Structure of Rhodolith Beds and Surrounding Habitats at the Doce River Shelf (Brazil)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
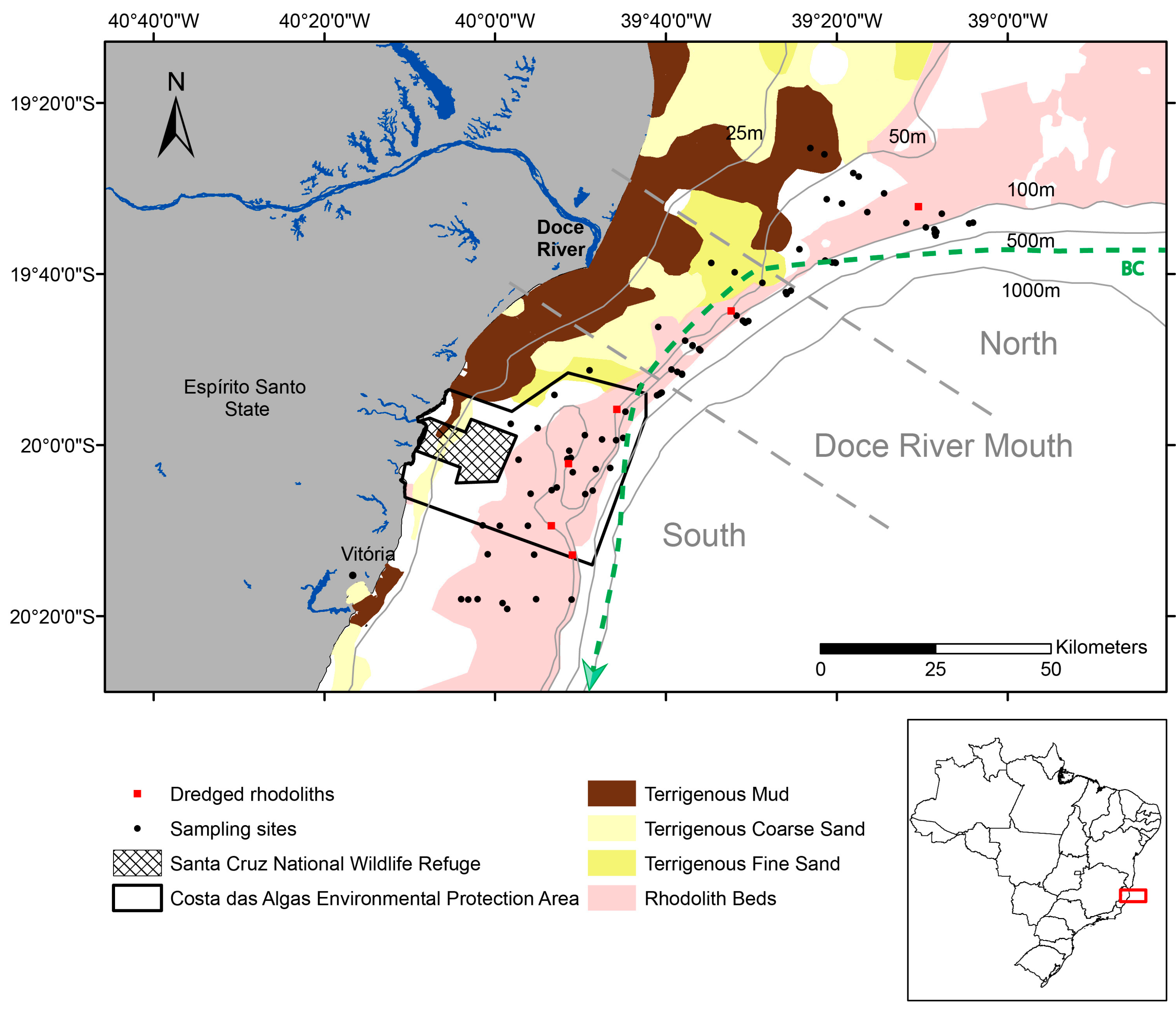
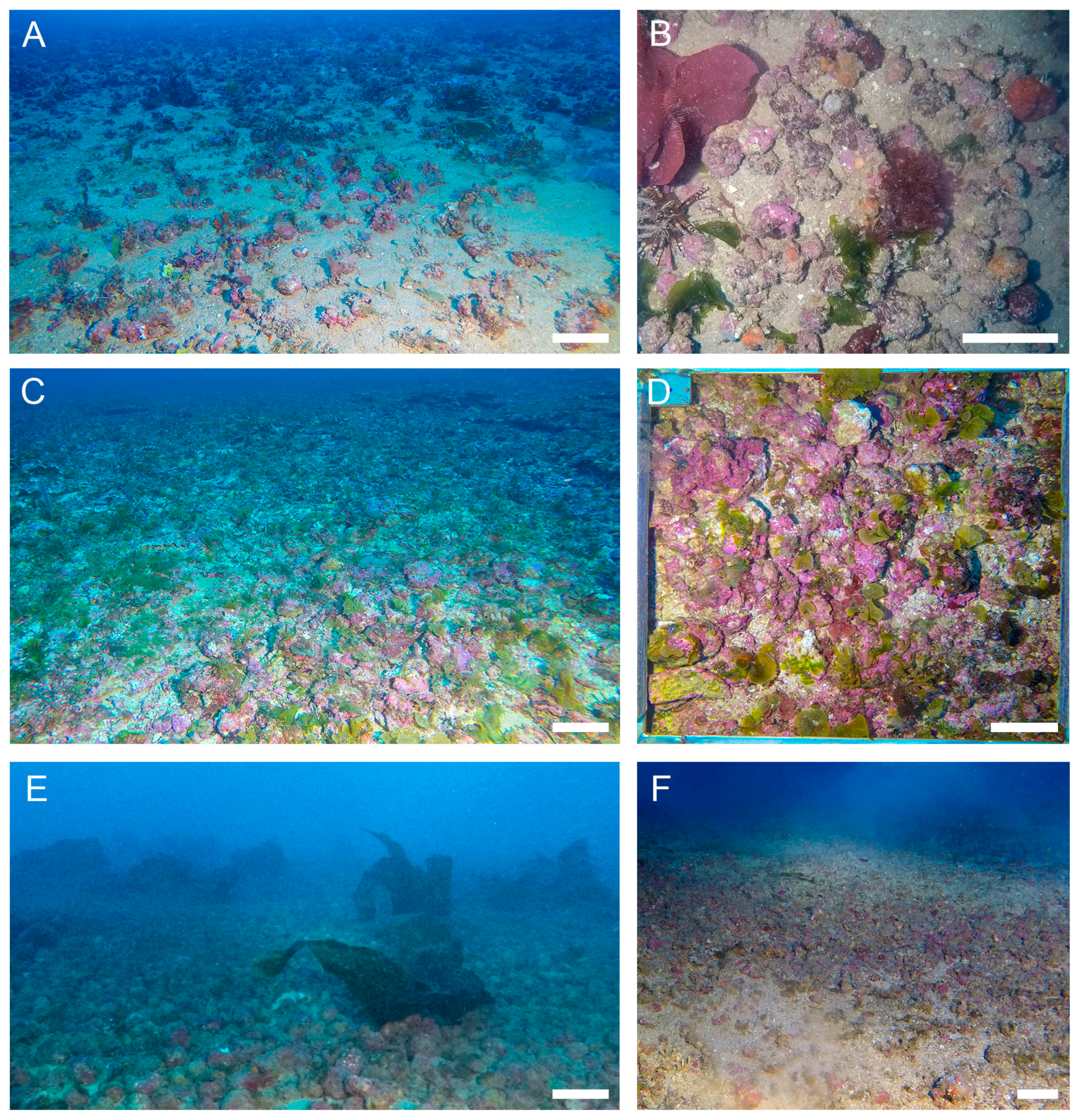
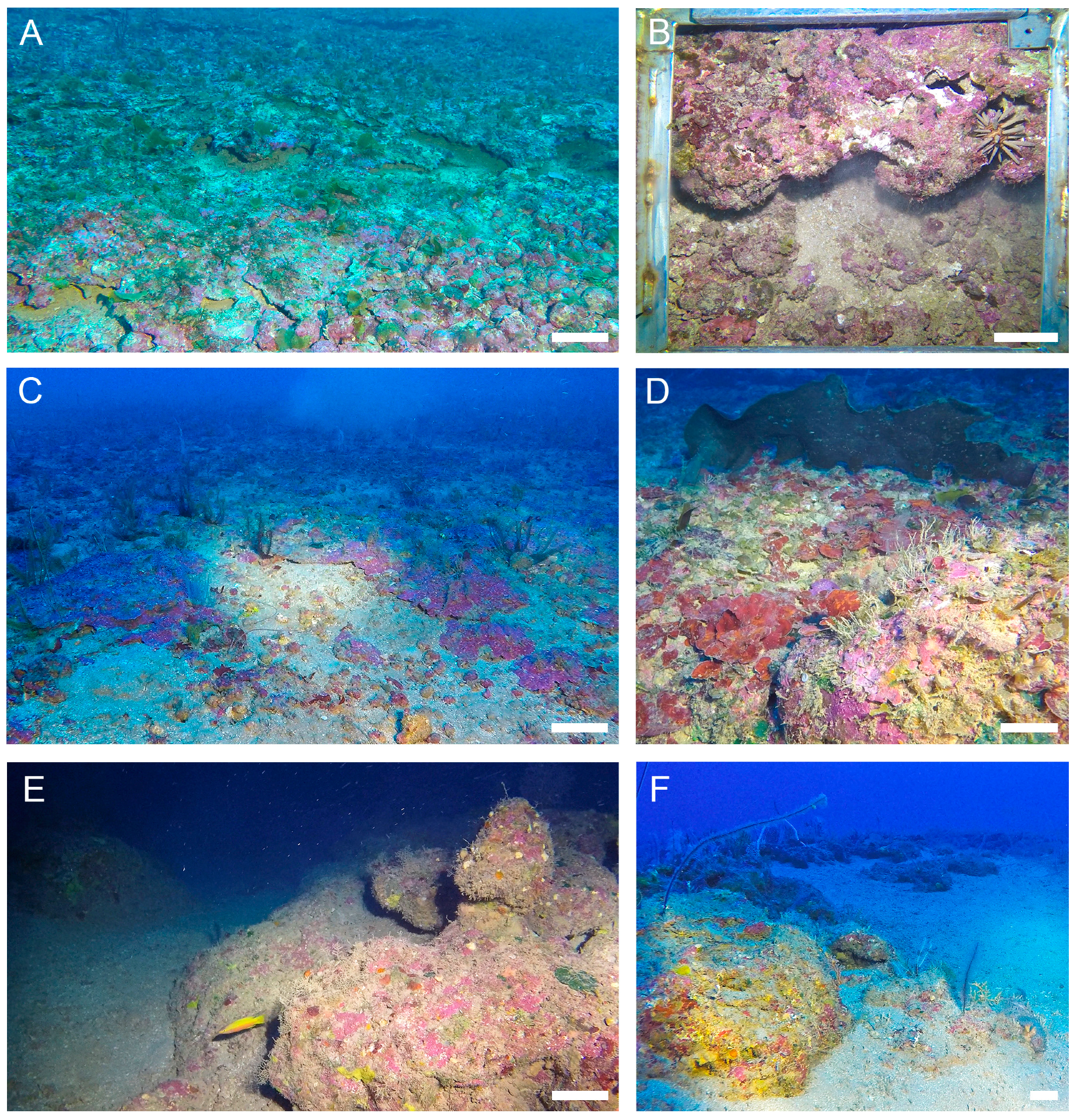
2.2. Rhodolith Sampling and Characterization
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Study Site | Zone | Habitat | Depth (m) | Latitude (S) | Longitude (W) |
|---|---|---|---|---|---|
| N1 | North | B | 50 | 19°28′9.30″ | 39°18′4.44″ |
| N2 | North | R | 100 | 19°33′59.34″ | 39°4′2.35″ |
| N3 | North | RB | 70 | 19°34′29.91″ | 39°9′33″ |
| N4 | North | RB | 90 | 19°35′13.53″ | 39°8′30.74″ |
| N5 | North | CC | 80 | 19°38′38.72″ | 39°20′32.96″ |
| DR1 | Doce River Mouth | S | 60 | 19°41′1.33″ | 39°28′43.62″ |
| DR2 | Doce River Mouth | S | 80 | 19°42′6.13″ | 39°25′57.87″ |
| DR3 | Doce River Mouth | RB | 50 | 19°44′18.99″ | 39°32′20.7″ |
| DR4 | Doce River Mouth | S | 68 | 19°45′8.94″ | 39°31′30.24″ |
| DR5 | Doce River Mouth | S | 40 | 19°46′19.64″ | 39°40′51.31″ |
| S1 | South | CC | 70 | 19°54′8.82″ | 39°41′2.12″ |
| S2 | South | R | 103 | 19°53′50.6″ | 39°40′29.88″ |
| S3 | South | B | 45 | 19°57′59.45″ | 39°55′1.25″ |
| S4 | South | RB/CC | 63 | 19°59′9.68″ | 39°45′2.9″ |
| S5 | South | B | 45 | 20°1′40.74″ | 39°57′42.74″ |
| S6 | South | S | 100 | 20°1′4.92″ | 39°56′11.12″ |
| S7 | South | S/B | 42 | 20°6′41.48″ | 39°59′47.4″ |
| S8 | South | B | 49 | 20°9′26.62″ | 39°53′25.52″ |
| S9 | South | B | 40 | 20°12′46.6″ | 40°3′10″ |
References
- Foster, M.S. Rhodoliths: Between rocks and soft places—Minireview. J. Phycol. 2001, 37, 659–667. [Google Scholar] [CrossRef]
- Steller, D.L.; Riosmena-Rodríguez, R.; Foster, M.S.; Roberts, C.A. Rhodolith bed diversity in the Gulf of California: The importance of rhodolith structure and consequences of disturbance. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S5–S20. [Google Scholar] [CrossRef]
- Basso, D.; Babbini, L.; Kaleb, S.; Bracchi, V.A.; Falace, A. Monitoring deep Mediterranean rhodolith beds. Aquatic. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 549–561. [Google Scholar] [CrossRef] [Green Version]
- Bosence, D.W.J. Description and classification of rhodoliths (rhodoids, rhodolites). In Coated Grains; Peryt, T.M., Ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 217–224. [Google Scholar]
