Variability of A Subterranean Prey-Predator Community in Space and Time
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Poulson, T.L.; White, W.B. The cave environment. Science 1969, 165, 971–981. [Google Scholar] [CrossRef]
- Culver, D.C. Cave Life; Harvard University Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Smithson, P.A. Inter-relationships between cave and outside air temperatures. Theor. Appl. Climatol. 1991, 44, 65–73. [Google Scholar] [CrossRef]
- Camp, C.D.; Jensen, J.B. Use of twilight zones of caves by plethodontid salamanders. Copeia 2007, 2007, 594–604. [Google Scholar] [CrossRef]
- Tobin, B.W.; Hutchins, B.T.; Schwartz, B.F. Spatial and temporal changes in invertebrate assemblage structure from the entrance to deep-cave zone of a temperate marble cave. Int. J. Speleol. 2013, 42, 203–214. [Google Scholar] [CrossRef] [Green Version]
- Mammola, S.; Isaia, M. Day–night and seasonal variations of a subterranean invertebrate community in the twilight zone. Subterr. Biol. 2018, 27, 31–51. [Google Scholar] [CrossRef]
- Poulson, T.L. Food sources. In Encyclopedia of Caves; Culver, D.C., White, W.B., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2007; pp. 255–264. [Google Scholar]
- Coulver, D.C. Entrances. In Encyclopedia of Caves; Culver, D.C., White, W.B., Eds.; Elsevier Academic Press: Burlington, MA, USA, 2007; pp. 206–208. [Google Scholar]
- Keith, J.H. Seasonal changes in a population of Pseudanophtalmus tenuis (Coleoptera, Carabidae) in Murray Spring Cave, Indiana: A preliminary report. Int. J. Speleol. 1975, 7, 33–44. [Google Scholar] [CrossRef]
- Crouau-Roy, B.; Crouau, Y.; Ferre, C. Dynamic and temporal structure if the troglobitic beetle Speonomus hydrophilus (Coleoptera: Bathysciinae). Ecography 1992, 15, 12–18. [Google Scholar] [CrossRef]
- Camp, C.D.; Wooten, J.A.; Jensen, J.B.; Bartek, D.F. Role of temperature in determining relative abundance in cave twilight zones by two species of lungless salamander (family Plethodontidae). Can. J. Zool. 2014, 92, 119–127. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Cave features, seasonality and subterranean distribution of non-obligate cave dwellers. PeerJ 2017, 5, e3169. [Google Scholar] [CrossRef]
- Nitzu, E.; Nae, A.; Băncilă, R.; Popa, I.; Giurginca, A.; Plăiaşu, R. Scree habitats: Ecological function, species conservation and spatial-temporal variation in the arthropod community. Syst. Biodivers. 2014, 12, 65–75. [Google Scholar] [CrossRef]
- Pellegrini, T.G.; Ferreira, R.L. Are inner cave communities more stable than entrance communities in Lapa Nova show cave? Subterr. Biol. 2016, 20, 15–37. [Google Scholar] [CrossRef] [Green Version]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Seasonal variation in microhabitat of salamanders: Environmental variation or shift of habitat selection? PeerJ 2014, 3, e1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Russo, C.; Carchini, G.; Rampini, M.; Lucarelli, M.; Sbordoni, V. Long term stability of a terrestrial cave communty. Int. J. Speleol. 1997, 26, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Poulson, T.L.; Lavoie, K.H.; Helf, K. Long-term effects of weather on the cricket (Hadanoecus subterraneus, Orthoptera, Raphidiophoridae) guano community in Mommouth cave National Park. Am. Mid. Nat. 1995, 134, 226–236. [Google Scholar] [CrossRef]
- Badino, G. Cave temperatures and global climatic change. Int. J. Speleol. 2004, 33, 103–114. [Google Scholar] [CrossRef]
- Scheneider, K.; Christman, M.C.; Faga, W.F. The influence of resource subsidies on cave invertebrates: Results from an ecosystem-level manipulation experiment. Ecology 2011, 92, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Salvidio, S.; Lattes, A.; Tavano, M.; Melodia, F.; Pastorino, M. Ecology of a Speleomantes ambrosii population inhabiting an artificial tunnel. Amphib. Reptil. 1994, 15, 35–45. [Google Scholar]
- Isaia, M.; Giachino, P.M.; Sapino, E.; Casale, A.; Badino, G. Conservation value of artificial subterranean systems: A case study in an abandoned mine in Italy. J. Nat. Conserv. 2011, 19, 24–33. [Google Scholar] [CrossRef]
- Istituto Superiore per la Protezione e la Ricerca Ambientale (2005). Carta Geologica d’Italia alla scala 1:50.000. Available online: http://www.isprambiente.gov.it/Media/carg/213_GENOVA/Foglio.html (accessed on 7 December 2019).
