Latitudinal and Cross-Shelf Patterns of Size, Age, Growth, and Mortality of a Tropical Damselfish Acanthochromis polyacanthus on the Great Barrier Reef
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling Design
2.2. Processing of Samples
2.3. Analysis of Growth Increments
2.4. Analyses of Size and Age
2.5. Growth
2.6. Mortality
3. Results
3.1. Color Morphs
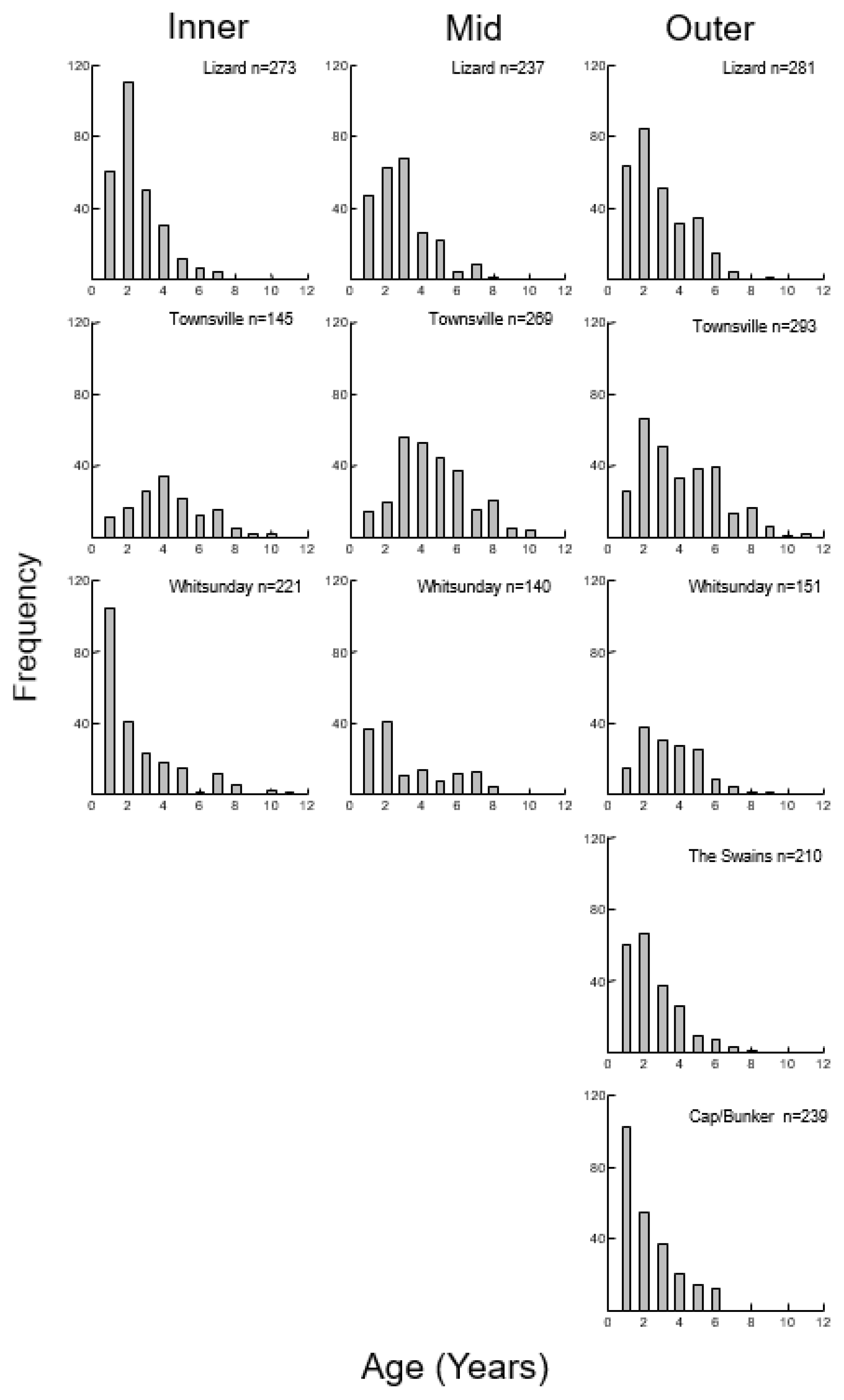
3.2. Size, Age, and Growth
3.3. Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conover, D.O.; Brown, J.J.; Ehtisham, A. Countergradient variation in growth of young striped bass (Morone saxatilis) from different latitudes. Can. J. Fish. Aquat. Sci. 1997, 54, 2401–2409. [Google Scholar] [CrossRef]
- Boehlert, G.W.; Kappenman, R.F. Variation of growth with latitude of two species of rock fishes (Sebastes pinniger and S. diploproa) from the North Eastern Pacific Ocean. Mar. Ecol. Prog. Ser. 1980, 3, 1–10. [Google Scholar] [CrossRef]
- Trip, E.L.; Choat, J.H.; Wilson, D.T.; Robertson, D.R. Inter-oceanic analysis of demographic variation in a widely distributed Indo-Pacific coral reef fish. Mar. Ecol. Prog. Ser. 2008, 373, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Hilborn, R.; Walters, C.J. Quantitative Fisheries Stock Assessment; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Boaden, A.E.; Kingsford, M.J. Predators drive community structure in coral reef fish assemblages. Ecosphere 2015, 6, 1–33. [Google Scholar] [CrossRef]
- Houde, E.D. Comparative growth, mortality, and energetics of marine fish larvae-temperature and implied latitudinal effects. Fish. Bull. 1989, 87, 471–495. [Google Scholar]
- Atkinson, D. Temperature and Organism Size: A Biological Law for Ectotherms. In Advances in Ecological Research; Begon, M., Fitter, A.H., Eds.; Academic Press Ltd-Elsevier Science Ltd.: London, UK, 1994; Volume 25, pp. 1–58. [Google Scholar]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Yamahira, K.; Conover, D.O. Intra-Vs. interspecific latitudinal variation in growth: Adaptation to temperature or seasonality? Ecology 2002, 83, 1252–1262. [Google Scholar] [CrossRef]