- Basso, D. Deep rhodolith distribution in the Pontian Islands, Italy: A model for the paleoecology of a temperate sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1998, 137, 173–187. [Google Scholar] [CrossRef]
- Amado-Fiho, G.M.; Bahia, R.G.; Pereira-Filho, G.H.; Longo, L.L. South Atlantic rhodolith beds: Latitudinal distribution, species composition, structure and ecosystem functions, threats and conservation status. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Geerbestrasse, Switzerland, 2017; pp. 299–317. [Google Scholar]
- Martin, S.; Gattuso, J.P. Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Glob. Chang. Biol. 2009, 15, 2089–2100. [Google Scholar] [CrossRef]
- Martin, S.; Cohu, S.; Vignot, C.; Zimmerman, G.; Gattuso, J.P. One-year experiment on the physiological response of the Mediterranean crustose coralline alga, Lithophyllum cabiochae, to elevated pCO2 and temperature. Ecol. Evol. 2013, 3, 676–693. [Google Scholar] [CrossRef] [Green Version]
- Ragazzola, F.; Foster, L.C.; Form, A.U.; Büscher, J.; Hansteen, T.H.; Fietzke, J. Phenotypic plasticity of coralline algae in a high CO2 world. Ecol. Evol. 2013, 3, 3436–3446. [Google Scholar]
- Blake, C.; Maggs, C.A. Comparative growth rates and internal banding periodicity of maerl species (Corallinales, Rhodophyta) from northern Europe. Phycologia 2003, 42, 606–612. [Google Scholar] [CrossRef]
- Nelson, W.A. Calcified macroalgae—Critical to coastal ecosystem and vulnerable to change: A review. Mar. Freshw. Res. 2009, 60, 781–801. [Google Scholar] [CrossRef]
- McCoy, S.J.; Kamenos, N.A. Coralline algae (Rhodophyta) in a changing world: Integrating ecological, physiological and geochemical responses to global change. J. Phycol. 2015, 51, 6–24. [Google Scholar] [CrossRef]
- Amado-Filho, G.M.; Maneveldt, G.W.; Pereira-Filho, G.H.; Manso, R.C.C.; Bahia, R.G.; Barros-Barreto, M.B.; Guimarães, S.M.P.B. Seaweed Diversity Associated with a Brazilian Tropical Rhodolith Bed. Cienc. Mar. 2010, 36, 371–391. [Google Scholar] [CrossRef] [Green Version]
