Abstract
The blue jack mackerel Trachurus picturatus (Bowdich, 1825) specimens (N = 155) were collected during the MEDITS survey, done along the eastern side, precisely, of the Croatian fishing ground in July 2018. Biometrical analysis of ten morphometric and five meristic characters, as well as genetic analysis proved that the collected specimens were blue jack mackerel. The total length (TL) and weight (W) of all observed specimens ranged from 9.2 to 33.7 cm (12.15 ± 2.95 cm) and from 5.79 to 384.94 g (17.64 ± 39.42 g), respectively. All calculated length–length relationships were linear (r > 0.923). Sex was determined only on two larger specimens (28 cm < TL < 32.8 cm), which were females. In the length–weight relationship, positive allometry was established (b = 3.1789). Based on 37 partial cytochrome b sequences, the overall haplotype diversity (h) of 0.812 ± 0.048 and nucleotide diversity (π) of 0.0064 ± 0.0007 indicated high levels of haplotype and low nucleotide diversity. The obtained sequences were compared to previously published research within the Northeast Atlantic Ocean and the Mediterranean Sea, confirming the absence of genetic structure among these populations.
1. Introduction
Over the years, environmental variables have changed, mostly due to increasing globalisation processes [1]. The modification of those variables directly influenced whole marine ecosystems. Consequently, fish assemblage has either accommodated or transformed. Appearance and increased abundance of new and/or rare species surely affected the structure and functioning of species communities; therefore, systematic monitoring is compulsory.
The blue jack mackerel Trachurus picturatus (Bowdich, 1825) is a benthopelagic fish species widely distributed and exploited in the area of East Atlantic (southern Bay of Biscay to south Morocco, including the Macaronesian archipelagos, Tristan de Cunha and Gough Islands). In the area of the Mediterranean Sea and its adjacent seas, this species can be found in its western part and the area of the Black Sea [2]. Within the distribution area of this species, its economic value is the lowest in the Mediterranean. In general, its specimens appear over the continental shelf and around offshore islands down to 400–500 m sea depth [3]. As with other pelagic fish species, it forms schools and tends to migrate from open sea to coastal areas [4]. In the area of the Adriatic, this species is rare and its specimens, in the past, were sporadically caught and noted [5,6,7,8,9,10]. Until now, only Bolognini et al. [11] reported the minor catch (N = 4) of blue jack mackerel in the Adriatic Sea.
Recently, during the scientific surveys, a Croatian scientist noticed a higher amount of not so common Trachurus species in the catches. As geographical expansion of this rare species might have an impact on Adriatic biodiversity, its monitoring is crucial. It is believed that ocean warming is among the main driving forces of species abundance and distribution changes and, consequently, of changes in species biodiversity and marine ecosystem functioning [12,13]. Žužul et al. [14] offered ocean warming as one of the explanations behind the increase in the number of wild gilthead seabream noted in the costal parts of the Adriatic Sea [15]. The authors further argued that it is possible that wild gilthead seabream, as a species that thrives in warmer areas, is becoming more numerous at its northern distribution areas [16], as a consequence of increased larval survival and recruitment success [14].
Hence, to better understand what could be expected from the possible emergence of a blue jack mackerel population in the Adriatic Sea, the aim of this study was to investigate the biometrical analysis, the length–weight relationship and genetic diversity of the blue jack mackerel caught in the Adriatic Sea and compare the results to previously published research within the Northeast Atlantic Ocean and the Mediterranean Sea. The present paper provides phenotypic and genetic characteristics of this rare fish species in the Adriatic Sea, which might help us define this population that has obviously entered the Adriatic over the last decade.
2. Materials and Methods
2.1. Sample Processing and Biometric Analysis
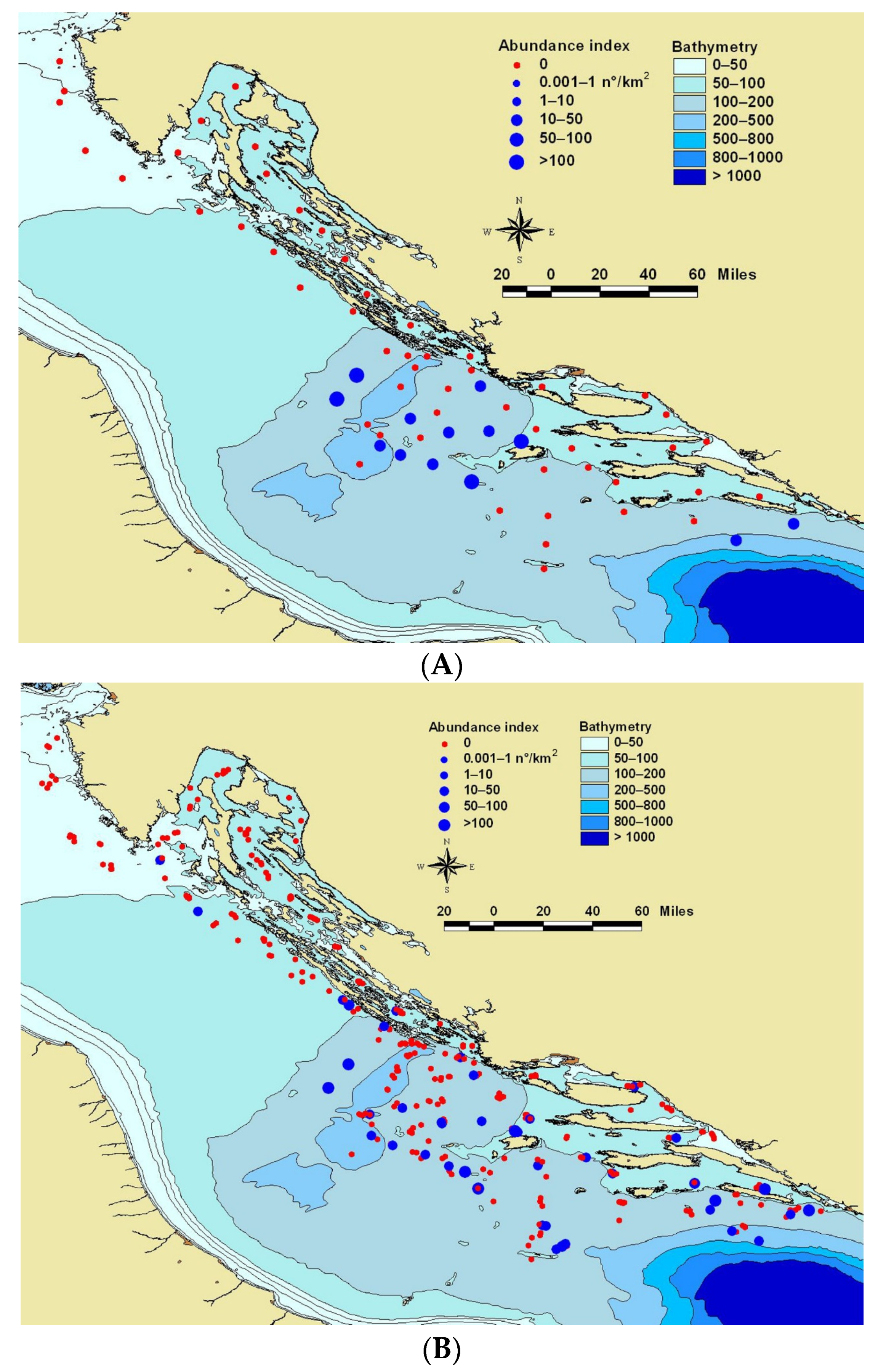
Specimens presumed to be blue jack mackerel (N = 155) and Mediterranean horse mackerel (N = 2) were collected by bottom trawl during the “MEDITS” research survey in July 2018 performed along the whole Croatian fishing ground. The tows were carried out over the continental shelf, between 40 to 800 m for a duration of 30 to 60 min, depending on the depth, and the mean trawl speed was 3 knots. Studied specimens were caught in 13 out of 63 hauls (Figure 1). Collected specimens were immediately frozen and by the end of the survey transported to the laboratory for detailed analysis.