- Choat, J.H.; Robertson, D.R. Age-Based Studies on Coral Reef Fishes. Coral Reef Fishes: Dynamics and Diversity in a Complex Ecosystem; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Cappo, M.; Marriott, R.J.; Newman, S.J. James’s rule and causes and consequences of a latitudinal cline in the demography of John’s Snapper (Lutjanus johnii) in coastal waters of Australia. Fish. Bull. 2013, 111, 309–324. [Google Scholar] [CrossRef]
- Piddocke, T.P.; Butler, G.L.; Butcher, P.A.; Stewart, J.; Bucher, D.J.; Christidis, L. Age and growth of mangrove red snapper Lutjanus argentimaculatus at its cool-water-range limits. J. Fish Biol. 2015, 86, 1587–1600. [Google Scholar] [CrossRef]
- Williams, A.J.; Davies, C.R.; Mapstone, B.D.; Currey, L.M.; Welch, D.J.; Begg, G.A.; Ballagh, A.C.; Choat, J.H.; Murchie, C.D.; Simpfendorfer, C.A. Age-based demography of humpback grouper Cromileptes altivelis: Implications for fisheries management and conservation. Endanger. Species Res. 2009, 9, 67–79. [Google Scholar] [CrossRef]
- Williams, D.M. Patterns of distribution of fish communities across the central Great Barrier Reef lagoon. Coral Reefs 1982, 1, 35–43. [Google Scholar] [CrossRef]
- Williams, D.M.; Russ, G.; Doherty, P.J. Reef Fish: Large scale distribution and recruitment. Oceanus 1986, 29, 76–82. [Google Scholar]
- Russ, G. Distribution and abundance of herbivorous grazing fishes in the central Great Barrier Reef. I. Levels of variability across the entire continental shelf. Mar. Ecol. Prog. Ser. 1984, 20, 23–34. [Google Scholar] [CrossRef]
- Williams, D.M.; Hatcher, A.I. Structure of fish communities on outer slopes of inshore, midshelf and outer shelf reefs of the Great Barrier Reef. Mar. Ecol. Prog. Ser. 1983, 10, 239–250. [Google Scholar] [CrossRef]
- Furnas, M.J. Catchments and Corals: Terrestrial Runoff to the Great Barrier Reef; Australian Institute of Marine Science: Townsville, Australia, 2003.