- Amado-Filho, G.M.; Moura, R.L.; Bastos, A.C.; Salgado, L.T.; Sumida, P.Y.; Guth, A.Z.; Francini-Filho, R.B.; Pereira-Filho, G.H.; Abrantes, D.P.; Brasileiro, P.S.; et al. Rhodolith beds are major CaCO3 bio-factories in the tropical south west atlantic. PLoS ONE 2012, 7, e35171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amado-Filho, G.M.; Pereira-Filho, G.H.; Bahia, R.G.; Abrantes, D.P.; Veras, P.C.; Matheus, Z. Occurrence and distribution of rhodolith beds on the Fernando de Noronha Archipelago of Brazil. Aquat. Bot. 2012, 101, 41–45. [Google Scholar] [CrossRef]
- Cavalcanti, G.S.; Gregoraci, G.B.; Santos, E.O.; Silveira, C.B.; Meirelles, P.M.; Longo, L.; Gotoh, K.; Naramura, S.; Iida, T.; Sawabe, T.; et al. Physiologic and metagenomic attributes of the rhodoliths forming the largest CaCO3 bed in the South Atlantic Ocean. ISME J. 2014, 8, 52–62. [Google Scholar] [CrossRef]
- Brasileiro, P.S.; Braga, J.C.; Amado-Filho, G.M.; Leal, R.N.; Bassi, D.; Franco, T.; Bastos, A.C.; Moura, R. Burial rate determines Holocene rhodolith development on the Brazilian Shelf. Palaios 2018, 33, 464–477. [Google Scholar] [CrossRef]
- Vale, N.F.; Amado-Filho, G.M.; Braga, J.C.; Brasileiro, P.S.; Karez, C.S.; Moraes, F.C.; Bahia, R.G.; Bastos, A.C.; Moura, R.L. Structure and composition of rhodoliths from the Amazon River mouth, Brazil. J. S. Am. Earth Sci. 2018, 84, 149–159. [Google Scholar] [CrossRef]
- Moura, R.M.; Amado-Filho, G.M.; Moraes, F.C.; Brasileiro, P.S.; Salomon, P.S.; Mahiques, M.M.; Bastos, A.C.; Almeida, M.G.; Silva, J.M.; Araujo, B.F.; et al. An extensive reef system at the Amazon River mouth. Sci. Adv. 2016, 2, e1501252. [Google Scholar] [CrossRef] [Green Version]
- Bastos, A.C.; Quaresma, V.S.; Marangoni, M.B.; D’Agostini, D.P.; Bourguignon, S.N.; Cetto, P.H.; Silva, A.E.; Amado-Filho, G.M.; Moura, R.L.; Collins, M. Shelf morphology as an indicator of sedimentary regimes: A synthesis from a mixed siliciclastic carbonate shelf on the eastern Brazilian margin. J. S. Am. Earth Sci. 2015, 63, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Berlandi, R.M.; Figueiredo, M.A.O.; Paiva, P.C. Rhodolith morphology and the diversity of polychaetes off the southeastern Brazilian coast. J. Coast. Res. 2012, 28, 280–287. [Google Scholar] [CrossRef]
- Dias, G.T.M.; Villaça, R.C. Coralline algae depositional environments on the Brazilian Central-South-Eastern shelf. J. Coast. Res. 2012, 28, 270–279. [Google Scholar] [CrossRef]
