier, J.; McGill, B.J.; Lechowicz, M.J. How do traits vary across ecological scales? A case for trait-based ecology. Ecol. Lett. 2010, 13, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Anderson, K.; Hoogenboom, M.O.; Widman, E.; Baird, A.H.; Pandolfi, J.M.; Edmunds, P.J.; Lough, J.M. Spatial, Temporal and Taxonomic Variation in Coral Growth–Implications for the Strucutre and Function of Coral Reef Ecosystems. Oceanogr. Mar. Biol. 2015, 53. [Google Scholar]
- McWilliam, M.; Chase, T.J.; Hoogenboom, M.O. Neighbor Diversity Regulates the Productivity of Coral Assemblages. Curr. Biol. 2018, 28, 3634–3639.e3. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Noriega, M.; Baird, A.H.; Dornelas, M.; Madin, J.S.; Cumbo, V.R.; Connolly, S.R. Fecundity and the demographic strategies of coral morphologies. Ecology 2016, 97, 3485–3493. [Google Scholar] [CrossRef] [Green Version]
- Madin, J.S.; Hoogenboom, M.O.; Connolly, S.R. Integrating physiological and biomechanical drivers of population growth over environmental gradients on coral reefs. J. Exp. Biol. 2012, 215, 968–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, J.B.; Hughes, T.P. Adaptive strategies of coral-reef invertebrates: Coral-reef environments that are regularly disturbed by storms and by predation often favor the very organisms most susceptible to damage by these processes. Am. Sci. 1985, 73, 265–274. [Google Scholar]
- Hixon, M.A.; Beets, J.B. Predation, prey refuges and the strugure of coral reef fish assemblages. Ecol. Monogr. 1993, 63, 77–101. [Google Scholar] [CrossRef]
- Dustan, P.; Doherty, O.; Pardede, S. Digital reef rugosity estimates coral reef habitat complexity. PLoS ONE 2013, 8, e57386. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nystrom, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef]
- McWilliam, M.; Hoogenboom, M.O.; Baird, A.H.; Kuo, C.Y.; Madin, J.S.; Hughes, T.P. Biogeographical disparity in the functional diversity and redundancy of corals. Proc. Natl. Acad. Sci. USA 2018, 115, 3084–3089. [Google Scholar] [CrossRef]
- Todd, P.A. Morphological plasticity in scleractinian corals. Biol. Rev. Camb. Philos. Soc. 2008, 83, 315–337. [Google Scholar] [CrossRef]
- Foster, A.B. Environmental variation in skeletal morphology within the Caribbean Reef corals Montastrea annularis and Siderastrea siderea. Bull. Mar. Sci. 1980, 30, 687–709. [Google Scholar]
- Willis, B.L. Phenotypic plasticity versus phenotypic stability in the reef corals Turbinaria mesenterina and Pavona catus. In Proceedings of the 5th International Coral Reef Congress, Tahiti, French Polynesia, 27 May–1 June 1985; pp. 107–112. [Google Scholar]
- Brown, B.E. Adaptations of reef corals to physical environmental stress. Adv. Mar. Biol. 1997, 31, 221–229. [Google Scholar]
- Anthony, K.R.N.; Hoogenboom, M.O.; Baird, A.H.; Kuo, C.Y.; Madin, J.S.; Hughes, T.P. Adaptive variation in coral geometry and the optimization of internal colony light climates. Biol. Rev. 2005, 19, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Hoogenboom, M.O.; Connolly, S.R.; Anthony, K.R. Interactions between morphological and physiological plasticity optimize energy acquisition in corals. Ecology 2008, 89, 1144–1154. [Google Scholar] [CrossRef]
- Lesser, M.P.; Weis, V.M.; Patterson, M.R.; Jokiel, P.L. Effects of morphology and water motion on carbon delivery and productivity in the reef coral, Pocillopora damicornis (Linnaeus): Diffusion barriers, inorganic carbon limitation, and biochemical plasticity. J. Exp. Mar. Biol. Ecol. 1994, 178, 153–179. [Google Scholar] [CrossRef]
