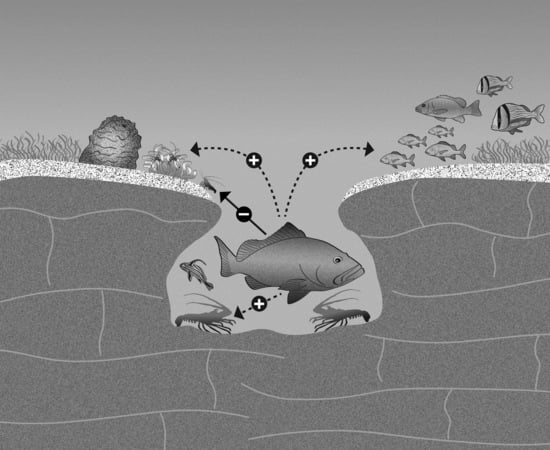
Red Grouper (Epinephelus morio) Shape Faunal Communities via Multiple Ecological Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Statistical Analysis
3. Results
4. Discussion
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Family | Species | Common Name | Functional Group | |
|---|---|---|---|---|
| Feeding | Habitat | |||
| Acanthuridae | Acanthurus chirurgus | Doctorfish | BA | MILL |
| Acanthurus coeruleus | Blue Tang | BA | MILL | |
| Apogonidae | Apogon binotatus | Barred Cardinalfish | PL | DEM |
| Apogon maculatus | Flamefish | ZB | DEM | |
| Balistidae | Balistes capriscus | Gray Triggerfish | ZB | MILL |
| Chaetodontidae | Chaetodon ocellatus | Spotfin Butterflyfish | ZB | MILL |
| Chaetodon sedentarius | Reef Butterflyfish | ZB | MILL | |
| Gerreidae | Eucinostomus melanopterus | Flagfin Mojarra | ZB | TRANS |
| Ginglymostomatidae | Ginglymostoma cirratum | Nurse Shark | INV | DEM |
| Gobiidae | Coryphopterus glaucofraenum | Bridled Goby | BA | DEM |
| Elacatinus oceanops | Neon Goby | CL | DEM | |
| Haemulidae | Anisotremus virginicus | Porkfish | CL/ZB | MILL |
| Haemulon chrysargyreum | Smallmouth Grunt | ZB | MILL | |
| Haemulon flavolineatum | French Grunt | ZB | MILL | |
| Haemulon plumierii | White Grunt | ZB | MILL | |
| Haemulon sciurus | Bluestriped Grunt | ZB | MILL | |
| Labridae | Lachnolaimus maximus | Hogfish | ZB | TRANS |
| Thalassoma bifasciatum | Bluehead Wrasse | CL/PL | TRANS | |
| Lutjanidae | Lutjanus griseus | Gray Snapper | ZB | TRANS |
| Lutjanus synagris | Lane Snapper | ZB | TRANS | |
| Ocyurus chrysurus | Yellowtail Snapper | PV | TRANS | |
| Mullidae | Pseudupeneus maculatus | Spotted Goatfish | ZB | TRANS |
| Muraenidae | Gymnothorax funebris | Green Moray | INV/PV | DEM |
| Gymnothorax vicinus | Purplemouth Moray | INV/PV | DEM | |
| Pomacanthidae | Holacanthus bermudensis | Blue Angelfish | ZB | MILL |
| Holacanthus ciliaris | Queen Angelfish | ZB | MILL | |
| Pomacanthus arcuatus | Gray Angelfish | ZB | MILL | |
| Pomacanthus paru | French Angelfish | CL/ZB | MILL | |
| Pomacentridae | Abudefdef saxatilis | Sergeant Major | ZB | MILL |
| Scaridae | Scarus coeruleus | Blue Parrotfish | BA | TRANS |
| Scarus iseri | Striped Parrotfish | BA | TRANS | |
| Sparisoma aurofrenatum | Redband Parrotfish | BA | TRANS | |
| Sciaenidae | Equetus lanceolatus | Jacknife Fish | ZB | DEM |
| Pareques acuminatus | High-Hat | ZB | DEM | |
| Scorpaenidae | Pterois miles/volitans | Lionfish | PV | DEM |
| Scorpaena plumieri | Spotted Scorpionfish | PV | DEM | |
| Serranidae | Diplectrum formosum | Sand Perch | ZB | TRANS |
| Epinephelus itajara | Goliath Grouper | INV | DEM | |
| Epinephelus morio | Red Grouper | INV | DEM | |
| Hypoplectrus puella | Barred Hamlet | ZB | MILL | |
| Mycteroperca bonaci | Black Grouper | PV | MILL | |
| Sparidae | Calamus calamus | Saucereye Porgy | ZB | TRANS |
| Phylum | Family | Species | Common Name | Functional Group | |
|---|---|---|---|---|---|
| RG Diet | CL | ||||
| Arthropoda | Hippolytidae | Lysmata spp. | Peppermint shrimp | N | Y |