- Salvidio, S.; Oneto, F.; Ottonello, D.; Pastorino, M.V. Monitoraggio a lungo termine del geotritone Speleomantes strinatii nella Stazione Biospeleologica di San Bartolomeo di Besolagno (GE). In Atti 22° Congresso Nazionale di Speleologia; De Nitto, L., Maurano, F., Parise, M., Eds.; Memorie dell’Istituto Italiano di Speleologia: Bologna, Italy, 2015; Volume II, pp. 29, 422–427. [Google Scholar]
- Pastorino, M.V.; Pedemonte, S. Nuove stazioni di raccolta del geotritone nell’Oltregiogo Genovese. Atti XI Congresso Nazionale di Speleologia 1974, 2, 81–82. [Google Scholar]
- Ravbar, N.; Kosutnik, J. Variations of karst underground air temperature induced by various factors (Cave of Županova jama, Central Slovenia). Theor. Appl. Climatol. 2014, 116, 327–341. [Google Scholar] [CrossRef]
- Lindström, L.; Reeve, R.; Salvidio, S. Bayesian salamanders: Analysing the demography of an underground population of the European plethodontid Speleomantes strinatii with state-space modelling. BMC Ecol. 2010, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvidio, S.; Oneto, F.; Ottonello, D.; Pastorino, M.V. Lagged influence of North Atlantic Oscillation on population dynamics of a Mediterranean terrestrial salamander. Inter. J. Biomet. 2016, 60, 475–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvidio, S.; Pastorino, M.V. Spatial segregation in the European plethodontid salamander Speleomantes strinatii in relation to age and sex. Amphib. Reptil. 2002, 23, 505–510. [Google Scholar]
- White, G.C.; Anderson, D.R.; Burnham, K.P.; Otis, D.L. Capture-Recapture Removal Methods for Sampling Closed Populations; Los Alamos National Laboratory 8787 NERP: Los Alamos, NM, USA, 1982; pp. 1–235. [Google Scholar]
- Lanza, B. Speleomantes strinatii (Aellen, 1958). In Fauna d’Italia. 42, Amphibia; Lanza, B., Andreone, F., Bologna, M.A., Corti, C., Razzetti, E., Eds.; Edizioni Calderini: Bologna, Italy, 2007; pp. 152–156. [Google Scholar]
- Salvidio, S. Life history of the European plethodontid salamander Speleomantes ambrosii. Herpetol. J. 1993, 3, 55–59. [Google Scholar]
- Gayanilo, F.C., Jr.; Sparre, P.; Pauly, D. The FAO-ICLARM Stock Assessment Tools (FiSAT) User’s Guide; Food and Agricolture Organisation of the United Nations: Rome, Italy, 1996; pp. 1–126. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 21 October 2019).