- Bainbridge, Z.T.; Wolanski, E.; Alvarez-Romero, J.G.; Lewis, S.E.; Brodie, J.E. Fine sediment and nutrient dynamics related to particle size and floc formation in a Burdekin River flood plume, Australia. Mar. Pollut. Bull. 2012, 65, 236–248. [Google Scholar] [CrossRef]
- Wolanski, E. Oceanographic Processes of Coral Reefs: Physical and Biological Links in the Great Barrier Reef; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Gust, N.; Choat, J.H.; Ackerman, J.L. Demographic plasticity in tropical reef fishes. Mar. Biol. 2002, 140, 1039–1051. [Google Scholar]
- Taylor, B.M.; Brandl, S.J.; Kapur, M.; Robbins, W.D.; Johnson, G.; Huveneers, C.; Renaud, P.; Choat, J.H. Bottom-up processes mediated by social systems drive demographic traits of coral-reef fishes. Ecology 2018, 99, 642–651. [Google Scholar] [CrossRef]
- Russ, G.R.; Alcala, A.C. Effects of intense fishing pressure on an assemblage of coral reef fish. Mar. Ecol. Prog. Ser. 1989, 56, 13–27. [Google Scholar] [CrossRef]
- Babcock, R.C.; Shears, N.T.; Alcala, A.C.; Barrett, N.S.; Edgar, G.J.; Lafferty, K.D.; McClanahan, T.R.; Russ, G.R. Decadal trends in marine reserves reveal differential rates of change in direct and indirect effects. Proc. Natl. Acad. Sci. USA 2010, 107, 18256–18261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, D.H.; Russ, G.R.; Ayling, A.M. No-take marine reserves increase abundance and biomass of reef fish on inshore fringing reefs of the Great Barrier Reef. Environ. Conserv. 2004, 31, 149–159. [Google Scholar] [CrossRef]
- Kingsford, M.J.; Hughes, J.M. Patterns of growth, mortality and size of a tropical damselfish Acanthochromis polyacanthus across the continental shelf of the Great Barrier Reef. Fish. Bull. 2005, 103, 561–573. [Google Scholar]
- Allen, G.R. Damselfishes of the World; Mergus: Melle, Germany, 1991. [Google Scholar]
- Allen, G.R. Damselfishes of the South Seas; TFH Publications: Neptune Cuty, NJ, USA, 1975. [Google Scholar]
- Planes, S.; Doherty, P.J.; Bernardi, G. Strong genetic divergence among populations of a marine fish with limited dispersal, Acanthochromis polyacanthus, within the Great Barrier Reef and the Coral Sea. Evolution 2001, 55, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, A.; Siegel, V.; Pakhomov, E.; Rothery, P. Long-term decline in krill stock and increase in salps within the southern ocean. Nature 2004, 432, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Underwood, A.J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Schnute, J. A versatile growth model with statistically stable parameters. Can. J. Fish. Aquat. Sci. 1981, 38, 1128–1140. [Google Scholar] [CrossRef]
- Kimura, D.K. Likelihood methods for comparison of von Bertalanffy growth curves. Fish. Bull. 1980, 77, 765–776. [Google Scholar]
- Pauly, D. Population dymanics in tropical waters. ICLARM Stud. Rev. 1984, 8, 1–325. [Google Scholar]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations; Department of the Environment: Canberra, Australia, 1975.