- Madin, J.S. Mechanical limitations of reef corals during hydrodynamic disturbances. Coral Reefs 2005, 24, 630–635. [Google Scholar] [CrossRef]
- Tebbett, S.B.; Goatley, C.H.R.; Bellwood, D.R. Algal turf sediments across the Great Barrier Reef: Putting coastal reefs in perspective. Mar. Pollut. Bull. 2018, 137, 518–525. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Logan, M.; Weeks, J.J.; Lewis, S.E.; Brodie, J. Changes in water clarity in response to river discharges on the Great Barrier Reef continental shelf: 2002–2013. Est. Coast. Shelf Sci. 2016, 173, A1–A15. [Google Scholar] [CrossRef] [Green Version]
- Storlazzi, C.D.; Norris, B.K.; Rosenberger, K.J. The influence of grain size, grain color, and suspended-sediment concentration on light attenuation: Why fine-grained terrestrial sediment is bad for coral reef ecosystems. Coral Reefs 2015, 34, 967. [Google Scholar] [CrossRef]
- Brodie, J.E.; Kroon, F.J.; Schaffelke, B.; Wolanski, E.C.; Lewis, S.E.; Devlin, M.J.; Bohnet, I.C.; Bainbridge, Z.T.; Waterhouse, J.; Davis, A.M. Terrestrial pollutant runoff to the Great Barrier Reef: An update of issues, priorities and management responses. Mar. Pollut. Bull. 2012, 65, 81–100. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Wainwright, P. Locomotion in labrid fishes: Implications for habitat use and cross-shelf biogeography on the Great Barrier Reef. Coral Reefs 2001, 20, 139–150. [Google Scholar] [CrossRef]
- Noonan, S.H.; Jones, G.P.; Pratchett, M.S. Coral size, health and structural complexity: Effects on the ecology of a coral reef damselfish. Mar. Ecol. Prog. Ser. 2012, 456, 127–137. [Google Scholar] [CrossRef]
- Jupiter, S.; Roff, G.; Marion, G.; Henderson, M.; Schramever, V.; McCulloch, M.; Hoegh-Guldberg, O. Linkages between coral assemblages and coral proxies of terrestrial exposure along a cross-shelf gradient on the southern Great Barrier Reef. Coral Reefs 2008, 27, 887–903. [Google Scholar] [CrossRef]
- Emslie, M.J.; Pratchett, M.S.; Cheal, A.J.; Osborne, K. Great Barrier Reef butterflyfish community structure: The role of shelf position and benthic community type. Coral Reefs 2010, 29, 705–715. [Google Scholar] [CrossRef]
- Burn, D.; Pratchett, M.S.; Heron, S.F.; Thompson, C.A.; Pratchett, D.; Hoey, A.S. Limited Cross-Shelf Variation in the Growth of Three Branching Corals on Australia’s Great Barrier Reef. Diversity 2018, 10, 122. [Google Scholar] [CrossRef]
- Hughes, T.P.; Day, J.C.; Brodie, J. Securing the future of the Great Barrier Reef. Nat. Clim. Change 2015, 5, 508–511. [Google Scholar] [CrossRef]
- Kroon, F.J.; Thorburn, P.; Schaffelke, B.; Whitten, S. Towards protecting the Great Barrier Reef from land-based pollution. Glob. Chang. Biol. 2016, 22, 1985–2002. [Google Scholar] [CrossRef] [Green Version]
- De Clippele, L.H.; Huvenne, V.A.I.; Lundalv, T.; Fox, A.; Hennig, S.J.; Roberts, J.M. The effect of local hydrodynamics on the spatial extent and morphology of cold-water coral habitats at Tisler Reef, Norway. Coral Reefs 2018, 37, 253–266. [Google Scholar] [CrossRef]
- Chindapol, N.; Kaandorp, J.A.; Cronemberger, C.; Mass, T.; Genin, A. Modelling growth and form of the scleractinian coral Pocillopora verrucosa and the influence of hydrodynamics. PLoS Comput. Biol. 2013, 9, e1002849. [Google Scholar] [CrossRef]
- Erftemeijer, P.A.L.; Riegl, B.; Hoeksema, B.W.; Todd, P.A. Environmental impacts of dredging and other sediment disturbanes on corals: A review. Mar. Pollut. Bull. 2012, 64, 1737–1765. [Google Scholar] [CrossRef]