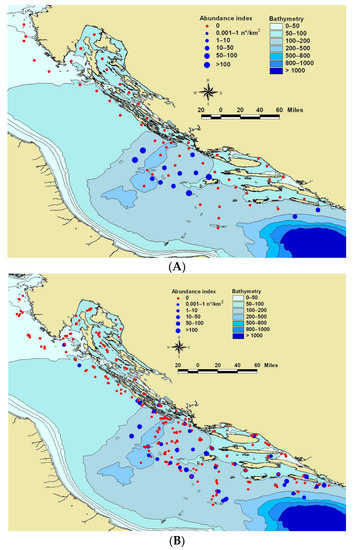
Figure 1.
Map of the Adriatic Sea showing the sampling stations of the survey MEDITS done in July 2018 (A) and MEDITS surveys done over the last six summers (2013–2018) (B) The red (negative stations) and blue (positive stations) dots indicate the sampling stations, where blue dot dimensions varied depending on the number of the blue jack mackerel Trachurus picturatus collected.
In the laboratory, fourteen morphometric variables were measured: total length (TL), standard length (SL), fork length (FL), preanal (PAL) distance, head length (LH), eye diameter (O), maximum (H) body height, length of first (LD1) and second dorsal fin (LD2), length of pectoral fin (LP), as well as five meristic characters: number of rays in first (D1) and second dorsal fin (D2), in pectoral fin (P), in ventral fin (V) and in anal fin (A), according to Jardas [6].
Each body length (±0.1 mm) and total body weight (W) (±0.01 g) was measured. Morphometric characters were expressed as % of TL, with the exception of eye diameter (O), which was expressed as % of LH.
Length–length relationships were determined by the method of least squares to fit a simple linear regression model. Length conversion equations were derived for total length (TL) and the head length (LH). The length–weight relationship was calculated by applying the exponential regression W = aTLb, where W = total fish weight in g; TL = total length in cm; a = proportionality constant; and b = allometric growth parameter. The hypotheses of isometric growth were tested using Student’s t-test (p < 0.05).
2.2. Genetic Analysis
For genetic analysis, the sub-samples of muscle tissue were collected from 40 individuals and stored in absolute ethanol until genomic DNA (gDNA) extraction. Total genomic DNA (gDNA) was isolated using standard proteinase K digestion and phenol–chloroform extraction protocols [17] and stored in TE buffer at −20 °C. Quantity and quality of isolated gDNA were assessed using Nanophotometer (IMPLEN), at 260 and 280 nm. Samples with a 260/280 ratio between 1.7 and 1.9 were used as templates in a polymerase chain reaction (PCR). The fragment of cytochrome b locus (cytb) was amplified by PCR using the universal primers L14841 and H15149, described by Kocher et al. [18]. PCR was run in 25 µL reactions combining 0.125 µL of HotStarTaq DNA Polymerase (5 u/µL), 2.5 µL of 10xPCR buffer, 1 µL of MgCl2 (25 mM), 0.5 µL of dNTP (0.25 mM each), 0.5 µL of each primer (10 µM) and DNase/RNase free PCR water to a volume of 25 µL. PCR conditions were as follows: 15 min at 95 °C, 35 cycles of 94 °C for 45 s, 51 °C for 45 s and 72 °C for 1 min, with a final extension at 72 °C for 10 min. PCR products were visualized on 1% agarose gel under UV transilluminator. Purification and Sanger sequencing was performed commercially by Macrogen Europe (Amsterdam, The Netherlands).
The obtained sequences were analysed for similarity with other known vertebrate sequences using Blast Local Alignment Search Tool (BLAST) [19]. The obtained sequences (~350 bp) were compiled along all publicly available partial cytb sequences of T. picturatus, as well as selected sequences of related Carangidae species, T. trachurus and T. mediterraneus in MEGA 7 [20] and aligned by ClustalW incorporated in the software, using default parameters. The alignment had to be shortened in order to overlap all publicly available cytb sequences of T. picturatus with those sequenced within this research. This resulted in 296 partial cytb sequences of 200 bp. Since the resulting fragment was sufficiently variable, it was deemed more beneficial to compare the new data to all the data collected thus far, than to use full length of the new sequences and not be able to compare them with a large part of the compiled dataset.
