Spatial Distribution of Phytoplankton Community Composition and Their Correlations with Environmental Drivers in Taiwan Strait of Southeast China
Abstract
:1. Introduction
2. Materials and Methods
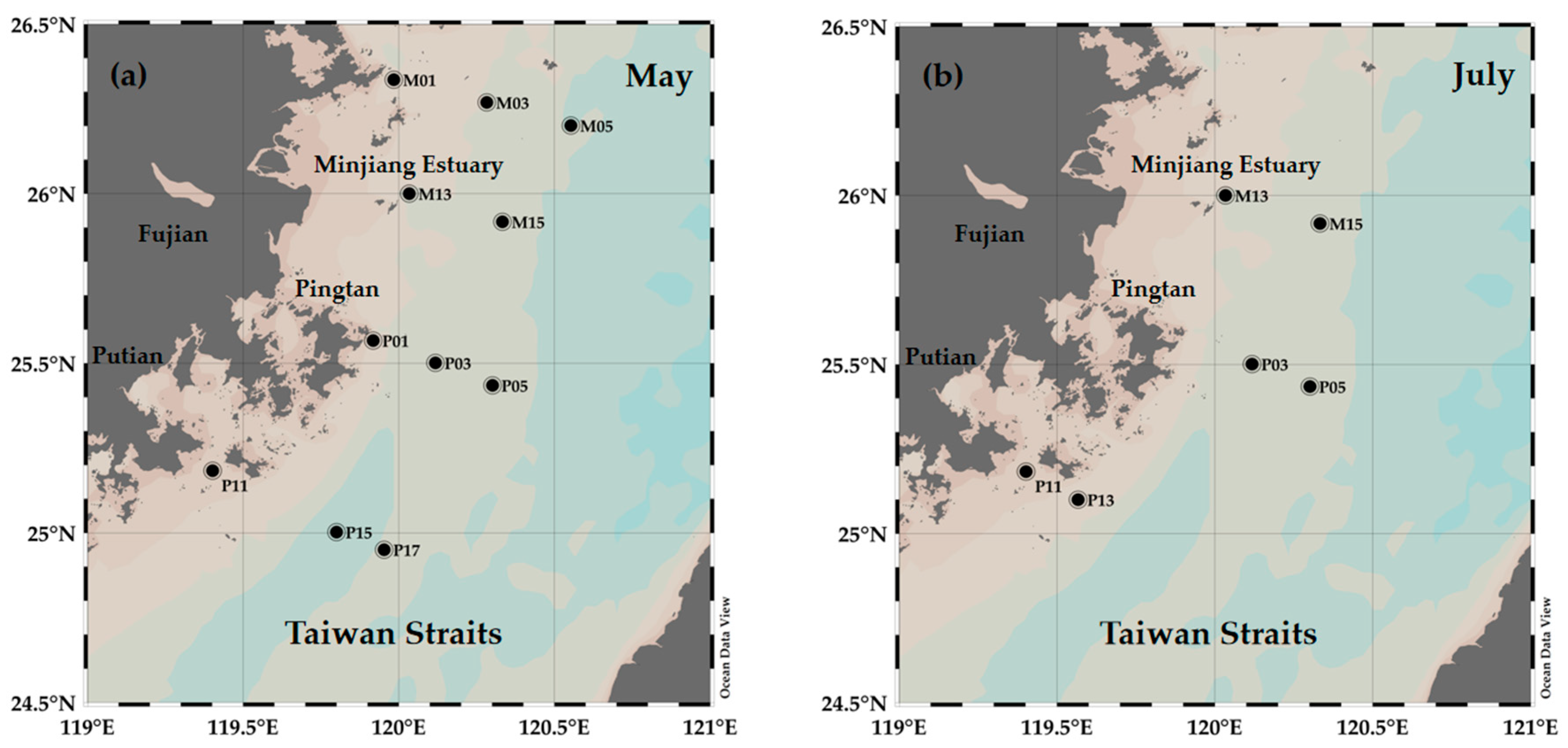
2.1. Study Area and Sampling Protocol
2.2. Nutrients Determination
2.3. Chlorophyll a Measurement, Phytoplankton Species Identification, and Diversity Analyses
2.4. Statistical Analysis
3. Results
3.1. Temperature, Salinity, Dissolved Oxygen Concentration and pH Value
3.2. Dissolved Inorganic Nitrogen (DIN) and Phosphorus (DIP) Concentrations, DIN:DIP Ratio and Chl a Concentration
3.3. Percentage of Chl a and Cell Density of Each Phytoplankton Group and Phytoplankton Diversity
3.4. Correlations of Cell Density of Dinoflagellates and Prorocentrum donghaiense with Environmental Drivers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hennon, G.M.M.; Dyhrman, S.T. Progress and promise of omics for predicting the impacts of climate change on harmful algal blooms. Harmful Algae 2020, 91, 101587. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Pitcher, G.C.; Probyn, T.A. Suffocating phytoplankton, suffocating water–red tides and anoxia. Front. Mar. Sci. 2016, 3, 186. [Google Scholar] [CrossRef]
- State Ocean Administration. Bulletin of Marine Environmental Quality of China; State Ocean Administration: Beijing, China, 2012. [Google Scholar]
- Yang, Y.; Huang, H.L.; Zeng, Y.D.; Guan, W.B.; Li, X.D.; Guo, M.Q. Comparison in temperature, salinity and circulation structures during spring of the years before and after Karenia mikinotoi bloom along Fujian coast in 2012. Oceanol. Limnol. Sin. 2019, 50, 553–562, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Shin, H.H.; Li, Z.; Mertens, K.N.; Seo, M.H.; Gu, H.; Lim, W.A.; Yoon, Y.H.; Soh, H.Y.; Matsuoka, K. Prorocentrum shikokuense Hada and P. donghaiense Lu are junior synonyms of P. obtusidens Schiller, but not of P. dentatum Stein (Prorocentrales, Dinophyceae). Harmful Algae 2019, 89, 101686. [Google Scholar] [CrossRef]
- Aissaoui, A.; Armi, Z.; Akrout, F.; Hassine, O.K.B. Environmental factors and seasonal dynamics of Prorocentrum lima population in coastal waters of the Gulf of Tunis, South Mediterranean. Water Environ. Res. 2014, 86, 2256–2270. [Google Scholar] [CrossRef]
- Sun, K.; Qiu, Z.; He, Y.; Fan, W.; Wei, Z. Vertical development of a Prorocentrum donghaiense bloom in the coastal waters of the East China Sea: Coupled biophysical numerical modeling. Acta Oceanol. Sin. 2017, 36, 23–33. [Google Scholar] [CrossRef]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, X.; Xiao, W.; Laws, E.A.; Chen, J.; Wang, L.; Liu, S.; Zhang, F.; Huang, B. Phytoplankton community patterns in the Taiwan Strait match the characteristics of their realized niches. Prog. Oceanogr. 2020, 186, 102–366. [Google Scholar] [CrossRef]
