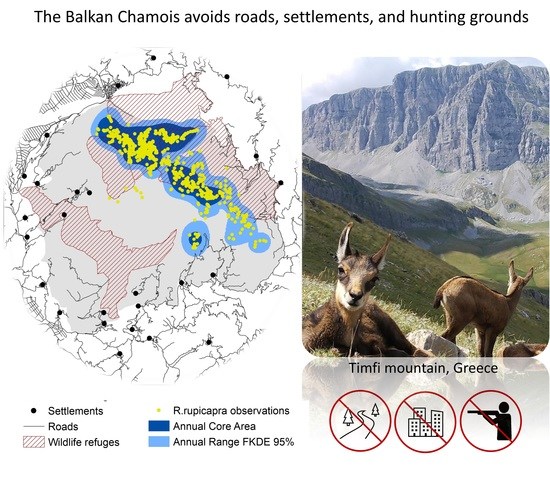
Balkan Chamois (Rupicapra rupicapra balcanica) Avoids Roads, Settlements, and Hunting Grounds: An Ecological Overview from Timfi Mountain, Greece
Abstract
:1. Introduction
2. Materials and Methods
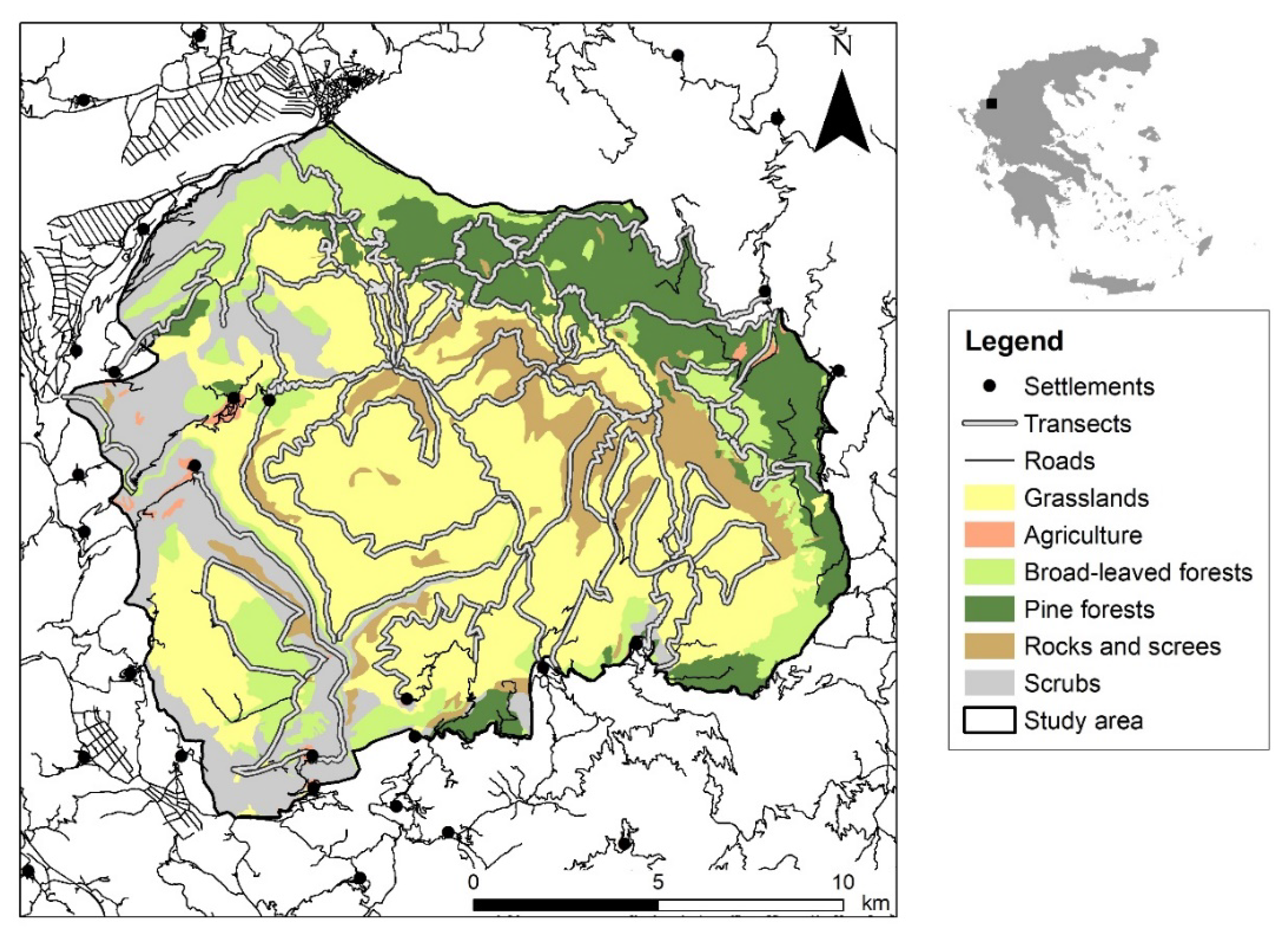
2.1. Study Area
2.2. Chamois Surveys
2.3. Environmental Variables
2.4. Data Analysis
2.4.1. Annual and Seasonal Ranges
2.4.2. Population Demography
2.4.3. Habitat Selection
2.4.4. Chamois Distribution and Hunting Ban Areas of Greece
3. Results
3.1. Annual and Seasonal Ranges
3.2. Population Demography
3.3. Habitat Selection
3.4. Chamois Distribution and Hunting Ban Area of Greece
4. Discussion
4.1. Range Pattern
4.2. Population Trend and Demography
4.3. Seasonal Habitat Selection
4.4. Anthropogenic Risk-Avoidance Pattern
4.5. Chamois Distribution and Hunting Grounds
4.6. Conservation Implications
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilson, E.O. Half-Earth: Our Planet’s Fight for Life; Liveright Publishing Corporation: New York, NY, USA, 2016; p. 272. [Google Scholar]
- Powers, R.P.; Jetz, W. Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Chang. 2019, 9, 323–329. [Google Scholar] [CrossRef]
- Morrison, M.L.; Marcot, B.G.; Mannan, R.W. Wildlife-habitat Relationships: Concepts and Applications, 3rd ed.; Island Press: Washington, DC, USA, 2006; p. 493. [Google Scholar]
- Krausman, P.R.; Cain, J.W. Wildlife Management and Conservation; The Johns Hopkins University Press: Baltimore, MD, USA, 2013; p. 342. [Google Scholar]
- Papaioannou, H.I.; Kati, V.I. Current status of the Balkan chamois (Rupicapra rupicapra balcanica) in Greece: Implications for conservation. Belg. J. Zool. 2007, 137, 33–39. [Google Scholar]
- Anderwald, P.; Ambarlı, H.; Avramov, S.; Ciach, M.; Corlatti, L.; Farkas, A.; Jovanovic, M.; Papaioannou, H.; Peters, W.; Sarasa, M.; et al. Rupicapra rupicapra. IUCN Red List Threat. Species 2018, (in press). [Google Scholar]
- Apollonio, M.; Andersen, R.; Putman, R. European Ungulates and Their Management in the 21th Century; Cambridge University Press: Cambridge, UK, 2010; p. 604. [Google Scholar]
- Papaioannou, H. Current status and conservation management of Balkan chamois (Rupicapra rupicapra balcanica) in Greece. In Chamois International Congress Proceedings, Lama dei Peligni—Majella National Park, Italy, 17–19 June 2014; Antonucci, A., Di Domenico, G., Eds.; Majambiente Edizioni: Lama dei Peligni, Italy, 2015; pp. 111–122. [Google Scholar]
- EEC. Council Directive 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora. 1992. Available online: http://data.europa.eu/eli/dir/1992/43/2013–07–01 (accessed on 20 February 2020).
- EIONET. Annex B—Report Format on the ‘Main Results of the Surveillance Under Article 11’ for Annex II, IV & V Species. Available online: http://cdr.eionet.europa.eu/gr/eu/art17/envxi9xsq (accessed on 2 March 2020).