| Majidae | Mithrax spinosissimus | Channel clinging crab | Y | N | |
| Palaemonidae | Ancylomenes pedersoni | Pederson’s cleaner shrimp | N | Y | |
| Periclimines yucatanicus | Spotted cleaner shrimp | N | Y | ||
| Palinuridae | Panulirus argus | Caribbean spiny lobster | Y | N | |
| Scyllaridae | Scyllarides aequinoctialis | Spanish lobster | Y | N | |
| Stenopodidae | Stenorhynchus seticornis | Yellowline arrow crab | N | N | |
| Xanthidae | Menippe mercenaria | Florida stone crab | N | N | |
| Echinodermata | Clypeasteridae | Clypeaster rosaceus | Inflated sea biscuit | N | N |
References
- Jones, C.G.; Lawton, J.H.; Shachak, M. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 1997, 78, 1946–1957. [Google Scholar] [CrossRef]
- Bruno, J.F.; Bertness, M.D. Habitat modification and facilitation in benthic marine communities. In Marine Community Ecology; Bertness, M.D., Gaines, S.D., Hay, M.E., Eds.; Sinauer Associates: Sunderland, MA, USA, 2001; pp. 201–218. [Google Scholar]
- Dayton, P.K. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound Antarctica. In Proceedings of the Colloquium on Conservation Problems in Antarctica; Parker, B.C., Ed.; Allen Press: Lawrence, KA, USA, 1972; pp. 81–96. [Google Scholar]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Naeem, S.; Thompson, L.J.; Lawler, S.P.; Lawton, J.H.; Woodfin, R.M. Declining biodiversity can alter the performance of ecosystems. Nature 1994, 368, 734–737. [Google Scholar] [CrossRef]
- Coleman, F.C.; Williams, S.L. Overexploiting marine ecosystem engineers: Potential consequences for biodiversity. Trends Ecol. Evol. 2002, 17, 40–44. [Google Scholar] [CrossRef]
- Stachowicz, J.J.; Bruno, J.F.; Duffy, J.E. Understanding the effects of marine biodiversity on communities and ecosystems. Ann. Rev. 2007, 38, 739–766. [Google Scholar] [CrossRef]
- Jackson, D.R.; Milstrey, E.G. The Fauna of Gopher Tortoise Burrows; Florida Game and Fresh Water Fish Commission Nongame Wildlife Program Technical Report No. 5; Florida Game and Fresh Water Fish Commission: Tallahassee, FL, USA, 1989. [Google Scholar]
- Kinlaw, A.; Grasmueck, M. Evidence for and geomorphologic consequences of a reptilian ecosystem engineer: The burrowing cascade initiated by the Gopher Tortoise. Geomorphology 2011, 157–158, 108–121. [Google Scholar] [CrossRef]
- Able, K.W.; Grimes, C.B.; Jones, R.S.; Twichell, D.C. Temporal and spatial variation in habitat characteristics of tilefish (Lopholatilus chamaeloeonticefs) off the east coast of Florida. Bull. Mar. Sci. 1993, 53, 1013–1026. [Google Scholar]
- Hixon, M.A. Predation as a process structuring coral-reef fish communities. In The Ecology of Fishes on Coral Reefs; Sale, P.F., Ed.; Academic Press: San Diego, CA, USA, 1991; pp. 475–508. [Google Scholar]
- Almany, G.R.; Webster, M.S. The predation gauntlet: Early post-settlement mortality in reef fishes. Coral Reefs 2006, 25, 19–22. [Google Scholar] [CrossRef]
- Stallings, C.D. Predator identity and recruitment of coral-reef fishes: Indirect effects of fishing. Mar. Ecol. Prog. Ser. 2009, 383, 251–259. [Google Scholar] [CrossRef]