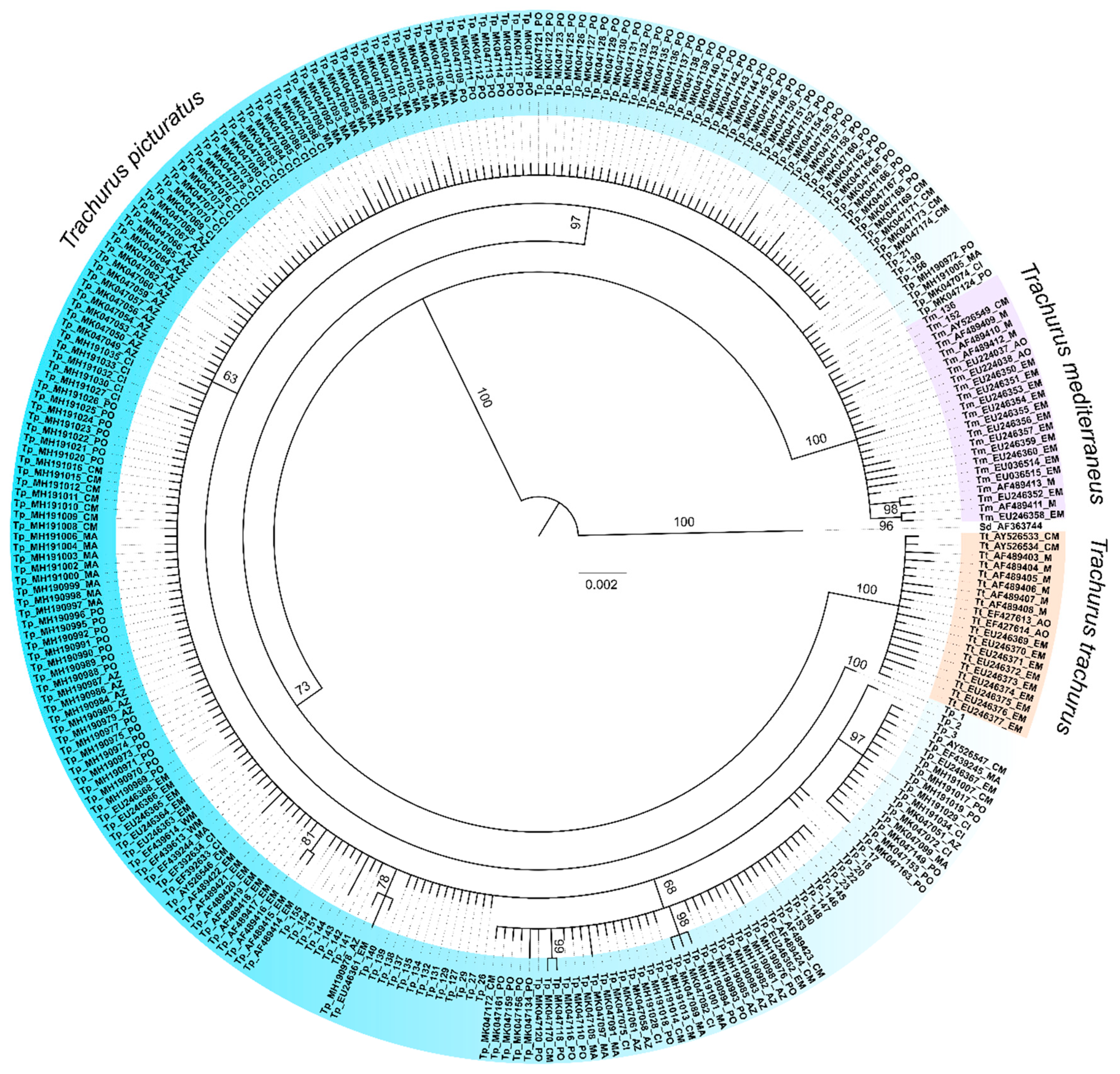
Bayesian inference (BI) [21] analysis was performed on 296 partial cytb sequences in MrBayes v3.2.7 [22] using a HKY evolutionary model of nucleotide substitution [23], previously selected in MEGA 7 [20] by AICc (Akaike Information Criterion, corrected) value. Four incrementally heated Markov chains were carried through 4,000,000 generations. Sampling frequency was set at 1000 generations, applying default discard of the first 25% samples from the cold chain. Markov chain Monte Carlo (MCMC) parameters were set at default. A 50% majority rule consensus tree produced in the BI analysis was illustrated using FigTree v1.4.4 [24]. The tree was rooted using Selene dorsalis as an outgroup, as S. dorsalis belongs to the same family as the genus Trachurus (Carangidae). GenBank accession numbers of all compiled sequences are incorporated in their IDs, for easier access and traceability.
DnaSP 5.19 [25] and Arlequin 3.5.2 [26] were used to calculate genetic diversity indices. The demographic history for T. picturatus populations in Mediterranean Sea and Atlantic Ocean were tested using Tajima’s D [27] and Fu’s FS [28], where negative values indicated population expansion or historical bottleneck [29]. 10,000 simulated samples were used to test the significance under a model of constant population size in Arlequin 3.5.2 [26]. A hierarchical spatial analysis of molecular variance (AMOVA; 10,000 permutations) was performed on two groups: Mediterranean (Adriatic Sea, Central and Eastern Mediterranean Sea) vs. Atlantic Ocean (mainland Portugal, Madeira, Canary Islands and Azores).