- National Plan for Energy and Climate. Available online: http://www.et.gr/index.php/ (accessed on 1 March 2020).
- UNFCCC. United Nations Framework Convention on Climate Change. Decision 1/CP.2. Adoption of the Paris Agreement. Available online: https://unfccc.int/resource/docs/2015/cop21/eng/l09r01.pdf (accessed on 20 February 2020).
- Bell, S.; Hampshire, K.; Topalidou, S. The political culture of poaching: A case study from northern Greece. Biodivers. Conserv. 2007, 16, 399–418. [Google Scholar] [CrossRef]
- Karris, G.; Martinis, A.; Kabassi, K.; Dalakiari, A.; Korbetis, M. Changing social awareness of the illegal killing of migratory birds in the Ionian Islands, western Greece. J. Biol. Educ. 2018. [Google Scholar] [CrossRef]
- Ibisch, P.L.; Hoffmann, M.T.; Kreft, S.; Pe’Er, G.; Kati, V.; Biber-Freudenberger, L.; Della Sala, D.A.; Vale, M.M.; Hobson, P.R.; Selva, N. A global map of roadless areas and their conservation status. Science 2016, 354, 1423–1427. [Google Scholar] [CrossRef]
- Kati, V. Fragmentation of natural and semi-natural areas—SEBI 13. In Greece: State of the Environment. Summary/2018; National Center of Environment and Sustainable Development (NCESD): Athens, Greece, 2018; pp. 430–433. [Google Scholar]
- Dulac, J. Global Land Transport Infrastructure Requirements: Estimating Road and Railway Infrastructure Capacity and Costs to 2050; International Energy Agency: Paris, France, 2013. [Google Scholar]
- Laurance, W.F.; Clements, G.R.; Sloan, S.; O’Connell, C.S.; Mueller, N.D.; Goosem, M.; Venter, O.; Edwards, D.P.; Phalan, B.; Balmford, A.; et al. A global strategy for road building. Nature 2014, 513, 229–232. [Google Scholar] [CrossRef]
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Kotjabopoulou, E. The mountainscapes of upper Palaeolithic epirus in NW Greece: A view from the bones. In The Palaeolithic of the Balkans, Proceedings of the XV World Congress, Lisbon, Portugal, 4–9 September 2006; Darlas, A., Mihailovic, D., Eds.; British Archaeological Reports, International Series 1819: Lisbon, Portugal, 2008; Volume 17, pp. 21–31. [Google Scholar]
- Papaioannou, H.; Fernández, M.; Pérez, T.; Domínguez, A. Genetic variability and population structure of chamois in Greece (Rupicapra rupicapra balcanica). Conserv. Genet. 2019, 20, 939–945. [Google Scholar] [CrossRef]
- Pepin, D.; Menaut, P.; Desneux, L.; Cargnelutti, B. Seasonal changes in the use of space by Isards (Rupicapra pyrenaica) in a protected area. In Ongules/Ungulates 91; Spitz, F., Janeau, G., Gonzales, G., Aulagnier, S., Eds.; IRGM-INRA: Toulouse, France, 1992; pp. 327–330. [Google Scholar]
- Clutton-Brock, T.H.; Guinness, F.E.; Albon, S.D. Red Deer: Behavior and Ecology of Two Sexes; Edinburgh University Press: Edinburgh, UK, 1982; p. 400. [Google Scholar]
- Houssin, H.; Loison, A.; Jullien, J.-M.; Gaillard, J.-M.; Taran, E.; Ebner, Ü.K. Validité de la méthode du pointage-flash pour l’estimation des effectifs de chamois (Rupicapra rupicapra). Gibier Faune Sauvage 1994, 11, 287–298. [Google Scholar]