- Steidinger, K.A. Implications of dinoflagellate life cycles on initiation of Gymnodinium breve red tides. Environ. Lett. 1975, 9, 129–139. [Google Scholar] [CrossRef]
- Li, R.S. Study on the ecology of red tide Prorocentrum donghaiense in the north of Fujian coast. Mar. Environ. Sci. 2009, 28, 65–69, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhou, Z.X.; Yu, R.C.; Zhou, M.J. Seasonal succession of microalgal blooms from diatoms to dinoflagellates in the East China Sea: A numerical simulation study. Ecol. Model. 2017, 360, 150–162. [Google Scholar] [CrossRef]
- Hu, Z.; Mulholland, M.R.; Duan, S.; Xu, N. Effects of nitrogen supply and its composition on the growth of Prorocentrum donghaiense. Harmful Algae 2012, 13, 72–82. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, M. Progress of the project “ecology and oceanography of harmful algal blooms in china”. Adv. Earth Sci. 2006, 21, 673–679, (in Chinese with English abstract). [Google Scholar]
- Sullivan, J.M.; Donaghay, P.L.; Rines, J.E.B. Coastal thin layer dynamics: Consequences to biology and optics. Cont. Shelf Res. 2010, 30, 50–65. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Li, M.; Wang, C.; Lin, X.; Li, L.; Shi, X.; Guo, C.; Lin, S. Comparative metatranscriptomic profiling and microRNA sequencing to reveal active metabolic pathways associated with a dinoflagellate bloom. Sci. Total Environ. 2019, 699, 134–323. [Google Scholar] [CrossRef]
- Yang, L.Y.; Cheng, Q.; Zhuang, W.E.; Wang, H.; Chen, W. Seasonal changes in the chemical composition and reactivity of dissolved organic matter at the land-ocean interface of a subtrophic river. Environ. Sci. Pollut. Res. 2019, 26, 24595–24608. [Google Scholar] [CrossRef]
- Chung, S.W.; Jan, S.; Liu, K.K. Nutrient fluxes through the Taiwan Strait in spring and Summer 1999. J. Oceanogr. 2001, 57, 47–53. [Google Scholar] [CrossRef]
- Pan, A.J.; Wan, X.F.; Guo, X.G.; Jing, C.S. Responses of the Zhe-Min coastal current adjacent to Pingtan Island to the wintertime monsoon relaxation in 2006 and its mechanism. Sci. China Earth Sci. 2013, 56, 386–396. [Google Scholar] [CrossRef]
- Zhou, M.J.; Yan, T.; Zou, J.Z. Preliminary analysis of the characteristics of red tide areas in Changjiang River estuary and its adjacent sea. Chin. J. Appl. Ecol. 2003, 14, 1031–1038, (in Chinese with English abstract). [Google Scholar]
- Zhuo, X. Research on the basic characteristics of red tide in Fuzhou coastal waters during the past 10 years. Mar. Forecast 2018, 35, 34–40, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhou, M.J.; Yu, R.C. Mechanisms and impacts of harmful algal blooms and the count measures. Chin. J. Nat. 2007, 29, 72–77, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Zhang, C.S.; Wang, J.T.; Zhu, D.D.; Shi, X.Y.; Wang, X.L. The preliminary analysis of nutrients in harmful algal blooms in the East China Sea in the spring and summer of 2005. Acta Oceanol. Sin. 2008, 30, 153–159, (in Chinese with English abstract). [Google Scholar]