- Stier, A.C.; Geange, S.W.; Hanson, K.M.; Bolker, B.M. Predator density and timing of arrival affect reef fish community assembly. Ecology 2013, 94, 1057–1068. [Google Scholar] [CrossRef]
- Heinlein, J.M.; Stier, A.C.; Steele, M.A. Predators reduce abundance and species richness of coral reef fish recruits via non-selective predation. Coral Reefs 2010, 29, 527–532. [Google Scholar] [CrossRef]
- Albins, M.A. Effects of invasive Pacific red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish communities. Biol. Invasions 2013, 15, 29–43. [Google Scholar] [CrossRef]
- Keough, M.J.; Downes, B.J. Recruitment of marine invertebrates: The role of active larval choice and early mortality. Oecologia 1982, 54, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Beukers, J.S.; Jones, G.P. Habitat complexity modifies the impact of piscivores on a coral reef fish population. Oceologia 1997, 114, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Aust. Ecol. 2001, 26, 32–46. [Google Scholar]
- Hixon, M.A.; Carr, M.H. Synergistic predation, density dependence, and population regulation in marine fish. Science 1997, 277, 946–949. [Google Scholar] [CrossRef]
- Stallings, C.D. Indirect effects of an exploited predator on recruitment of coral-reef fishes. Ecology 2008, 89, 2090–2095. [Google Scholar] [CrossRef]
- Paine, R.T. Food web complexity and species diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Paine, R.T. A note on trophic complexity and community stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- McLean, J.H. Sublittoral ecology of kelp beds of the open coast near Carmel, California. Biol. Bull. 1962, 122, 95–114. [Google Scholar] [CrossRef]
- Estes, J.A.; Palmisano, J.F. Sea otters: Their role in structuring nearshore communities. Science 1974, 185, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Estes, J.A.; Smith, N.S.; Palmisano, J.F. Sea otter predation and community organization in the Western Aleutian Islands, Alaska. Ecology 1978, 59, 822–833. [Google Scholar] [CrossRef]
- Estes, J.A.; Duggins, D.O. Sea otters and kelp forests in Alaska: Generality and variation in a community ecological paradigm. Ecol. Monogr. 1995, 65, 75–100. [Google Scholar] [CrossRef]
- Coleman, F.C.; Koenig, C.C.; Scanlon, K.M.; Heppell, S.; Heppell, S.; Miller, M.W. Benthic habitat modification through excavation by red grouper, Epinephelus morio, in the northeastern Gulf of Mexico. Open Fish Sci. J. 2010, 3, 1–15. [Google Scholar] [CrossRef]
- Ellis, R.D.; Coleman, F.C.; Koenig, C.C. Effects of habitat manipulation by red grouper, Epinephelus morio, on faunal communities associated with excavations in Florida Bay. Bull. Mar. Sci. 2017, 93, 961–983. [Google Scholar] [CrossRef]
- Herrnkind, W.F.; Butler IV, M.J.; Hunt, J.H.; Childress, M. Role of physical refugia: Implications from a mass sponge die-off in a lobster nursery in Florida. Mar. Fresh. Res. 1997, 48, 759–769. [Google Scholar] [CrossRef]
- Randall, J.E. Food habits of reef fishes of the West Indies. Stud. Trop. Ocean. 1967, 5, 665–847. [Google Scholar]