- ter Braak, C.J.F.; Verdonschot, F.M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat. Sci. 1995, 57, 255–289. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 1998; pp. 612–616. [Google Scholar]
- Mammola, S.; Isaia, M. Cave communities and species interactions. In Cave Ecology; Ecological Studies 235; Moldovan, O.T., Kováč, L., Halse, S., Eds.; Springer Nature: Cham, Switzerland, 2018; pp. 255–267. [Google Scholar]
- Sket, B. Can we agree on an ecological classification of subterranean animals? J. Nat. Hist. 2008, 42, 1549–1563. [Google Scholar] [CrossRef]
- Bellés, X. Survival, opportunism and convenience in the processes of cave colonization by terrestrial faunas. Oecol. Aquat. 1991, 10, 325–335. [Google Scholar]
- Culver, D.C.; Pipan, T. Shallow Subterranean Habitats: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2014; pp. 188–199. [Google Scholar]
- Salvidio, S.; Palumbi, G.; Romano, A.; Costa, A. Safe caves and dangerous forests? Predation risk may contribute to salamander colonization of subterranean habitats. Sci. Nat. 2017, 104, 20. [Google Scholar] [CrossRef]
- Lunghi, E.; Manenti, R.; Ficetola, G.F. Do cave features affect underground habitat exploitation by non-troglobite species? Acta Oecol. 2014, 55, 29–35. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Pennati, R.; Manenti, R. Spatial segregation among age classes in cave salamanders: Habitat selection or social interactions? Popul. Ecol. 2013, 55, 217–226. [Google Scholar] [CrossRef]
- Romero, A. Cave Biology: Life in Darkness; Cambridge University Press: Cambridge, UK, 2009; pp. 1–291. [Google Scholar]
- Romero, A. Caves as biological spaces. Polymath 2012, 2, 1–15. [Google Scholar]
- Mammola, S. Finding answers in the dark: Caves as models in ecology fifty years after Poulson and White. Ecography 2019, 42, 1331–1351. [Google Scholar] [CrossRef] [Green Version]
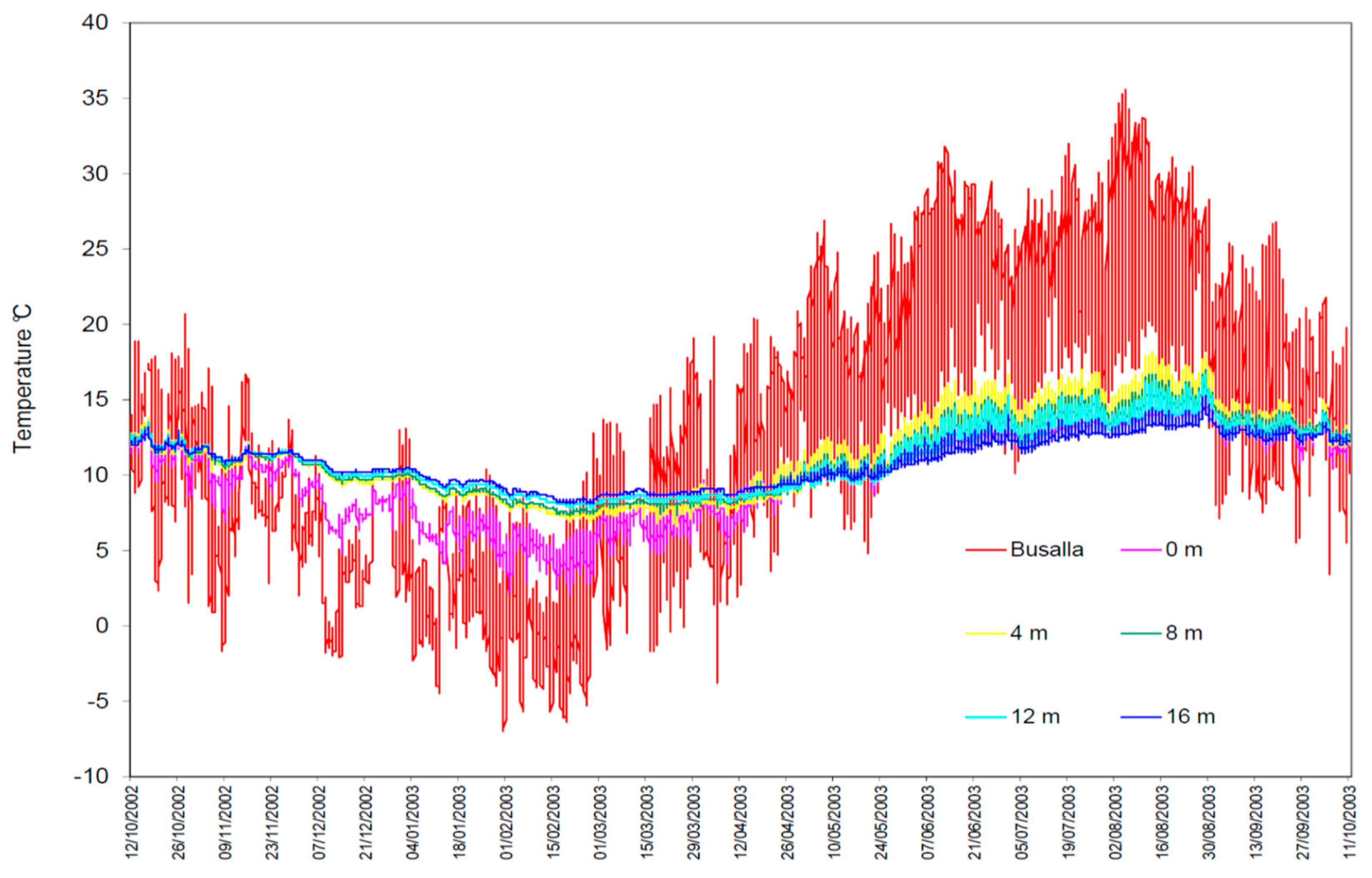

| Busalla Meteo-Station | Biospelological Station of Besolagno | |||||
|---|---|---|---|---|---|---|
| 0 m from entrance | 4 m from entrance | 8 m from entrance | 12 m from entrance | 16 m from entrance | ||
| T record number | 7306 | 7600 | 7600 | 7600 | 7600 | 7600 |
| T minimum (°C) | −7.0 | 2.1 | 6.6 | 7.3 | 7.8 | 8.1 |
| T maximum (°C) | 35.6 | 15.3 | 18.2 | 17.0 | 16.7 | 15.3 |
| T mean (°C) | 12.6 | 10.0 | 11.3 | 11.2 | 11.1 | 10.9 |
| Standard deviation | 8.9 | 2.9 | 2.7 | 2.4 | 2.1 | 1.7 |
| Coefficient of variation | 68.4 | 29.4 | 24.3 | 21.6 | 18.9 | 15.1 |
| Outside | Entrance | 3 m | 6 m | 9 m | 12 m | 15 m | 18 m | 21 m | |
|---|---|---|---|---|---|---|---|---|---|
| Year 2013 | |||||||||
| Diptera | 62 | 5 | 1 | 0 | 0 | 2 | 0 | 1 | 1 |
| Acarina | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hymenoptera | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coleoptera | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Homoptera | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Araneida | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Undetermined insects | 5 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Salamander males | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Salamander females | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 |