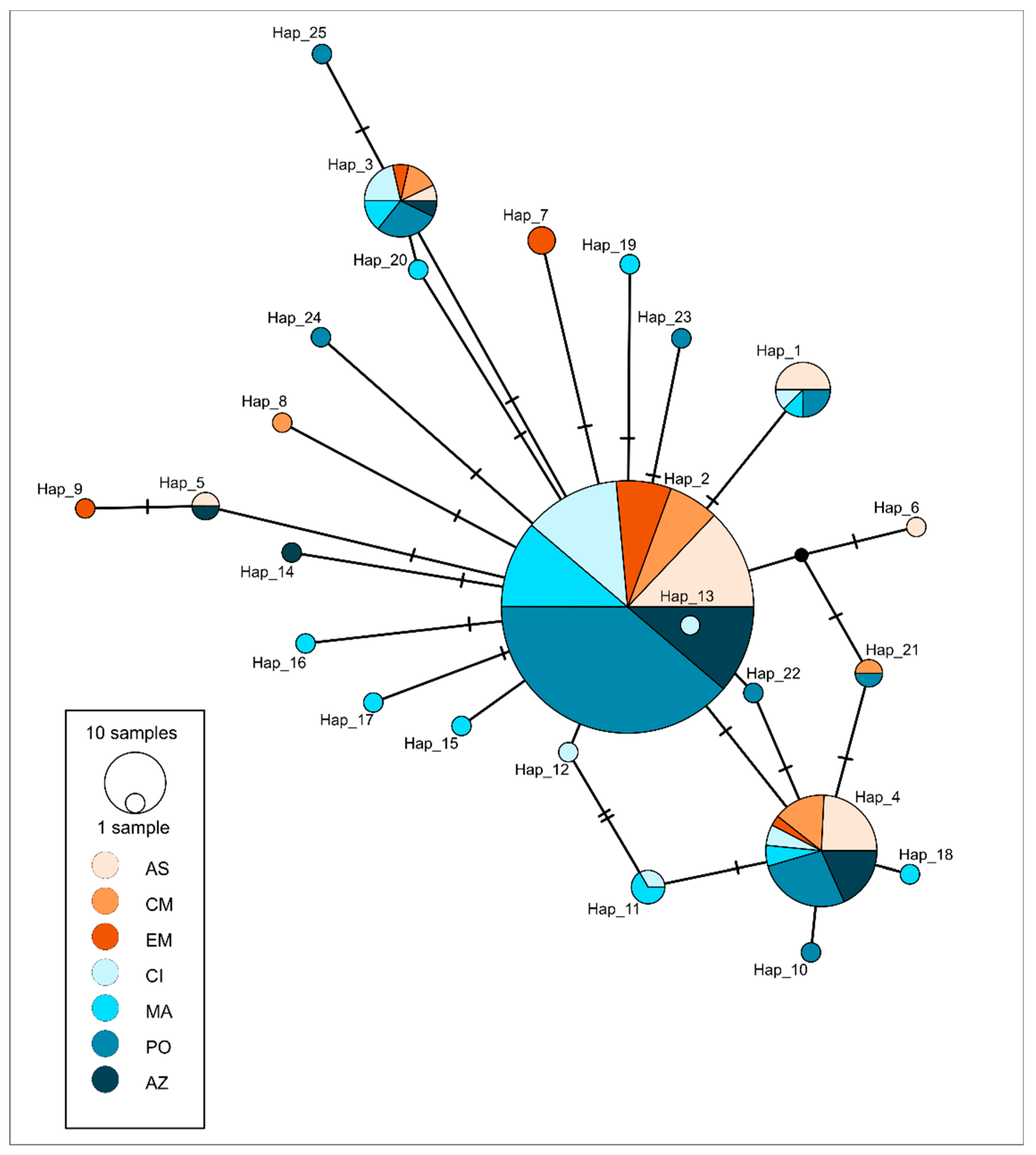
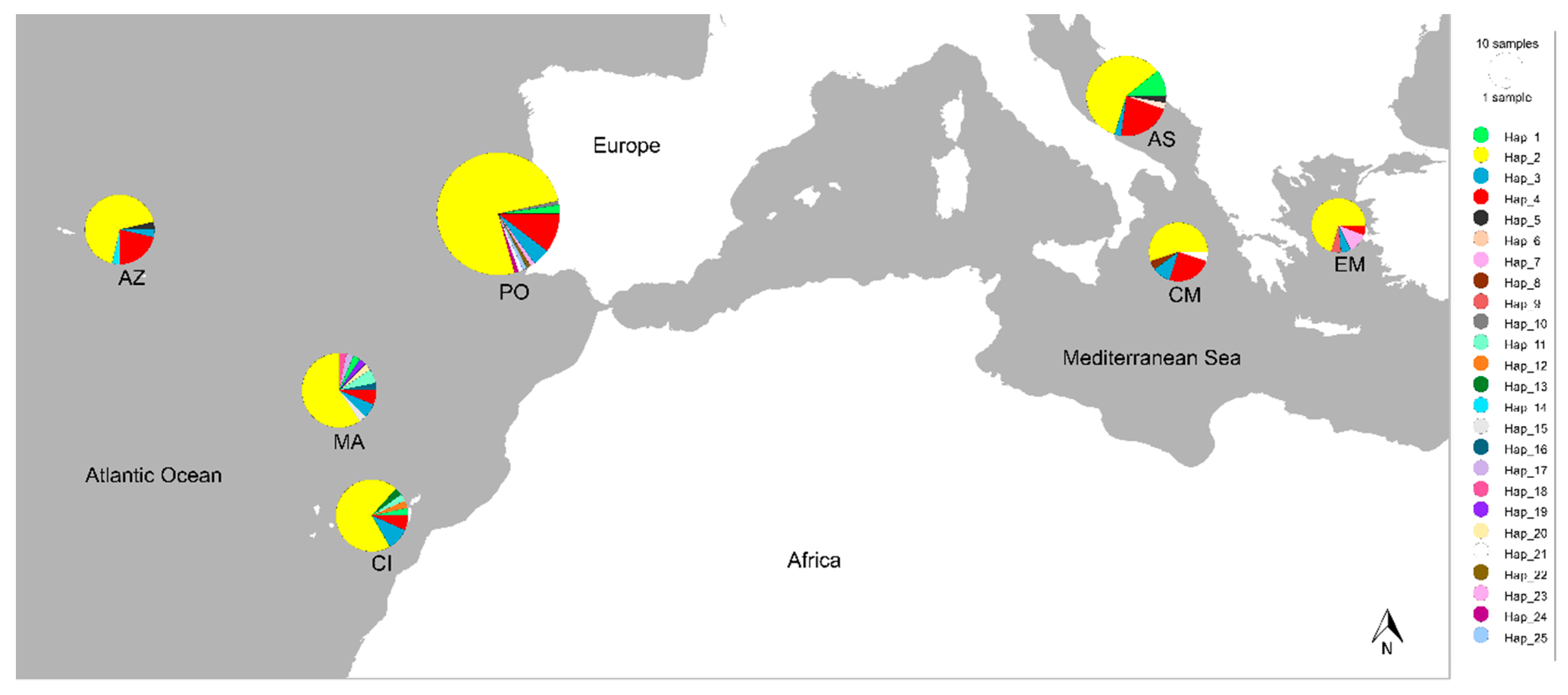
Network was constructed in PopART using the integer neighbour-joining (IntNJ) method and sampling localities were used to depict geographical distribution of the sequenced haplotypes [30]. The IntNJ method computes a distance matrix from which a neighbour-joining tree is inferred, while the integer edge lengths are chosen using integer linear programming [30]. Two sequences were excluded from Arlequin analysis and network construction, as they were the only two belonging to Western Mediterranean locality (N = 251 partial cytb sequences). The figures were prepared for publication in Inkscape version 1.0.