- Moe, M.A. Biology of the Red Grouper (Epinephelus morio Valenciennes) from the Eastern Gulf of Mexico; Professional Papers Series; Florida Department of Natural Resources, Marine Science Laboratory: St. Petersburg, FL, USA, 1967. [Google Scholar]
- Bullock, L.H.; Smith, G.B. Seabasses (Pisces: Serranidae). Memoirs of the Hourglass Cruises,X; Florida Marine Research Institute, Department of Natural Resources: St. Petersburg, FL, USA, 1991; Volume VIII, Part II. [Google Scholar]
- Brulé, T.; Rodriguez Canche, L.G. Food habits of juvenile red groupers, Epinephelus morio (Valenciennes, 1828), from Campeche Bank, Yucatan, Mexico. Bull. Mar. Sci. 1993, 52, 772–779. [Google Scholar]
- Weaver, D.C. Feeding Ecology and Ecomorphology of Three Sea Basses (Pisces: Serranidae) in the Gulf of Mexico. Master’s thesis, University of Florida, Gainesville, FL, USA, 1996. [Google Scholar]
- Brulé, T.; Avila, D.O.; Crespo, M.S.; Deniel, C. Seasonal and diel changes in diet composition of juvenile red grouper (Epinephelus morio) from Campeche Bank. Bull. Mar. Sci. 1994, 55, 255–262. [Google Scholar]
- Hacker, S.; Gaines, S.D. Some implications of direct positive interactions for community species diversity. Ecology 1997, 78, 1990–2003. [Google Scholar] [CrossRef]
- Dill, L.M.; Heithaus, M.R.; Walters, C.J. Behaviorally mediated indirect interactions in marine communities and their conservation implications. Ecology 2003, 84, 1151–1157. [Google Scholar] [CrossRef]
- Ellis, R.D. Ecological effects of Red Grouper (Epinephelus morio) in Florida Bay. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 2015. [Google Scholar]
- Ellis, R.D.; Faletti, M.E. Native grouper indirectly ameliorates the negative effects of invasive lionfish. Mar. Ecol. Prog. Ser. 2016, 558, 267–279. [Google Scholar] [CrossRef]
- Rhyne, A.L.; Lin, J. A western Atlantic peppermint shrimp complex: Redescription of Lysmata wurdemanni, description of four new species, and remarks on Lysmata rathbunae (Crustacea: Decapoda: Hipploytidae). Bull. Mar. Sci. 2006, 79, 165–204. [Google Scholar]
- Hill, M.O. Diversity and evenness: A unifying notation and its consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef]
- Hill, M.O. An evenness statistic based on the abundance-weighted variance of species proportions. Oikos 1997, 79, 413–416. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities, 1st ed.; MjM Software Design: Gleneden Beach, OR, USA, 2002. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package. R package version 2.0-10, 2013. Available online: https://CRAN.R-project.org/package=vegan (accessed on 13 September 2013).
- Paine, R.T. Food-web analysis through field measurement of per capita interaction strength. Nature 1992, 355, 73–75. [Google Scholar] [CrossRef]
- Berlow, E.L.; Navarrete, S.A.; Briggs, C.J.; Power, M.E.; Menge, B.A. Quantifying variation in the strengths of species interactions. Ecology 1999, 80, 2206–2224. [Google Scholar] [CrossRef]
- Hesterberg, T. Resample: Resampling Functions. R package version 0.4, 2015. Available online: https://CRAN.R-project.org/package=resample (accessed on 25 April 2015).