| Salamander subadults | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| Salamander juveniles | 0 | 0 | 5 | 2 | 2 | 1 | 0 | 0 | 0 |
| Year 2014 | |||||||||
| Diptera | 31 | 3 | 0 | 2 | 0 | 1 | 0 | 0 | 1 |
| Acarina | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hymenoptera | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coleoptera | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Homoptera | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Araneida | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Undetermined insects | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 |
| Salamander males | 0 | 0 | 0 | 1 | 0 | 4 | 1 | 0 | 0 |
| Salamander females | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Salamander subadults | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Salamander juveniles | 0 | 0 | 7 | 12 | 0 | 1 | 0 | 0 | 0 |
| Year 2015 | |||||||||
| Diptera | 30 | 1 | 2 | 3 | 0 | 2 | 3 | 5 | 2 |
| Acarina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hymenoptera | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coleoptera | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Homoptera | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Araneida | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Undetermined insects | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Salamander males | 0 | 0 | 1 | 2 | 2 | 1 | 0 | 2 | 1 |
| Salamander females | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Salamander subadults | 0 | 0 | 0 | 2 | 4 | 1 | 0 | 1 | 0 |
| Salamander juveniles | 0 | 0 | 6 | 2 | 0 | 1 | 0 | 0 | 0 |
| Year 2016 | |||||||||
| Diptera | 41 | 10 | 2 | 2 | 4 | 3 | 8 | 10 | 9 |
| Acarina | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hymenoptera | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coleoptera | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Homoptera | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Araneida | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Undetermined insects | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Salamander males | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| Salamander females | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Salamander subadults | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Salamander juveniles | 0 | 0 | 4 | 4 | 0 | 1 | 0 | 0 | 0 |
| Year 2017 | |||||||||
| Diptera | 64 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Acarina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hymenoptera | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coleoptera | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Homoptera | 32 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Araneida | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Undetermined insects | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Salamander males | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 1 |