3. Results
All collected specimens were mainly caught above the continental shelf of the open sea area of the middle eastern Adriatic, precisely from 50–200 m of the sea depth (Figure 1A.). Total length of all T. picturatus analysed specimens (N = 155) varied from 9.2 to 33.7 cm (mean ± SD: 12.15 ± 2.95 cm), while their total body weight ranged from 5.79 to 384.94 g (mean ± SD: 17.64 ± 39.42 g). Length frequency distribution was unimodal with very few specimens whose total length was above 14 cm. The results of relative morphometric relationships of measured body proportions expressed in percentages, as well as five meristic characteristics, are given in Table 1. Obtained coefficients of variation (CV) values were relatively low (CV < 10%) for all phenotypic characteristics (Table 1). Obtained relative morphometric relationships were also analysed as function of total body length (TL) and head length (LH). Those analyses pointed out that, with the increase in blue jack mackerel total body length (TL) morphometric relationships of FL/TL (FL/TL = 92.44 − 0.095TL; p < 0.05) and PAL/TL (PAL/TL = 61.35 − 0.499TL; p < 0.05) significantly decrease, while the morphometric relationship of LP/TL (LP/TL = 16.74 + 0.238TL; p < 0.05) significantly increases. According to obtained data, blue jack mackerel has the following set of meristic characters: D1: VI–IX, D2: I + 25–35, A: II + 24–32, P: 17–23, V: 5.

Table 1.
Morphometric (relative relationships of measured body proportions) and meristic characteristics of blue jack mackerel Trachurus picturatus collected during the MEDITS survey in July 2018, eastern Adriatic Sea.
All calculated length–length relationships and their coefficient of determination (r2) are presented in Table 2. Overall, obtained length–length regressions were statistically significant (p < 0.05) with a high value of the coefficient of correlation (r > 0.923).

Table 2.
Length–length regression parameters (a,b) and determination coefficient (r2) of blue jack mackerel Trachurus picturatus (N = 155) collected during the MEDITS survey in July 2018, eastern Adriatic Sea.
Length–weight relationships of investigated species pointed out that blue jack mackerel has positive allometric growth (W = 0.0048TL3.1789, r2 = 0.9801), since its allometric coefficient (b) was statistically significant different (p < 0.05) from isometric value (b = 3).
Sequencing of the fragment of the cytochrome b locus revealed that specimens belonged to the Trachurus picturatus (Bowdich, 1825) (N = 37, length of the sequence 319 bp, GenBank accession numbers MN412596 to MN412632). Two specimens caught in the same batch were confirmed as T. mediterraneus (Steindachner, 1868) (GenBank accession numbers MN412633 and MN412634). Among 11 polymorphic sites observed in cytb fragment of T. picturatus, 4 were singleton variable sites and 7 parsimony informative sites, giving total of 10 haplotypes. Overall haplotype diversity (h) of 0.812 ± 0.048 and nucleotide diversity (π) of 0.0064 ± 0.0007 indicated high levels of haplotype diversity and low nucleotide diversity. When the sequences obtained within this research where aligned with all publicly available data (N = 251) on T. picturatus cytb mitochondrial gene, the overall h was 0.521 ± 0.036 and π was 0.0033 ± 0.0003. Descriptive statistics of genetic diversity and demographic history of T. picturatus for each sampling locality, based on collected cytb sequence data, is summarised in Table 3.

Table 3.
Descriptive statistics of genetic diversity and demographic history of Trachurus picturatus, based on cytb sequence data. Sequences from the Adriatic Sea were obtained within this study, while the rest of the sequences were collected from GenBank, for comparison purposes.
Phylogenetic reconstruction showed the presence of three principal clades (Figure 2) corresponding to the three Carangidae species analysed. T. picturatus specimens collected within this research were represented with six haplotypes and branched together with other referent T. picturatus sequences, separate from T. mediterraneus and T. trachurus. The most frequent haplotype, Hap_2, was detected on all sampling localities and it is an ancestral haplotype (Figure 3). Besides Hap_1, Hap_3 and Hap_4, which were also present on most or all sampling localities in the Northeast Atlantic and Mediterranean Sea, the majority of other haplotypes were unique for each sampling locality (Figure 4; Tables S1 and S2). The results of hierarchical analysis of molecular variation (AMOVA) indicated that most of the variation was found within analysed populations and not between groups (Northeast Atlantic vs. Mediterranean Sea) or populations within groups (Table S3), but the findings were not statistically significant.
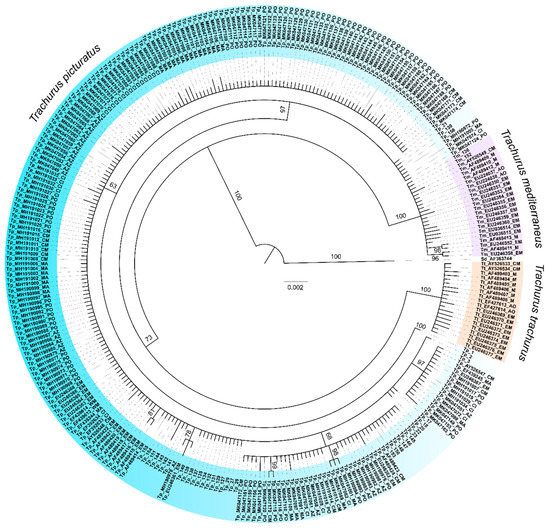
Figure 2.