- Marins, B.V.; Amado-Filho, G.M.; Barreto, M.B.B.; Longo, L.L. Taxonomy of the southwestern Atlantic endemic kelp: Laminaria abyssalis and Laminaria brasiliensis (Phaeophyceae, Laminariales) are not different species. Phycol. Res. 2012, 60, 51–60. [Google Scholar] [CrossRef]
- Magris, R.A.; Marta-Almeida, M.; Monteiro, J.A.F.; Ban, N.C. A modelling approach to assess the impact of land mining on marine biodiversity: Assessment in coastal catchments experiencing catastrophic events (SW Brazil). Sci. Total. Environ. 2019, 659, 828–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, L.E.O.; Correa, L.B.; Sá, F.; Rodrigues-Neto, R.; Bernardino, A.F. The impacts of the Samarco mine tailing spill on the Rio Doce estuary, Eastern Brazil. Mar. Pollut. Bull. 2017, 120, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Golder. Avaliação do Estado de Conservação dos Bancos de Rodolitos Adjacentes à Foz do Rio Doce; Report prepared for Samarco Mineração S.A.; Golder Associates Ltda: Rio de Janeiro, Brazil, 2016; p. 46. [Google Scholar]
- Oliveira, K.S.S.; Quaresma, V.S. Temporal variability in the suspended sediment load and streamflow of the Doce River. J. S. Am. Earth Sci. 2017, 78, 101–115. [Google Scholar] [CrossRef]
- Quaresma, V.S.; Catabriga, G.; Bourguignon, S.N.; Godinho, E.; Bastos, A.C. Modern sedimentary processes along the Doce river adjacent continental shelf. Brazlian J. Geol. 2015, 45, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Guimarães, S.M.P.B. Uma análise da diversidade da flora marinha bentônica do Estado do Espírito Santo, Brasil. Hoehnea 2003, 30, 11–19. [Google Scholar]
- Guimarães, S.M.P.B. A revised checklist of benthic marine Rhodophyta from the State of Espírito Santo, Brazil. Boletim do Instituto de Botânica 2006, 17, 143–194. [Google Scholar]
- Horta, P.A.; Amancio, E.; Coimbra, C.S.; Oliveira, E.C. Considerações sobre a distribuição e origem da flora de macroalgas marinhas brasileiras. Hoehnea 2001, 28, 243–265. [Google Scholar]