| Salamander females | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Salamander subadults | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Salamander juveniles | 0 | 0 | 4 | 3 | 0 | 0 | 0 | 0 | 0 |
| Year 2018 | |||||||||
| Diptera | 40 | 3 | 7 | 2 | 2 | 20 | 21 | 25 | 44 |
| Acarina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hymenoptera | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coleoptera | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Homoptera | 7 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Araneida | 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Undetermined insects | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Salamander males | 0 | 0 | 0 | 3 | 1 | 1 | 1 | 0 | 0 |
| Salamander females | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Salamander subadults | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| Salamander juveniles | 0 | 0 | 1 | 4 | 2 | 0 | 0 | 0 | 0 |
| Year 2019 | |||||||||
| Diptera | 11 | 1 | 0 | 1 | 3 | 3 | 4 | 3 | 0 |
| Acarina | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hymenoptera | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coleoptera | 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Homoptera | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Araneida | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
| Undetermined insects | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 |
| Salamander males | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 1 |
| Salamander females | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Salamander subadults | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Salamander juveniles | 0 | 0 | 4 | 3 | 0 | 0 | 0 | 0 | 0 |
| Sum of Squares | df | Mean Square | F | p | |
|---|---|---|---|---|---|
| Year | 1.7389 | 6 | 0.2898 | 0.9060 | 0.1336 |
| Cave zone | 3.0232 | 2 | 1.5116 | 4.7254 | 0.0001 |
| Year * Cave zone | −2.4987 | 12 | −0.2082 | −0.6509 | 0.9338 |
| Residual | 13.115 | 41 | 0.3199 | ||
| Total | 15.379 | 61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvidio, S.; Costa, A.; Oneto, F.; Pastorino, M.V. Variability of A Subterranean Prey-Predator Community in Space and Time. Diversity 2020, 12, 17. https://doi.org/10.3390/d12010017
Salvidio S, Costa A, Oneto F, Pastorino MV. Variability of A Subterranean Prey-Predator Community in Space and Time. Diversity. 2020; 12(1):17. https://doi.org/10.3390/d12010017
Chicago/Turabian StyleSalvidio, Sebastiano, Andrea Costa, Fabrizio Oneto, and Mauro Valerio Pastorino. 2020. "Variability of A Subterranean Prey-Predator Community in Space and Time" Diversity 12, no. 1: 17. https://doi.org/10.3390/d12010017
APA StyleSalvidio, S., Costa, A., Oneto, F., & Pastorino, M. V. (2020). Variability of A Subterranean Prey-Predator Community in Space and Time. Diversity, 12(1), 17. https://doi.org/10.3390/d12010017