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Behav. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Nagkawa, S.; Cuthill, I.C. Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 2007, 82, 591–605. [Google Scholar] [CrossRef]
- Osenberg, C.W.; Sarnelle, O.; Cooper, S.D. Effect size in ecological experiments: The application of biological models in meta-analysis. Am. Nat. 1997, 150, 798–812. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. Available online: http://www.fishbase.org (accessed on 25 April 2015).
- Kirby, K.N.; Gerlanc, D. BootES: An R package for bootstrap confidence intervals on effect sizes. Behav. Res. Meth. 2013, 45, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Ellison, A.M. Partitioning diversity. Ecology 2010, 91, 1962–1963. [Google Scholar] [CrossRef] [PubMed]
- Jost, L. Independence of alpha and beta diversities. Ecology 2010, 91, 1969–1974. [Google Scholar] [CrossRef] [PubMed]
- Bertelsen, R.D.; Cox, C. Sanctuary roles in population and reproductive dynamics of Caribbean spiny lobster. In Spatial Processes and Management of Marine Populations; Kruse, G.H., Bez, N., Booth, A., Dorn, M.W., Hills, S., Lipcius, R.N., Pelletier, D., Roy, C., Smith, S.J., Witherells, D., Eds.; University of Alaska Sea Grant: Fairbanks, AK, USA, 2001; pp. 591–605. [Google Scholar]
- Ellis, R.D. Species interactions through ontogeny: Effects of size-selective predation by red grouper on Caribbean spiny lobster in solution holes of Florida Bay. J. Exp. Mar. Biol. Ecol. 2018, 506, 115–123. [Google Scholar] [CrossRef]
- Jones, C.G.; Gutiérrez, J.L.; Byers, J.E.; Crooks, J.A.; Lambrinos, J.G.; Talley, T.S. A framework for understanding physical ecosystem engineering by organisms. Oikos 2010, 119, 1862–1869. [Google Scholar] [CrossRef]
- Thayer, G.W.; Powell, A.B.; Hoss, D.E. Composition of larval, juvenile, and small adult fishes relative to changes in environmental conditions in Florida Bay. Estuaries 1999, 22, 518–533. [Google Scholar] [CrossRef]
- Sponaugle, S.; Paris, C.; Walter, K.D.; Kourafalou, V.; Alessandro, E.D. Observed and modeled larval settlement of a reef fish to the Florida Keys. Mar. Ecol. Prog. Ser. 2012, 453, 201–212. [Google Scholar] [CrossRef]
- Silbiger, N.J.; Childress, M.J. Interspecific variation in anemone shrimp distribution and host selection in the Florida Keys (USA): Implications for marine conservation. Bull. Mar. Sci. 2008, 83, 329–345. [Google Scholar]
- Wicksten, M.K. Associations of fishes and their cleaners on coral reefs of Bonaire, Netherlands Antilles. Copeia 1995, 2, 477–481. [Google Scholar] [CrossRef]
- Zieman, J.; Fourqurean, J.W.; Iverson, R.L. Distribution, abundance and productivity of seagrasses and macroalgae in Florida Bay. Bull. Mar. Sci. 1989, 44, 292–311. [Google Scholar]