- Vieira, F.; Bastos, A.C.; Quaresma, V.S.; Leite, M.D.; Costa A., Jr.; Oliviera, K.S.S.; Dalvi, C.; Bahia, R.G.; Holz, V.L.; Moura, R.L.; et al. Along-shelf changes in mixed carbonate-siliciclastic sedimentation patterns. Cont. Shelf Res. 2019, 187, 1039642. [Google Scholar] [CrossRef]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- Bahia, R.G.; Abrantes, D.P.; Brasileiro, P.S.; Amado-Filho, G.M.; Pereira-Filho, G.H. Rhodolith bed structure along a depth gradient on the northern coast of Bahia state, Brazil. Brazlian J. Oceanogr. 2010, 58, 323–337. [Google Scholar] [CrossRef]
- Abramoff, M.D.; Magalhaes, P.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Bosence, D.W.J.; Pedley, H.M. Sedimentology and palaeoecology of Miocene coralline algal biostrome from the Maltese Islands. Palaeogeog. Palaeoclim. Palaeoecol. 1982, 38, 9–43. [Google Scholar] [CrossRef]
- Graham, D.J.; Midgley, N.G. Graphical representation of particle shape using triangular diagrams: An Excel spreadsheet method. Earth Surf. Process. Landf. 2000, 25, 1473–1477. [Google Scholar] [CrossRef]
- Basso, D.; Nalin, R.; Nelson, C.S. Shallow-water Sporolithon rhodoliths from North Island (New Zealand). Palaios 2009, 24, 92–103. [Google Scholar] [CrossRef]
- Maneveldt, G.W.; van der Merwe, E. Heydrichia cerasina sp. nov. (Sporolithales, Corallinophycidae, Rhodophyta) from the southernmost tip of Africa. Phycologia 2012, 51, 11–21. [Google Scholar] [CrossRef]
- Bahia, R.G. Algas Coralináceas Formadoras de Rodolitos da Plataforma Continental Tropical e Ilhas Oceânicas do Brasil: Levantamento Florístico e Taxonomia. Ph.D. Thesis, Escola Nacional de Botânica Tropical, Rio de Janeiro, Brazil, 2014. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. Permanova+ for Primer: Guide to Software and Statistical Methods; Primer-E: Plymouth, UK, 2008. [Google Scholar]
- Bastos, A.C.; Oliveria, K.S.S.; Fernandes, L.F.; Pereira, J.B.; Demoner, L.E.; Neto, R.R.; Costa, E.S.; Sá, F.; Silva, C.A.; Lerhback, B.D.; et al. Monitoramento da Influência da Pluma do Rio Doce após o Rompimento da Barragem de Rejeitos em Mariana/MG, Novembro de 2015: Processamento, Interpretação e Consolidação de Dados; Universidade Federal do Espírito Santo: Vitoria, Brasil, 2017; p. 254. [Google Scholar]
- Grilo, C.F.; Quaresma, V.S.; Amorim, G.F.L.; Bastos, A.C. Changes in flocculation patterns of cohesive sediments after an iron ore mining dam failure. Mar. Geol. 2018, 400, 1–11. [Google Scholar] [CrossRef]