- Figueira, W.; Ferrari, R.; Weatherby, E.; Porter, A.; Hawes, S.; Byrne, M. Accuracy and Precision of Habitat Structural Complexity Metrics Derived from Underwater Photogrammetry. Remote Sensing 2015, 7, 16883–16900. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, R.; Figueira, W.F.; Pratchett, M.S.; Boube, T.; Adam, A.; Kobelkowsky-Vidrio, T.; Doo, S.S.; Atwood, T.B.; Byrne, M. 3D photogrammetry quantifies growth and external erosion of individual coral colonies and skeletons. Sci. Rep. 2017, 7, 16737. [Google Scholar] [CrossRef] [Green Version]
- Gochfeld. Predation-induced morphological and behavioral defenses in a hard coral: Implications for foraging behavior of coral-feeding butterflyfishes. Mar. Ecol. Prog. Ser. 2004, 267, 145–158. [Google Scholar] [CrossRef]
- Roberts, C.M.; Ormond, R.F.G. Habitat complexity and coral reef fish diversity and abundance on Red Sea fringing reefs. Mar. Ecol. Prog. Ser. 1987, 41, 1–8. [Google Scholar] [CrossRef]
- Kerry, J.T.; Bellwood, D.R. The effect of coral morphology on shelter selection by coral reef fishes. Coral Reefs 2012, 31, 415–424. [Google Scholar] [CrossRef]
- Valadez-Rocha, V.; Ortiz-Lozano, L. Spatial and temporal effects of port facilities expansion on the surface area of shallow coral reefs. Environ. Manag. 2013, 52, 250–260. [Google Scholar] [CrossRef]
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L., Jr.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [Green Version]
- Airoldi, L. The effects of sedimentation on rocky coast assemblages. Oceanogr Mar Biol Ann Rev 2003, 41. [Google Scholar]
- Pratchett, M.S.; Bay, L.K.; Gehrke, P.C.; Koehn, J.D.; Osborne, K.; Pressey, R.L.; Sweatman, H.P.A.; Wachenfeld, D. Contribution of climate change to degradation and loss of critical fish habitats in Australian marine and freshwater environments. Mar. Freshw. Res. 2011, 62, 1062–1081. [Google Scholar] [CrossRef]
- Osborne, K.; Dolman, A.M.; Burgess, S.C.; Johns, K.A. Disturbance and the Dynamics of Coral Cover on the Great Barrier Reef (1995–2009). PLoS ONE 2011, 6, e17516. [Google Scholar] [CrossRef]
- Palardy, J.E.; Grottoli, A.G.; Matthews, K.A. Effects of upwelling, depth, morphology and polyp size on feeding in three species of Panamanian corals. Mar. Ecol. Prog. Ser. 2005, 300, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Duckworth, A.; Giofre, N.; Jones, R. Coral morphology and sedimentation. Mar. Pollut. Bull. 2017, 125, 289–300. [Google Scholar] [CrossRef]
- Anthony, K.R.; Fabricius, K.E. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J. Exp. Mar. Bio. Ecol. 2000, 252, 221–253. [Google Scholar] [CrossRef]
- Amaral, F.D. Morphological variation in the reef coral Montastrea cavernosa in Brazil. Coral Reefs 1994, 13, 113–117. [Google Scholar] [CrossRef]
- Porter, J.W. Autotrophy, heterotrophy and resource partitioning in Caribbean reef-building corals. Am. Nat. 1976. [Google Scholar] [CrossRef]
- Madin, J.S.; Connolly, S.R. Ecological consequences of major hydrodynamic disturbances on coral reefs. Nature 2006, 444, 477–480. [Google Scholar] [CrossRef]
- Baldock, T.E.; Karampour, H.; Sleep, R.; Vyltla, A.; Albermani, F.; Golshani, A.; Callaghan, D.P.; Roff, G.; Mumby, P.J. Resilience of branching and massive corals to wave loading under sea level rise—A coupled computational fluid dynamics-structural analysis. Mar. Pollut. Bull. 2014, 86, 91–101. [Google Scholar] [CrossRef]