- Haraguchi, L.; Carstensen, J.; Abreu, P.C.; Odebrecht, C. Long-term changes of the phytoplankton community and biomass in the subtropical shallow Patos Lagoon Estuary, Brazil. Estuar. Coast. Shelf S 2015, 162, 76–87. [Google Scholar] [CrossRef]
- Hansen, H.P.; Koroleff, F. Determination of nutrients. In Methods of Seawater Analysis; Grasshoff, K., Kremling, K., Ehrhardt, M., Eds.; Wiley-VCH Publishers: Weinheim, Germany, 1999; pp. 159–228. [Google Scholar]
- Catherine, A.; Escoffier, N.; Belhocine, A.; Nasri, A.B.; Hamlaoui, S.; Yéprémian, C.; Bernard, C.; Troussellier, M. On the use of the FluoroProbe®, a phytoplankton quantification method based on fluorescence excitation spectra for large-scale surveys of lakes and reservoirs. Water Res. 2012, 46, 1771–1784. [Google Scholar] [CrossRef]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon Press: Toronto, ON, Canada, 1984; pp. 1–137. [Google Scholar]
- Utermöhl, H. Toward the improvement of the quantitative phytoplankton method. Mitt. Int. Ver. Limnol. 1958, 9, 138. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; Illinois University Press: Urbana, IL, USA, 1963; pp. 20–31. [Google Scholar]
- Pielou, E.C. Ecological Diversity; John Wiley: New York, NY, USA, 1975; Volume 1, pp. 1–318. [Google Scholar]
- Terbraak, C.J.F.; Smilauer, P. Canoco Reference Manual and Canodraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: New York, NY, USA, 2002. [Google Scholar]
- Legendre, P.; Oksanen, J.; Braak, C.J.F.T. Testing the significance of canonical axes in redundancy analysis. Methods Ecol. Evol. 2011, 2, 269–277. [Google Scholar] [CrossRef]
- Moal, J.; Martin-Jezequel, V.; Harris, R.P.; Samain, J.F.; Poulet, S.A. Interspecific and intraspecific variability of the chemical composition of marine phytoplankton. Oceanol. Acta 1987, 10, 339–346. [Google Scholar]
- Wang, Y.; Li, R.X.; Dong, S.L.; Li, Y.; Sun, P.; Wang, X.D. Relationship between cell volume and cell carbon and cell nitrogen for ten common dinoflagellates. Acta Ecol. Sin. 2011, 31, 6540–6550, (in Chinese with English abstract). [Google Scholar] [CrossRef]
- Mercado, J.; Ramírez, T.; Cortés, D.; Sebastián, M.; Liger, E.; Bautista, B. Partitioning the effects of changes in nitrate availability and phytoplankton community structure on relative nitrate uptake. Mar. Ecol. Prog. Ser. 2008, 359, 51–68. [Google Scholar] [CrossRef] [Green Version]
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Duan, S.; Li, A.; Zhang, C.; Cai, Z.; Hu, Z. Effects of temperature, salinity and irradiance on the growth of the harmful dinoflagellate Prorocentrum donghaiense Lu. Harmful Algae 2010, 9, 13–17. [Google Scholar] [CrossRef]