- Fabricius, K.; D’eath, G. Environmental factors associated with the spatial distribution of crustose coralline algae on the Great Barrier Reef. Coral Reefs 2001, 19, 303–309. [Google Scholar] [CrossRef]
- Tâmega, F.T.S.; Bassi, D.; Figueiredo, M.A.O.; Cherkinsky, A. Deep-water rhodolith bed from central Brazilian continental shelf, Campos Basin: Coralline algal and faunal taxonomic composition. Galaxea 2014, 16, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, M.A.O.; Coutinho, R.; Villas-Boas, A.B.; Tâmega, F.T.S.; Mariath, R. Deep-water rhodolith productivity and growth in the southwestern Atlantic. J. Appl. Phycol. 2012, 24, 487–493. [Google Scholar] [CrossRef]
- Marins, B.V.; Amado-Filho, G.M.; Barbarino, E.; Pereira-Filho, G.H.; Longo, L.L. Seasonal chnages in population structure of the tropical deep-water kelp Laminaria abyssalis. Phycol. Res. 2014, 62, 55–62. [Google Scholar]
- Mazzini, P.L.F.; Barth, J.A. A comparison of mechanisms generating vertical transport in the Brazilian coastal upwelling regions. J. Geophys. Res. Ocean. 2013, 118, 5977–5993. [Google Scholar] [CrossRef]
- Palóczy, A.; Brink, K.H.; Silveira, I.C.A.; Arruda, W.Z.; Martins, R.P. Pathways and mechanisms of offshore water intrusions on the Espírito Santo Basin shelf (18° S–22° S, Brazil). J. Geophys. Res. Ocean. 2016, 121, 5134–5163. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.F.; Belem, A.L.; Castro, B.M.; Geremias, R. Tide–topography interaction along the eastern Brazilian shelf. Cont. Shelf Res. 2005, 25, 1521–1539. [Google Scholar] [CrossRef]
- Ovalle, A.R.C.; Resende, C.E.; Carvalho, C.E.V.; Jennerjahn, T.C.; Ittekkot, V. Biogeochemical characteristics of coastal waters adjacent to small river-mangrove systems, East Brazil. Geo. Mar. Lett. 1999, 19, 179–185. [Google Scholar] [CrossRef]
- Rudorff, N.; Rudorff, C.N.; Kampel, M.; Ortiz, G. Remote sensing monitoring of the impact of a major mining wastewater disaster on the turbidity of the Doce River plume off the eastern Brazilian coast. ISPRS J. Photogramm. Remote Sens. 2018, 145, 349–361. [Google Scholar] [CrossRef]
- Marrack, E.C. The relationship between water motion and living rhodolith beds in the southwestern Gulf of California, Mexico. Palaios 1999, 14, 159–171. [Google Scholar] [CrossRef]