| Common Name | Species | Individual Contribution | Cumulative Contribution | SIMPER Rank | Rank Abundance |
|---|---|---|---|---|---|
| Juvenile grunts | Haemulon spp. | 0.276 | 0.276 | 1 | 3 |
| White grunt | Haemulon plumierii | 0.216 | 0.492 | 2 | 2 |
| Caribbean spiny lobster | Panulirus argus | 0.217 | 0.709 | 3 | 1 |
| French grunt | Haemulon flavolineatum | 0.072 | 0.781 | 4 | 5 |
| Pederson’s cleaner shrimp | Ancylomenes pedersoni | 0.061 | 0.842 | 5 | 4 |
| Gray snapper | Lutjanus griseus | 0.034 | 0.876 | 6 | 6 |
| Porkfish | Anisotremus virginicus | 0.015 | 0.891 | 7 | 7 |
| Channel clinging crab | Mithrax spinosissimus | 0.013 | 0.904 | 8 | 8 |
| High-hat | Pareques acuminatus | 0.012 | 0.916 | 9 | 9 |
| French angelfish | Pomacanthus arcuatus | 0.010 | 0.926 | 10 | 13 |
| Blue angelfish | Holacanthus bermudensis | 0.009 | 0.935 | 11 | 10 |
| Florida stone crab | Menippe mercenaria | 0.009 | 0.944 | 12 | 11 |
| Peppermint shrimp | Lysmata spp. | 0.007 | 0.951 | 13 | 16 |
| Queen angelfish | Holacanthus ciliaris | 0.007 | 0.958 | 14 | 15 |
| Hogfish | Lachnolaimus maximus | 0.006 | 0.964 | 15 | 14 |
| Spotted cleaner shrimp | Periclimenes yucatanicus | 0.005 | 0.969 | 16 | 19 |
| Doctorfish | Acanthurus chirurgus | 0.005 | 0.974 | 17 | 20 |
| Striped parrotfish | Scarus iseri | 0.004 | 0.978 | 18 | 17 |
| Sand perch | Diplectrum formosum | 0.004 | 0.982 | 19 | 22 |
| Bluestriped grunt | Haemulon sciurus | 0.003 | 0.985 | 20 | 18 |
| Species | Common Name | Experimental Effect [PI] | Bootstrapped Effect (± SE) | 95% CI |
|---|---|---|---|---|
| Haemulon plumierii | White grunt | 4.96 | 4.97 (± 2.38) | 0.206, 9.737 |
| Panulirus argus | Caribbean spiny lobster | 3.39 | 3.29 (± 2.57) | −1.85, 8.44 |
| Haemulon spp. | Juvenile grunts | 1.57 | 1.56 (± 1.19) | −0.811, 3.95 |
| Ancylomenes pedersoni | Pederson’s cleaner shrimp | 0.520 | 0.512 (± 0.257) | −0.0031, 1.03 |
| Lutjanus griseus | Gray snapper | 0.482 | 0.482 (± 0.255) | −0.0282, 0.992 |
| Anisotremus virginicus | Porkfish | 0.362 | 0.356 (± 0.161) | 0.0332, 0.679 |
| Haemulon flavolineatum | French grunt | 0.281 | 0.290 (± 0.449) | −0.607, 1.19 |
| Pareques acuminatus | High-hat | 0.0191 | 0.0198 (± 0.082) | −0.159, 0.198 |
| Holacanthus bermudensis | Blue angelfish | −0.0125 | −0.0119 (± 0.0408) | −0.0945, 0.0697 |
| Lachnolaimus maximus | Hogfish | −0.0333 | −0.0331 (± 0.0149) | −0.0629, −0.00329 |
| Haemulon chrysargyreum | Smallmouth grunt | −0.0375 | −0.0368 (± 0.0362) | −0.109, 0.0356 |
| Mycteroperca bonaci | Black grouper | −0.0500 | −0.0515 (± 0.0485) | −0.148, 0.0455 |
| Lutjanus synagris | Lane snapper | −0.0583 | −0.0572 (± 0.0563) | −0.170, 0.0554 |
| Scarus coeruleus | Blue parrotfish | −0.0625 | −0.0624 (± 0.0607) | −0.184, 0.0590 |
| Pomacanthus paru | French angelfish | −0.0625 | −0.0634 (± 0.0563) | −0.183, 0.0558 |