- Wang, D.; Xia, W.; Lu, S.; Wang, G.; Liu, Q.; Moore, W.S.; Chen, C.T.A. The nonconservative property of dissolved molybdenum in the western Taiwan Strait: Relevance of submarine groundwater discharges and biological utilization. Geochem. Geophys. Geosyst. 2016, 17, 28–43. [Google Scholar] [CrossRef]
- Lin, G.; Yang, Q.; Lin, W.; Wang, Y. Distribution characteristic and variation trend of planktonic dinoflagellate in the Taiwan Strait from 2006 to 2007. Mar. Sci. Bull. 2012, 14, 68–79, (in Chinese with English abstract). [Google Scholar]
- Wang, Y.; Kang, J.; Ye, Y.; Lin, G.; Yang, Q.; Lin, M. Phytoplankton community and environmental correlates in a coastal upwelling zone along western Taiwan Strait. J. Mar. Syst. 2016, 154, 252–263. [Google Scholar] [CrossRef]
- Thomas, M.K.; Kremer, C.T.; Klausmeier, C.A.; Litchman, E. A global pattern of thermal adaptation in marine phytoplankton. Science 2012, 338, 1085–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, P.W.; Rynearson, T.A.; Armstrong, E.A.; Fu, F.; Hayashi, K.; Hu, Z.; Hutchins, D.A.; Kudela, R.M.; Litchman, E.; Mulholland, M.R.; et al. Marine phytoplankton temperature versus growth responses from polar to tropical waters—Outcome of a scientific community-wide study. PLoS ONE 2013, 8, e63091. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.H.; Huang, X.Q. Ecolgical characteristics of Prorocentrum dentatum and the cause of harmful algal bloom formation in China. Chin. J. Appl. Ecol. 2003, 14, 1065–1069, (in Chinese with English abstract). [Google Scholar]
- Li, X.D. Analysis on characteristics of red tide in Fujian coastal waters during the last 10 years. Environ. Sci. 2012, 33, 2210–2216. [Google Scholar] [CrossRef]
- Ran, Z.; Sun, J.; Song, S. Phytoplankton in the Yangtze River Estuary and its adjacent waters in spring 2006. Mar. Sci. Bull. 2013, 32, 421–428. [Google Scholar] [CrossRef]
- Phillips, K.A.; Fawley, M.W. Winter phytoplankton blooms under ice associated with elevated oxygen levels. J. Phycol. 2002, 38, 1068–1073. [Google Scholar] [CrossRef]
- Chen, M. Marine Chemistry; Ocean Press: Beijing, China, 2009; pp. 90–130. [Google Scholar]
- Rodríguez, J.J.G.; Mirón, A.S.; García, M.C.C.; Belarbi, E.H.; Camacho, F.G.; Chisti, Y.; Grima, E.M. Macronutrients requirements of the dinoflagellate Protocetatum reticulatum. Harful Algae 2009, 8, 239–246. [Google Scholar] [CrossRef]
- Gobler, C.J. Climate change and harmful algal blooms: Insights and perspective. Harmful Algae 2020, 91, 101731. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.A. Distributions of nutrients in the East China Sea and the South China Sea connection. J. Oceanogr. 2008, 64, 737–751. [Google Scholar] [CrossRef]
- Ye, X.; Chen, J.; Ji, W.; Li, D. Research the biogeochemical processes of nutrients in Minjiang Estuary. Environ. Sci. 2011, 32, 375–383, (in Chinese with English abstract). [Google Scholar]