- Harris, P.T.; Tsuji, Y.; Marshall, J.F.; Davies, P.J.; Honda, N.; Matsuda, H. Sand and rhodolith-gravel entrainment on the mid- to outer-shelf under a western boundary current: Fraser Island continental shelf, eastern Australia. Mar. Geol. 1996, 129, 313–330. [Google Scholar] [CrossRef]
- Halfar, J.; Eisele, M.; Riegl, B.; Hetzinger, S.; Godinez-Orta, L. Modern Rhodolith dominated carbonates at Punta Chivato, Mexico. Geodiversitas 2012, 34, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Bracchi, V.A.; Angeletti, L.; Marchese, F.; Taviani, M.; Cardone, F.; Hajdas, I.; Grande, V.; Prampolini, M.; Caragnano, A.; Corselli, C.; et al. A resilient deep-water rhodolith bed off the Egadi Archipelago (Mediterranean Sea) and its actuopaleontological significance. Alp. Mediterr. Quat. 2019, 32, 1–20. [Google Scholar]
- Brasileiro, P.; Pereira-Filho, G.; Bahia, R.; Abrantes, D.; Guimarães, S.; Moura, R.; Francini-Filho, R.; Bastos, A.; Amado-Filho, G. Macroalgal composition and community structure of the largest rhodolith beds in the world. Mar. Biodivers. 2016, 46, 407–420. [Google Scholar] [CrossRef]
- Sissini, M.N.; Oliveira, M.C.; Gabrielson, P.W.; Robinson, N.M.; Okolodkov, Y.B.; Riosmena-Rodríguez, R.; Horta, P.A. Mesophyllum erubescens (Corallinales, Rhodophyta)—So many species in one epithet. Phytotaxa 2014, 190, 299–319. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Kantun, J.J.; Gabrielson, P.W.; Hughey, J.R.; Pezzolesi, L.; Rindi, F.; Robinson, N.M.; Peña, V.; Riosmena-Rodriguez, R.; le Gall, L.; Adey, W.H. Reassessment of branched Lithophyllum spp. (Corallinales, Rhodophyta) in the Caribbean Sea with global implications. Phycologia 2016, 55, 619–639. [Google Scholar] [CrossRef] [Green Version]
- Richards, J.L.; Sauvage, T.; Schmidt, W.E.; Fredericq, S.; Hughey, J.R.; Gabrielson, P.W. The coralline genera Sporolithon and Heydrichia (Sporolithales, Rhodophyta) clarified by sequencing type material of their generitypes and other species. J. Phycol. 2017, 53, 1044–1059. [Google Scholar] [CrossRef]
- Bastos, A.C.; Moura, R.L.; Moraes, F.C.; Vieira, L.S.; Braga, J.B.; Ramalho, L.V.; Amado-Filho, G.M.; Magdalena, U.R.; Webster, J.M. Bryozoans are major modern builders of South Atlantic oddly shaped reefs. Sci. Rep. 2018, 8, 9638. [Google Scholar] [CrossRef] [Green Version]