Rooted phylogenetic tree inferred by Bayesian analysis of mtDNA cytochrome b sequences, with posterior probability percentage shown for main nodes. Selene dorsalis (Carangidae) was used as an outgroup. Blue jack mackerel Trachurus picturatus was presented with 25 haplotypes. Sequence IDs of each Trachurus species were coloured differently for easier interpretation of the phylogenetic tree. IDs of all publicly available Genbank sequences were created in the same way: first two letters are species abbreviation (Tp—Trachurus picturatus, Tm—Trachurus mediterraneus, Tt—Trachurus trachurus, Sd—Selene dorsalis), followed by GenBank accession number and locality abbreviation (AO—Atlantic Ocean, PO—Portugal mainland, MA—Madeira, CI—Canary Islands, AZ—Azores, WM—Western Mediterranean, CM—Central Mediterranean, EM—Eastern Mediterranean and M—Mediterranean Sea). Information about localities was collected in published research papers/supplementary data or sequence description on GenBank. Sequence IDs collected from Adriatic Sea in this research contain the same species abbreviation.
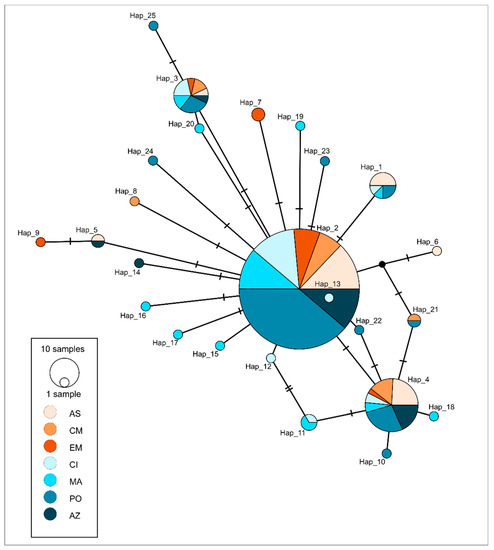
Figure 3.
Integer neighbour-joining (IntNJ) network depicting mutational relationships between haplotypes of blue jack mackerel Trachurus picturatus (represented by circles in the lower left corner and coloured according to sampling locality (shades of orange represent Mediterranean Sea: AS—Adriatic Sea, CM—Central Mediterranean and EM—Eastern Mediterranean, while shades of blue represent Atlantic Ocean: CI—Canary Islands, MA—Madeira, PO—Portugal mainland and AZ—Azores). Samples collected from the Adriatic Sea were sequenced within this research, while the rest of the sequences were publicly available from GenBank. The integer edge lengths are chosen using integer linear programming by default, when IntNJ is selected in PopART [30].
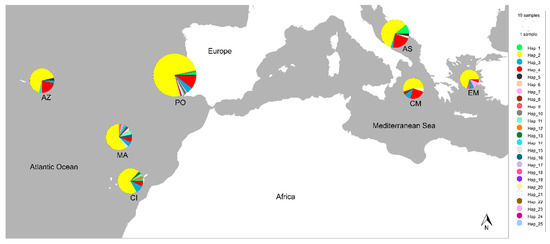
Figure 4.
Sampling localities of sequenced blue jack mackerels Trachurus picturatus were placed on a map using PopART and they were named in the same manner as on the IntNJ network (Mediterranean Sea: AS—Adriatic Sea, CM—Central Mediterranean and EM—Eastern Mediterranean; Atlantic Ocean: CI—Canary Islands, MA—Madeira, PO—Portugal mainland and AZ—Azores). Colours indicate different mitochondrial haplotypes (N = 25), and the size of the circles reflects the observed haplotype frequency. Note the presence of the most frequent haplotype Hap_2 on each sampling locality. Samples collected from the Adriatic Sea were sequenced within this research, while the rest of the sequences were publicly available from GenBank. GPS of the localities was approximated according to the description from published research papers/supplementary data or sequence description on GenBank.
4. Discussion
This is the first time that specimens of blue jack mackerel were collected in sufficient quantity that allowed for the analysis of its phenotypic and genetic characteristics and the area of its distribution in the Adriatic Sea. Specimens of blue jack mackerel occurred over the continental shelf (depth range: 50–200 m, Figure 1) of the middle-Eastern Adriatic, characterised mostly by the muddy sea bottom [31] during the investigated period. According to the available literature, this species was also found in the areas of the shallow Northern Adriatic [11], which coincided with the distribution pattern obtained for this species over the last six MEDITS surveys (2013–2018; Figure 1B). The area of blue jack mackerel appearance in the Adriatic was in line with the studies done in the area of the Macaronesian archipelagos, where this species is common and represents an important fishery resource [3,32,33]. Due to the length frequency distribution, it was obvious that, throughout this survey, the majority of collected specimens were smaller (TL < 14.0 cm) and immature, which was in accordance with reported length at first maturity for this species [34]. The possible confirmation of blue jack mackerel spawning in the Adriatic Sea was through the genetic identification of blue jack mackerel larva collected (42°56′21″ N, 15°10′5″ E; near Jabuka/Pomo pit) during the scientific survey, performed along the Eastern Adriatic (Croatia) in June–July 2019 (GenBank accession number MT795964). A few larger specimens (TL > 28.0 cm) had gonads and were determined as females. Blue jack mackerel morphological values obtained within this study were in line with those provided by Bolognini et al. [11], for the same species inhabiting the same geographical area, as well as the ones reported via www.fishbase.org. Meristic characters of studied species collected in the Adriatic Sea fit within the ranges of the same ones provided through determination keys of [2] and Jardas [6]. The only meristic difference between this study and Jardas [6] was observed in ventral fin, as Jardas [6] noted the presence of one spine in mentioned fin.
All length–length relationships were linear and coincide with the previously reported length–length relationship in the Adriatic [11] and other areas. Concerning the length–weight relationship, blue jack mackerel specimens observed within this study had positive allometric growth (b = 3.1789) which was in line with all the previous studies analysing this relationship along the blue jack mackerel area of distribution [35,36,37].