- Ou, L.; Huang, X.; Huang, B.; Qi, Y.; Lu, S. Growth and competition for different forms of organic phosphorus by the dinoflagellate Prorocentrum donghaiense with the dinoflagellate Alexandrium catenella and the diatom Skeletonema costatum s. l. Hydrobiologia 2015, 754, 29–41. [Google Scholar] [CrossRef]
- Flynn, J.; Fasham, R. A modelling exploration of vertical migration by phytoplankton. J. Theor. Biol. 2002, 218, 471–484. [Google Scholar] [CrossRef]
- Lou, X.; Hu, C. Diurnal changes of a harmful algal bloom in the East China Sea: Obervations from GOCI. Remote Sens. Environ. 2014, 140, 562–572. [Google Scholar] [CrossRef]
- Anderson, D.A.; Gliber, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 2002, 25, 562–584. [Google Scholar] [CrossRef]
- Huang, T.H.; Chen, C.T.A.; Lee, J.; Wu, C.R.; Wang, Y.L.; Bai, Y.; He, X.; Wang, S.L.; Kandasamy, S.; Luo, J.Y.; et al. East China Sea increasingly gains limiting nutrient P from South China Sea. Sci. Rep. 2019, 9, 5648. [Google Scholar] [CrossRef] [Green Version]
- Brennan, G.; Collins, S. Growth responses of a green alga to multiple environmental drivers. Nat. Clim. Chang. 2015, 5, 892–897. [Google Scholar] [CrossRef]
- Brennan, G.; Colegrave, N.; Collins, S. Evolutionary consequences of multidriver environmental change in an aquatic primary producer. Proc. Natl. Acad. Sci. USA 2017, 114, 9930–9935. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| May Cruise | July Cruise | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M 01 | M 03 | M 05 | M 13 | M 15 | P 01 | P 03 | P 05 | P 11 | P 15 | P 17 | M 13 | M 15 | P 03 | P 05 | P 11 | P 13 | |
| Alexandrium sp. | + | + | + | + | |||||||||||||
| Gonyaulax sp. | + | + | + | + | + | + | + | ||||||||||
| Prorocentrum donghaiense | + | + | + | + | + | + | + | + | + | ||||||||
| Prorocentrum minimum | + | + | + | + | + | + | |||||||||||
| Scrippsiella trochoidea | + | + | + | + | + | + | + | + | + | + | |||||||
| Chaetoceros sp. | + | + | + | + | + | + | + | ||||||||||
| Coscinodiscus sp. | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| Dactyliosolen sp. | + | + | + | ||||||||||||||
| Guinardia sp. | + | + | + | + | |||||||||||||
| Leptocylindrus sp. | + | + | + | ||||||||||||||
| Navicula sp. | + | + | + | + | + | + | |||||||||||
| Pinnularia sp. | + | + | + | ||||||||||||||
| Pleurosigma sp. | + | + | + | ||||||||||||||
| Pseudo-nitzschia sp. | + | + | + | + | + | ||||||||||||
| Rhizosolenia sp. | + | + | + | + | + | + | + | + | + | + | + | ||||||
| Thalassionema sp. | + | + | + | + | + | + | + | + | |||||||||
| Identified number of species per sample | 6 | 6 | 5 | 9 | 8 | 6 | 4 | 7 | 11 | 5 | 2 | 2 | 4 | 5 | 4 | 9 | 9 |
| Evenness | 53.7 | 80.3 | 79.4 | 46.5 | 60.9 | 73.3 | 59.4 | 68.7 | 90.7 | 85.6 | 80.8 | 77.3 | 79.8 | 84.1 | 89.2 | 58.9 | 67.7 |
| Shannon | 1.1 | 1.6 | 1.3 | 1.1 | 1.3 | 1.3 | 1.0 | 1.4 | 2.6 | 1.5 | 1.1 | 0.8 | 1.1 | 1.6 | 1.2 | 1.5 | 1.7 |