- Basso, D.; Tavares de Macedo, D.G.; Henriques, M.C. The occurrence of coralligène de plateau in Brazilian coastal waters. In Proceedings of the IV International Rhodolith Workshop, Granada, Spain, 17–21 September 2012. [Google Scholar]
- Ballesteros, E. Mediterranean coralligenous assemblages: A synthesis of present knowledge. Oceanogr. Mar. Biol. 2006, 44, 123–195. [Google Scholar]
- Martin, C.S.; Giannoulaki, M.; Leo, F.; Scardi, M.; Salomidi, M.; Knittweis, L.; Pace, M.L.; Garofalo, G.; Gristina, M.; Ballesteros, E.; et al. Coralligenous and maërl habitats: Predictive modelling to identify their spatial distributions across the Mediterranean Sea. Sci. Rep. 2014, 4, 5073. [Google Scholar] [CrossRef]
- Sissini, M.N.; Berchez, F.; Hall-Spencer, J.; Ghilardi-Lopes, N.; Carvalho, V.F.; Schubert, N.; Koerich, G.; Diaz-Pulido, G.; Silva, J.; Serrão, E.; et al. Brazil oil spill response: Protect rhodolith beds. Science 2020, 367, 156. [Google Scholar]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holz, V.L.; Bahia, R.G.; Karez, C.S.; Vieira, F.V.; Moraes, F.C.; Vale, N.F.; Sudatti, D.B.; Salgado, L.T.; Moura, R.L.; Amado-Filho, G.M.; et al. Structure of Rhodolith Beds and Surrounding Habitats at the Doce River Shelf (Brazil). Diversity 2020, 12, 75. https://doi.org/10.3390/d12020075
Holz VL, Bahia RG, Karez CS, Vieira FV, Moraes FC, Vale NF, Sudatti DB, Salgado LT, Moura RL, Amado-Filho GM, et al. Structure of Rhodolith Beds and Surrounding Habitats at the Doce River Shelf (Brazil). Diversity. 2020; 12(2):75. https://doi.org/10.3390/d12020075
Chicago/Turabian StyleHolz, Vitória L., Ricardo G. Bahia, Cláudia S. Karez, Fernanda V. Vieira, Fernando C. Moraes, Nicholas F. Vale, Daniela B. Sudatti, Leonardo T. Salgado, Rodrigo L. Moura, Gilberto M. Amado-Filho, and et al. 2020. "Structure of Rhodolith Beds and Surrounding Habitats at the Doce River Shelf (Brazil)" Diversity 12, no. 2: 75. https://doi.org/10.3390/d12020075
APA StyleHolz, V. L., Bahia, R. G., Karez, C. S., Vieira, F. V., Moraes, F. C., Vale, N. F., Sudatti, D. B., Salgado, L. T., Moura, R. L., Amado-Filho, G. M., & Bastos, A. C. (2020). Structure of Rhodolith Beds and Surrounding Habitats at the Doce River Shelf (Brazil). Diversity, 12(2), 75. https://doi.org/10.3390/d12020075