- Kritzer, J.P. Variation in the population biology of stripey bass Lutjanus carponotatus within and between two island groups on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002, 243, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Walther, B.D.; Kingsford, M.J.; McCulloch, M.T. Environmental Records from Great Barrier Reef Corals: Inshore versus Offshore Drivers. PLoS ONE 2013, 8, 11. [Google Scholar]
- Kingsford, M.J.; Hughes, J.M.; Patterson, H.M. Otolith chemistry of the non-dispersing reef fish Acanthochromis polyacanthus: Cross-shelf patterns from the central Great Barrier Reef. Mar. Ecol. Prog. Ser. 2009, 377, 279–288. [Google Scholar] [CrossRef]
- Fry, G.C.; Milton, D.A. Age, growth and mortality estimates for populations of red snappers Lutjanus erythropterus and L. malabaricus from northern Australia and eastern Indonesia. Fish. Sci. 2009, 75, 1219–1229. [Google Scholar] [CrossRef]
- Williams, A.J.; Davies, C.R.; Mapstone, B.D.; Russ, G.R. Scales of spatial variation in demography of a large coral-reef fish - an exception to the typical model? Fish. Bull. 2003, 101, 673–683. [Google Scholar]
- Williams, A.J.; Mapstone, B.D.; Davies, C.R. Spatial patterns in cohort-specific mortality of red throat emperor, Lethrinus miniatus, on the Great Barrier Reef. Fish Res. 2007, 84, 328–337. [Google Scholar] [CrossRef]
- Ong, J.J.L.; Rountrey, A.N.; Marriott, R.J.; Newman, S.J.; Meeuwig, J.J.; Meekan, M.G. Cross-continent comparisons reveal differing environmental drivers of growth of the coral reef fish, Lutjanus bohar. Coral Reefs 2017, 36, 195–206. [Google Scholar] [CrossRef]
- Munday, P.L.; Kingsford, M.J.; O’Callaghan, M.D.; Donelson, J.M. Elevated temperature restricts growth potential of the coral reef fish Acanthochromis polyacanthus. Coral Reefs 2008, 27, 927–931. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Baird, A.H.; Connolly, S.R.; Dietzel, A.; Eakin, C.M.; Heron, S.F.; Hoey, A.S.; Hoogenboom, M.O.; Liu, G.; et al. Global warming transforms coral reef assemblages. Nature 2018, 556, 492–496. [Google Scholar] [CrossRef]
- Wolanski, E.; Richmond, R.; McCook, L.; Sweatman, H. Mud, marine snow and coral reefs-The survival of coral reefs requires integrated watershed-based management activities and marine conservation. Am. Sci. 2003, 91, 44–51. [Google Scholar] [CrossRef]
- Wenger, A.S.; Johansen, J.L.; Jones, G.P. Increasing suspended sediment reduces foraging, growth and condition of a planktivorous damselfish. J. Exp. Mar. Biol. Ecol. 2012, 428, 43–48. [Google Scholar] [CrossRef]
- Gillanders, B.M. Comparison of growth rates between estuarine and coastal reef populations of Achoerodus viridis (Pisces: Labridae). Mar. Ecol. Prog. Ser. 1997, 146, 283–287. [Google Scholar] [CrossRef]