- Brazeau, D.A.; Lasker, H.R. Inter- and intraspecific variation in gorgonian colony morphology: Quantifying branching patterns in arborescent animals. Coral Reefs 1988, 7, 139–143. [Google Scholar] [CrossRef]
- Wolanski, E.C.; Drew, E.; Abel, K.M.; O’Brien, J. Tidal jets, nutrient upwelling and their influence on the productivity of the alga Halimeda in the Ribbon Reefs, Great Barrier Reef. Estuar. Coast Shelf Sci. 1988, 26, 169–2001. [Google Scholar] [CrossRef]
- Bay, L.K.; Ulstrup, K.E.; Nielsen, H.B.; Jarmer, H.; Goffard, N.; Willis, B.L.; Miller, D.J.; Van Oppen, M.J. Microarray analysis reveals transcriptional plasticity in the reef building coral Acropora millepora. Mol. Ecol. 2009, 18, 3062–3075. [Google Scholar] [CrossRef]
- Marti-Puig, P.; Forsman, Z.H.; Haverkort-Yeh, R.D.; Knapp, I.S.S.; Maragos, J.E.; Toonen, R.J. Extreme phenotypic polymorphism in the coral genus Pocillopora; micro-morphology corresponds to mitochondrial groups, while colony morphology does not. Bull of Mar. Sci. 2014, 90, 211–231. [Google Scholar] [CrossRef]
- Schmidt-Roach, S.; Miller, K.J.; Andreakis, N. Pocillopora aliciae: A new species of scleractinian coral (Scleractinia, Pocilloporidae) from subtropical Eastern Australia. Zootaxa 2013, 3626, 576–582. [Google Scholar] [CrossRef]
- Prada, C.; Schizas, N.V.; Yoshioka, P.M. Phenotypic plasticity or speciation? A case from a clonal marine organism. BMC Evol. Biol. 2008, 8, 47. [Google Scholar] [CrossRef]
- Madin, J.S.; Hoogenboom, M.O.; Connolly, S.R.; Darling, E. A trait-based approach to advance coral reef science. Trends Ecol. Evol. 2016, 31, 419–428. [Google Scholar] [CrossRef]


| Species | Inner-Shelf | Mid-Shelf | Outer-Shelf |
|---|---|---|---|
| Acropora nasuta | 16 | 4 | 12 |
| Pocillopora spp | 18 | 17 | 16 |
| Stylophora pistillata | 1 | 0 | 10 |
| Process | Settings |
|---|---|
| Photo alignment | High accuracy, pair and reference preselection disabled, key point limit 40,000, tie point limit 4000, features not constrained by mask, no adaptive camera model fitting |
| Sparse point cloud | All optimisation properties ‘yes’ except fit b1, b2, k4, p3, p4, adaptive camera model fitting |
| Dense point cloud | High quality *, moderate depth filtering, do not reuse depth maps, calculate point colors |
| Mesh | Arbitrary surface type, source data-dense cloud, face count high, interpolation enabled, all point classes, don’t reuse depth maps |
| Texture | Generic mapping mode, texture from all cameras, mosaic blending mode, texture size 8192, texture count 1, hole filling |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doszpot, N.E.; McWilliam, M.J.; Pratchett, M.S.; Hoey, A.S.; Figueira, W.F. Plasticity in Three-Dimensional Geometry of Branching Corals Along a Cross-Shelf Gradient. Diversity 2019, 11, 44. https://doi.org/10.3390/d11030044
Doszpot NE, McWilliam MJ, Pratchett MS, Hoey AS, Figueira WF. Plasticity in Three-Dimensional Geometry of Branching Corals Along a Cross-Shelf Gradient. Diversity. 2019; 11(3):44. https://doi.org/10.3390/d11030044
Chicago/Turabian StyleDoszpot, Neil E., Michael J. McWilliam, Morgan S. Pratchett, Andrew S. Hoey, and Will F. Figueira. 2019. "Plasticity in Three-Dimensional Geometry of Branching Corals Along a Cross-Shelf Gradient" Diversity 11, no. 3: 44. https://doi.org/10.3390/d11030044
APA StyleDoszpot, N. E., McWilliam, M. J., Pratchett, M. S., Hoey, A. S., & Figueira, W. F. (2019). Plasticity in Three-Dimensional Geometry of Branching Corals Along a Cross-Shelf Gradient. Diversity, 11(3), 44. https://doi.org/10.3390/d11030044