| Balistes capriscus | Gray triggerfish | −0.0625 | −0.0643 (± 0.0606) | −0.185, 0.0568 |
| Haemulon sciurus | Bluestriped grunt | −0.0667 | −0.0685 (± 0.0438) | −0.156, 0.0190 |
| Menippe mercenaria | Florida stone crab | −0.0750 | −0.0730 (± 0.0670) | −0.207, 0.0611 |
| Scarus iseri | Striped parrotfish | −0.0833 | −0.0812 (± 0.0551) | −0.191, 0.0290 |
| Holacanthus ciliaris | Queen angelfish | −0.100 | −0.0999 (± 0.0518) | −0.204, 0.00373 |
| Acanthurus chirurgus | Doctorfish | −0.104 | −0.103 (± 0.0686) | −0.241, 0.0339 |
| Equetus lanceolatus | Jackknife fish | −0.108 | −0.107 (± 0.0712) | −0.250, 0.0350 |
| Mithrax spinosissimus | Channel clinging crab | −0.145 | −0.147 (± 0.0873) | −0.321, 0.0279 |
| Ocyurus chrysurus | Yellowtail snapper | −0.163 | −0.161 (± 0.0812) | −0.323, 0.00199 |
| Pomacanthus arcuatus | Gray angelfish | −0.169 | −0.172 (± 0.0632) | −0.298, −0.0458 |
| Lysmata spp. | Peppermint shrimp | −0.175 | −0.178 (± 0.0694) | −0.317, −0.0395 |
| Diplectrum formosum | Sand perch | −0.215 | −0.211 (± 0.0911) | −0.393, −0.0290 |
| Periclimenes yucatanicus | Spotted cleaner shrimp | −0.244 | −0.244 (± 0.0794) | −0.402, −0.0848 |
| Functional Group | Effect Size g, Experimental | 95% CI | Effect Size g, Observational | 95% CI |
|---|---|---|---|---|
| All Fishes | 0.538 | −0.281, 1.09 | 1.41 | 1.11, 1.70 |
| Herbivores | 0.224 | −0.550, 1.03 | 0.626 | 0.216, 0.943 |
| Planktivores | 0.012 | −0.650, 0.823 | 1.02 | 0.792, 1.23 |
| Benthivores | 0.546 | −0.194, 1.07 | 0.645 | 0.375, 0.865 |
| Invertivores | 0.677 | 0.362, 1.11 | 0.439 | 0.043, 0.734 |
| Piscivores | 0.151 | −0.622, 0.751 | 0.276 | −0.127, 0.716 |
| Demersal Fishes | 0.469 | −0.215, 1.19 | 0.768 | 0.249, 1.23 |
| Water Column Fishes | 0.499 | −0.152, 1.10 | 1.34 | 1.07, 1.60 |
| Transient Fishes | 0.396 | −0.340, 1.04 | 0.0239 | −0.393, 0.376 |
| All Invertebrates | 0.346 | −0.446, 1.15 | 1.12 | 0.682, 1.51 |
| Red grouper Prey | 0.309 | −0.464, 1.10 | 0.771 | 0.375, 1.16 |
| Not Prey | 0.184 | −0.479, 1.05 | 0.959 | 0.561, 1.38 |
| Cleaners | 0.395 | −0.427, 1.16 | 0.993 | 0.539, 1.39 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ellis, R.D. Red Grouper (Epinephelus morio) Shape Faunal Communities via Multiple Ecological Pathways. Diversity 2019, 11, 89. https://doi.org/10.3390/d11060089
Ellis RD. Red Grouper (Epinephelus morio) Shape Faunal Communities via Multiple Ecological Pathways. Diversity. 2019; 11(6):89. https://doi.org/10.3390/d11060089
Chicago/Turabian StyleEllis, Robert D. 2019. "Red Grouper (Epinephelus morio) Shape Faunal Communities via Multiple Ecological Pathways" Diversity 11, no. 6: 89. https://doi.org/10.3390/d11060089
APA StyleEllis, R. D. (2019). Red Grouper (Epinephelus morio) Shape Faunal Communities via Multiple Ecological Pathways. Diversity, 11(6), 89. https://doi.org/10.3390/d11060089