The genetic identification and phylogenetic reconstruction in this study was expanded and put in a wider context of the Mediterranean Sea and the Northeast Atlantic Ocean. These results offered an additional confirmation of the genetic distances of the three European species of the genus Trachurus (T. trachurus, T. mediterraneus and T. picturatus). Furthermore, recurring evidence of high levels of haplotype diversity and low levels of nucleotide diversity across the blue jack mackerel’s distribution [38,39,40,41], now including the Adriatic Sea results, suggested no established genetic structure. This observed lack of significant variation between populations [39,41] indicated considerable gene flow and the existence of a single panmictic population. In contrast, other approaches, such as using geometric morphometrics and otolith phenotypic variability in the Northeast Atlantic have established the existence of a complex population structure [42] or indicated towards distinguishable populations [43]. Nevertheless, the most recent assessment of the population genetic structure of T. picturatus using microsatellites, a much more sensitive tool to detect recent changes than mitochondrial genes, often applied thus far, revealed a lack of genetic structure in the Northeast Atlantic and Mediterranean Sea [44]. The authors concluded that the most probable explanation is the faster evolutionary rate of environmentally dependent phenotypic traits (for example Tanner et al. [45]). Although at this point in time there lacks genetic evidence of separate stocks, it is possible a more comprehensive study design and greater number of analysed blue jack mackerels would have revealed these subtle genetic changes in population structures or they will follow the divergence of environmentally-dependent phenotypic traits. What can be claimed with certainty is that the local spawning aggregations do exist. They have been confirmed in the Azores [3] and Canary Islands [42] and now it seems the spawning might have occurred in the Adriatic Sea as well.
5. Conclusions
Bearing in mind that, over the last few decades, climate regimes in the Adriatic [46] have changed, the occurrence of new species or increased abundance of very rare species is not surprising. Hence, awareness of the role of this within an ecosystem and the possible effect that it has on local populations should be monitored and well documented.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/12/12/463/s1, Table S1: Number of cytb haplotypes obtained in each population of Trachurus picturatus, Table S2: List of cytb haplotypes obtained from Trachurus picturatus and the sequences that belong to each haplotype, Table S3: Hierarchical analysis of molecular variation (AMOVA) for Trachurus picturatus populations, based on cytb sequences data.
Author Contributions
B.Z. and N.V. designed the concept of the study, V.Č.K. and I.I. analysed the biometric data, V.V. performed field work and measurements, I.L.P. and I.R. isolated D.N.A. and edited sequence data, I.B. analysed genetic data, B.Z. and I.B. wrote the majority of the manuscript, while all authors reviewed the text and contributed to writing within their area of specialty. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Ministry of Agriculture of the Republic of Croatia, as a part of the research project “Data Collection Framework (DCF)”, and throughout the projects “Local Ecological Knowledge and Fisheries Research in Croatia (LEKFishResCRO)” and “Exploration of ecologically sensitive areas with special emphasis on growth, development and protection of commercially important maritime organisms (ESAmar)” founded by Croatian Science Foundation.
Conflicts of Interest
The authors declare no conflict of interest.
Ethical Statement
The sampling within this research was conducted during “MEDITS” research survey, supported by the European Commission (Directorate for Fisheries) jointly with the contribution of the partner countries. It complies with the Marine Fisheries Act in Croatia (NN 62/17; NN 130/17; NN 14/19). The animals collected through this scientific survey, and subsequently used in the research presented here, were not exposed to any unnecessary pain, injuries or suffering. Obtaining specialised permission was not necessary for the type of research conducted.
References
- Lynch, R. Globalisation and Official Statistics, ONS UK. In Proceedings of the Conference of European Statisticians, Paris, France, 13–15 June 2006. [Google Scholar]
- Smith-Vaniz, W.F. Carangidae. In Fishes of the North-Eastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; pp. 649–674, 1009–1031. [Google Scholar]
- Menezes, G.M.; Sigler, M.F.; Silva, H.M.; Pinho, M.R. Structure and zonation of demersal fish assemblages off the Azores Archipelago (mid-Atlantic). Mar. Ecol. Prog. Ser. 2006, 324, 241–260. [Google Scholar] [CrossRef]
- FAO. World Review of Highly Migratory Species and Straddling Stocks; FAO: Rome, Italy, 1994. [Google Scholar]
- Binni, G. Atlante dei Pesci delle Coste Italiane; Mondo Sommerso: Milano, Italy, 1968. [Google Scholar]
- Jardas, I. The Adriatic Ichthyofauna; Školska Knjiga d.d.: Zagreb, Croatia, 1996. [Google Scholar]
- Šoljan, T. I Pesci dell’Adriatico; A. Mondadori: Verona, Italy, 1975. [Google Scholar]
- Tortonese, E. Osteichtyes (Pesci ossei). Fauna d’Italia; XI Calderini: Bologna, Italy, 1975. [Google Scholar]
- Relini, G.; Lanteri, L. Osteichthyes. Biol. Mar. Mediterr. 2010, 17, 649–674. [Google Scholar]
- Županović, Š.; Jardas, I. Fauna i Flora Jadrana, Jabučka Kotlina, II; Logos: Split, Croatia, 1987. [Google Scholar]
- Bolognini, L.; Domenichetti, F.; Grati, F.; Polidori, P.; Scarcella, G.; Fabi, G. New record of the blue jack mackerel, Trachurus picturatus, T.E. Bowdich, 1825 (Osteichthyes: Carangidae) from the Northern Adriatic Sea. Acta Adriat. 2015, 56, 305–308. [Google Scholar]
- Bianchi, C.N.; Morri, C. Marine biodiversity of the Mediterranean Sea: Situation, problems and prospects for future research. Mar. Pollut. Bull. 2000, 40, 367–376. [Google Scholar] [CrossRef]
- Perry, A.L.; Low, P.J.; Ellis, J.R.; Reynolds, J.D. Climate change and distribution shifts in marine fishes. Science 2005, 308, 1912–1915. [Google Scholar] [CrossRef]
- Žužul, I.; Šegvić-Bubić, T.; Talijančić, I.; Džoić, T.; Lepen Pleić, I.; Beg Paklar, G.; Ivatek-Šahdan, S.; Katavić, I.; Grubišić, L. Spatial connectivity pattern of expanding gilthead seabream populations and its interactions with aquaculture sites: A combined population genetic and physical modelling approach. Sci. Rep. 2019, 9, 14718. [Google Scholar] [CrossRef]
- Šegvić-Bubić, T.; Talijančić, I.; Grubišić, L.; Izquierdo-Gomez, D.; Katavić, I. Morphological and molecular differentiation of wild and farmed gilthead sea bream Sparus aurata: Implications for management. Aquac. Environ. Interact. 2014, 6, 43–54. [Google Scholar] [CrossRef]
- Avignon, S.; Tastard, E.; Weston, S.; Duhamel, G.; Denis, F. Morphological identification and DNA barcoding used for diet analysis of gilthead seabream (Sparus aurata) in its expanding northerly range. Aquat. Living Resour. 2017, 30, 1. [Google Scholar] [CrossRef]
- Taggart, J.B.; Hynes, R.A.; Prodöuhl, P.A.; Terguson, A. A simplified protocol for routine total DNA isolation from salmonid fishes. J. Fish Biol. 1992, 40, 963–965. [Google Scholar] [CrossRef]
- Kocher, T.D.; Thomas, W.K.; Meyer, A.; Edwards, S.V.; Pääbo, S.; Villablanca, F.X.; Wilson, A.C. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proc. Natl. Acad. Sci. USA 1989, 86, 6196–6200. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Larget, B.; Simon, D.L. Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Mol. Biol. Evol. 1999, 16, 750–759. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kishino, H.; Yano, T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985, 22, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. Figtree Version 1.4.4. 2018. Available online: https://github.com/rambaut/figtree/releases (accessed on 3 December 2020).