- Robertson, D.R.; Ackerman, J.L.; Choat, J.H.; Posada, J.M.; Pitt, J. Ocean surgeonfish Acanthurus bahianus. I. The geography of demography. Mar. Ecol. Prog. Ser. 2005, 295, 229–244. [Google Scholar] [CrossRef]
- Vigliola, L.; Meekan, M.G. Size at hatching and planktonic growth determine post-settlement survivorship of a coral reef fish. Oecologia 2002, 131, 89–93. [Google Scholar] [CrossRef]






| Latitude | Shelf | Total n | Reef | n | Dates Collected |
|---|---|---|---|---|---|
| Lizard | Inner | 278 | Martin | 162 | Nov 2004 |
| Linnet | 116 | Nov 2004 | |||
| Mid | 237 | Eagle Islet | 99 | Dec 2001 | |
| North Direction Is. | 57 | Dec 2001 | |||
| MacGillivray | 111 | Dec 2001 | |||
| Outer | 336 | Yonge | 155 | Dec 2001 | |
| Day | 138 | Dec 2001 | |||
| No Name* | 43 | Dec 2001 | |||
| Townsville | Inner | 156 | Havannah Island | 70 | Sep 2001 |
| Orpheus Island | 41 | Sep 2001 | |||
| Pandora | 45 | Sep 2001 | |||
| Mid | 286 | Britomart | 88 | Oct 2001 | |
| Bramble | 105 | Oct 2001 | |||
| Slashers | 93 | Oct 2001 | |||
| Outer | 298 | Barnett Patches | 116 | Oct 2001 | |
| Pith | 100 | Oct 2001 | |||
| Myrmidon | 82 | Oct 2001 | |||
| Whitsunday | Inner | 232 | Hook Island | 105 | Sep 2003 |
| South Mole | 127 | Sep 2003 | |||
| Mid | 173 | Line | 84 | Sep 2003 | |
| Net | 89 | Sep 2003 | |||
| Outer | 169 | Elizabeth | 79 | Sep 2003 | |
| Ellen | 90 | Sep 2003 | |||
| Swains | Outer | 218 | Hixson Cay | 82 | Jan 2006 |
| Sweetlips | 70 | Jan 2006 | |||
| Sandshoe | 66 | Jan 2006 | |||
| Cap/Bunker | Outer | 256 | One Tree Island | 97 | Jan/Feb 2002 |
| Lamont | 73 | Jan/Feb 2002, Feb 2006 | |||
| Heron Island | 86 | Jan/Feb 2002 |
| Latitude | Shelf | LAT (°S) | n | Linf | K | T0 | Mortality m(r2) | ASR (%) | AMAX | Mean Age 10% | SLMAX | Mean SL 10% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lizard Island | Inner | 14° | 273 | 88.64 | 1.38 | −0.04 | 0.681 (0.61) | 51 | 7 | 5.9 | 98 | 94 |
| Mid | 14° | 237 | 94.53 | 1.52 | −0.04 | 0.578 (0.52) | 47 | 8 | 6.0 | 105 | 102 | |
| Outer | 14° | 281 | 93.13 | 1.55 | −0.04 | 0.536 (0.54) | 50 | 9 | 5.8 | 105 | 100 | |
| Townsville | Inner | 18° | 145 | 82.01 | 1.06 | −0.06 | 0.506 (0.55) | 60 | 10 | 8.0 | 100 | 92 |
| Mid | 18° | 269 | 97.85 | 1.06 | −0.05 | 0.398 (0.47) | 67 | 10 | 8.5 | 120 | 112 | |
| Outer | 18° | 293 | 101.16 | 1.16 | −0.04 | 0.432 (0.65) | 65 | 11 | 8.3 | 116 | 110 | |
| Whitsundays | Inner | 19° | 221 | 83.17 | 1.77 | −0.04 | 0.438 (0.35) | 65 | 11 | 7.6 | 98 | 92 |
| Mid | 19° | 140 | 94.96 | 1.14 | −0.05 | 0.245 (0.55) | 80 | 8 | 7.4 | 111 | 104 | |
| Outer | 19° | 151 | 90.40 | 1.64 | −0.03 | 0.398 (0.41) | 56 | 9 | 6.6 | 104 | 99 | |