| Time | Period (Day) | Dominant Species | Temperature (°C) | Salinity (psu) | Reference |
|---|---|---|---|---|---|
| 2002. 04–05 | 16 | Prorocentrum donghaiense, Noctiluca scintillans, Gymnodinium spp. | 18.8–24.0 | 24.7–30.3 | [44] |
| 2003. 04–06 | 34 | P. donghaiense, G. mikimotoi | N | N | [12] |
| 2004. 04–05 | 26 | P. donghaiense, Karenia mikimotoi | N | N | [12] |
| 2005. 04–05 | 8 | P. donghaiense | N | N | [12] |
| 2006. 03 or 06 | 9 | P. donghaiense, | N | N | [12] |
| 2007. 01 or 06 | 20 | P. donghaiense, | 17.0–25.2 | 29.5–33.4 | [12] |
| 2008. 04–05 | 42 | P. donghaiense, K. mikimotoi | 18.2–24.5 | 29.8–31.5 | [12] |
| 2009. 02 | 22 | Akashiwo sanguinea | N | N | [45] |
| 2010. 04–05 | 27 | P. donghaiense, K. mikimotoi | 18.8–19.9 | 22.7–26.1 | [22,45] |
| 2011.05 | 10 | P. donghaiense, A. sanguinea, | 20.0–24.0 | 30.0–34.0 | [5] |
| 2012. 04–06 | 40 | P. donghaiense, K. mikimotoi, | 20.5–24.5 | 32.0–34.0 | [5] |
| 2013. 05 | 27 | P. donghaiense, N. scintillans, Eucampia zodiacus | 21.0–24.5 | 30.0–33.0 | [5] |
| 2014. 05 | 27 | P. donghaiense, N. scintillans, K. mikimotoi | 19.0–20.2 | 26.4–30.3 | [22] |
| 2015. 04–05 | 12 | K. mikimotoi, | N | N | [22] |
| 2016. 04–05 | 34 | P. donghaiense, N. scintillans, P. globosa, | 17.0–20.8 | 25.2–29.0 | [22] |
| 2017. 05–07 | 37 | P. donghaiense, N. scintillans,G. catenatum, K. mikimotoi, | 21.8–23.2 | 28.6–32.0 | [22] |
| 2018. 04–06 | 21 | P. donghaiense, G. catenatum, K. mikimotoi | N | N | [22] |
| 2019. 04–05 | 32 | P. donghaiense, N. scintillans, K. mikimotoi, A. sanguinea, | 21.8–24.1 | 27.2–32.6 | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Su, J.-Z.; Su, Y.-P.; Lin, H.; Xu, Y.-C.; Barathan, B.P.; Zheng, W.-N.; Schulz, K.G. Spatial Distribution of Phytoplankton Community Composition and Their Correlations with Environmental Drivers in Taiwan Strait of Southeast China. Diversity 2020, 12, 433. https://doi.org/10.3390/d12110433
Zhang Y, Su J-Z, Su Y-P, Lin H, Xu Y-C, Barathan BP, Zheng W-N, Schulz KG. Spatial Distribution of Phytoplankton Community Composition and Their Correlations with Environmental Drivers in Taiwan Strait of Southeast China. Diversity. 2020; 12(11):433. https://doi.org/10.3390/d12110433
Chicago/Turabian StyleZhang, Yong, Jin-Zhu Su, Yu-Ping Su, Hong Lin, Yang-Chun Xu, Balaji P. Barathan, Wan-Ning Zheng, and Kai G. Schulz. 2020. "Spatial Distribution of Phytoplankton Community Composition and Their Correlations with Environmental Drivers in Taiwan Strait of Southeast China" Diversity 12, no. 11: 433. https://doi.org/10.3390/d12110433
APA StyleZhang, Y., Su, J.-Z., Su, Y.-P., Lin, H., Xu, Y.-C., Barathan, B. P., Zheng, W.-N., & Schulz, K. G. (2020). Spatial Distribution of Phytoplankton Community Composition and Their Correlations with Environmental Drivers in Taiwan Strait of Southeast China. Diversity, 12(11), 433. https://doi.org/10.3390/d12110433