- Librado, P.; Rozas, J. DNASP V5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Res. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar]
- Ciampalini, A.; Forli, M.; Guerrini, A.; Sammartino, F. The marine fossils malacofauna in a Plio-Pleistocene section from Vallin Buio (Livorno, Italy). Biodivers. J. 2014, 5, 9–18. [Google Scholar]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- HIJRM. (Adriatic Sea. General Map of the Bottom Dediments) 1: 1 000 000; Jadransko More. Generalna Karta Sedimenta dna; Hidrografski Institut Jugoslavenske Ratne Mornarice: Split, Croatia, 1985. [Google Scholar]
- ICES. Report of the Working Group on Southern Horse Mackerel, Anchovy and Sardine (WGHANSA); ICES: Copenhagen, Denmark, 2018. [Google Scholar]
- Menezes, G.M.; Rosa, A.; Melo, O.; Pinho, M.R. Demersal fish assemblages off the Seine and Sedlo seamounts (Northeast Atlantic). Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 2683–2704. [Google Scholar] [CrossRef]
- Jurado-Ruzafa, A.; Santamaria, M.T.G. Reproductive biology of the blue jack mackerel, Trachurus picturatus (Bowdich, 1825), off the Canary Islands. J. Appl. Ichthyol. 2013, 29, 526–531. [Google Scholar] [CrossRef]
- Bilge, G.; Yapıcı, S.; Filiz, H.; Cerim, H. Weight–length relations for 103 fish species from the southern Aegean Sea, Turkey. Acta Ichthyol. Piscat. 2014, 44, 263–269. [Google Scholar] [CrossRef]
- Garcia, A.; Pereira, J.G.; Canha, Â.; Reis, D.; Diogo, H. Life history parameters of blue jack mackerel Trachurus picturatus (Teleostei: Carangidae) from north-east Atlantic. J. Mar. Biol. Assoc. UK 2015, 95, 401–410. [Google Scholar] [CrossRef]
- Jurado-Ruzafa, A.; Santamaria, M.T.G. Age, growth and natural mortality of blue jack mackerel Trachurus picturatus (Carangidae) from the Canary Islands, Spain (NW Africa). Afr. J. Mar. Sci. 2018, 40, 451–460. [Google Scholar] [CrossRef]
- Bektas, Y.; Belduz, A.O. Molecular phylogeny of Turkish Trachurus species (Perciformes: Carangidae) inferred from mitochondrial DNA analyses. J. Fish Biol. 2008, 73, 1228–1248. [Google Scholar] [CrossRef]
- Karaiskou, N.; Triantafyllidis, A.; Triantaphyllidis, C. Shallow genetic structure of three species of the genus Trachurus in European waters. Mar. Ecol. Prog. Ser. 2004, 281, 193–205. [Google Scholar] [CrossRef]
- Karaiskou, N.; Apostolidis, A.P.; Triantafyllidis, A.; Kouvatsi, A.; Triantaphyllidis, C. Genetic identification and phylogeny of three species of the genus Trachurus based on mitochondrial DNA analysis. Mar. Biotechnol. 2003, 5, 493–504. [Google Scholar]
- Moreira, C.; Correia, A.T.; Vaz-Pires, P.; Froufe, E. Genetic diversity and population structure of the blue jack mackerel Trachurus picturatus across its western distribution. J. Fish Biol. 2019, 94, 725–731. [Google Scholar] [CrossRef]
- Tuset, V.M.; Jurado-Ruzafa, A.; Otero-Ferrer, J.L.; Santamaría, M.T.G. Otolith phenotypic variability of the blue jack mackerel, Trachurus picturatus, from the Canary Islands (NE Atlantic): Implications in its population dynamic. Fish. Res. 2019, 218, 48–58. [Google Scholar] [CrossRef]
- Vasconcelos, J.; Vieira, A.R.; Sequeira, V.; González, J.A.; Kaufmann, M.; Gordo, L.S. Identifying populations of the blue jack mackerel (Trachurus picturatus) in the Northeast Atlantic by using geometric morphometrics and otolith shape analysis. Fish. Bull. 2018, 116, 81–92. [Google Scholar] [CrossRef]
- Moreira, C.; Presa, P.; Correia, A.T.; Vaz-Pires, P.; Froufe, E. Spatio-temporal microsatellite data suggest a multidirectional connectivity pattern in the Trachurus picturatus metapopulation from the Northeast Atlantic. Fish. Res. 2020, 225, 105499. [Google Scholar] [CrossRef]
- Tanner, S.E.; Pérez, M.; Presa, P.; Thorrold, S.R.; Cabral, H.N. Integrating microsatellite DNA markers and otolith geochemistry to assess population structure of European hake (Merluccius merluccius). Estuar. Coast. Shelf Sci. 2014, 142, 68–75. [Google Scholar] [CrossRef]
- Grbec, B.; Morović, M.; Matić, F.; Ninčević Gladan, Ž.; Marasović, I.; Vidjak, O.; Bojanić, N.; Čikeš Keč, V.; Zorica, B.; Kušpilić, G.; et al. Climate regime shifts and multi-decadal variability of the Adriatic Sea pelagic ecosystem. Acta Adriat. 2014, 55, 117–126. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).