| Swains | Outer | 22° | 210 | 94.05 | 1.45 | −0.04 | 0.617 (0.64) | 51 | 8 | 5.7 | 112 | 100 |
| Capricorn Bunker | Outer | 23° | 239 | 80.84 | 1.58 | −0.04 | 0.414 (0.49) | 66 | 6 | 5.5 | 102 | 93 |
| SL 10% C = 0.21, ns | Age 10% C = 0.14, ns | ||||
|---|---|---|---|---|---|
| Source of variation | df | MS | F | MS | F |
| Latitude | 2 | 15.7 | 0.35 | 18.0 | 19.6 *** |
| Distance | 2 | 874.4 | 19.3 *** | 1.43 | 1.55 |
| L × D | 4 | 264.5 | 5.8 * | 5.0 | 5.4 * |
| Reef (L × D) | 9 | 45.2 | 10.3 *** | 0.92 | 1.15 |
| Residual | 60 | 4.4 | 0.79 |
| SLMAX 10% | AMAX 10% | ||||
|---|---|---|---|---|---|
| Source of variation | df | MS | Variance Component % | MS | Variance Component % |
| Latitude | 4 | 486.7 ns | 29 | 12.95 ns | 21 |
| Reef(Lat) | 5 | 247.3 *** | 58 | 4.67 * | 15 |
| Residual | 60 | 7.9 | 13 | 1.76 | 64 |
| (a) The top 10% by size (SLMAX 10%); the factor location (Distance × Z) was significant. | |||
| Distance | Latitude | MPA | Fished |
| Inner (Location 1) | Lizard | 95.3 (0.7) 3 | 97.5 (1.3) 6 |
| Inner (Location 2) | Townsville | 87.5 (0.9) 4 | 91.5 (0.6) 3 |
| Outer (Location1) | Townsville | 111.3 (1.3) 5 | 113 (1.1) 5 |
| Outer (Location 2) | Capricorn Bunker | 82 (0.9) 4 | 99 (1.5) 6 |
| (b) The top 10% of fish by age (AMAX 10%); the interaction Distance × Zone was significant. | |||
| Distance | Latitude | MPA | Fished |
| Inner (Location 1) | Lizard | 6 (0) 0 | 6.8 (1) 2 |
| Inner (Location 2) | Townsville | 6.8 (0.3) 1 | 7.8 (1) 2 |
| Outer (Location 1) | Townsville | 9.5 (0.6) 3 | 9 (0.7) 3 |
| Outer (Location 2) | Capricorn Bunker | 6 (0) 0 | 6 (0) 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kingsford, M.J.; Welch, D.; O’Callaghan, M. Latitudinal and Cross-Shelf Patterns of Size, Age, Growth, and Mortality of a Tropical Damselfish Acanthochromis polyacanthus on the Great Barrier Reef. Diversity 2019, 11, 67. https://doi.org/10.3390/d11050067
Kingsford MJ, Welch D, O’Callaghan M. Latitudinal and Cross-Shelf Patterns of Size, Age, Growth, and Mortality of a Tropical Damselfish Acanthochromis polyacanthus on the Great Barrier Reef. Diversity. 2019; 11(5):67. https://doi.org/10.3390/d11050067
Chicago/Turabian StyleKingsford, Michael J., David Welch, and Mark O’Callaghan. 2019. "Latitudinal and Cross-Shelf Patterns of Size, Age, Growth, and Mortality of a Tropical Damselfish Acanthochromis polyacanthus on the Great Barrier Reef" Diversity 11, no. 5: 67. https://doi.org/10.3390/d11050067
APA StyleKingsford, M. J., Welch, D., & O’Callaghan, M. (2019). Latitudinal and Cross-Shelf Patterns of Size, Age, Growth, and Mortality of a Tropical Damselfish Acanthochromis polyacanthus on the Great Barrier Reef. Diversity, 11(5), 67. https://doi.org/10.3390/d11050067