- Catusse, M.; Corti, R.; Cugnasse, J.M.; Dubray, D.; Gibert, P.; Michallet, J. La Grande Faune de Montagne; Hatier Litterature Generale: Paris, France, 1996; p. 260. [Google Scholar]
- GEODATA.gov.gr. Available online: http://geodata.gov.gr/ (accessed on 23 February 2020).
- ESRI. ArcGISDesktop; Environmental Systems Research Institute: Redlands, CA, USA, 2018. [Google Scholar]
- Worton, B.J. Kernel methods for estimating the utilization distribution in home range studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Hooge, P.N.; Eichenlaub, B. Animal Movement Extension to Arcview, Ver. 1.1. Geological Survey; Alaska Science Center—Biological Science Office, USA Geological Survey: Anchorage, AK, USA, 1997. [Google Scholar]
- Powell, R.A. Animal home ranges and territories and home range estimators. In Research Techniques in Animal Ecology: Controversies and Consequences; Boitani, L., Fuller, T., Eds.; Columbia University Press: New York, NY, USA, 2000; pp. 65–110. [Google Scholar]
- Hirzel, A.H.; Hausser, J.; Chessel, D.; Perrin, N. Ecological-niche factor analysis: How to compute habitat-suitability maps without absence data? Ecology 2002, 83, 2027–2036. [Google Scholar] [CrossRef]
- Basille, M.; Calenge, C.; Marboutin, E.; Andersen, R.; Gaillard, J.M. Assessing habitat selection using multivariate statistics: Some refinements of the ecological-niche factor analysis. Ecol. Model. 2008, 211, 233–240. [Google Scholar] [CrossRef]
- Thomas, D.; Taylor, E. Study designs and tests for comparing resource use and availability. J. Wildl. Manag. 1990, 54, 322–330. [Google Scholar] [CrossRef]
- Jackson, D.A. Stopping rules in principal components analysis: A comparison of heuristical and statistical approaches. Ecology 1993, 74, 2204–2214. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 15 December 2019).
- Calenge, C. The package “adehabitat” for the R software: A tool for the analysis of space and habitat use by animals. Ecol. Model. 2006, 197, 516–519. [Google Scholar] [CrossRef]
- CLC 2018. Available online: https://land.copernicus.eu/pan-european/corine-land-cover/clc2018 (accessed on 20 February 2020).
- Crampe, J.P.; Bon, R.; Gerard, J.F.; Serrano, E.; Caens, P.; Florence, E.; Gonzalez, G. Site fidelity, migratory behaviour, and spatial organization of female isards (Rupicapra pyrenaica) in the Pyrenees National Park, France. Can. J. Zool. 2007, 85, 16–25. [Google Scholar] [CrossRef]
- Garcia-Gonzalez, R.; Hidalgo, R.; Ameztoy, J.M.; Herrero, J. Census, population structure and habitat use of a Chamois population in Ordesa, N.P. living in sympatry with Pyrenean wild goat In Ongules/Ungulates 91; Spitz, F., Janeau, G., Gonzales, G., Aulagnier, S., Eds.; IRGM—INRA: Toulouse, France, 1992; pp. 321–325. [Google Scholar]
- Lovari, S.; Cosentino, R. Seasonal habitat selection and group size of the Abruzzo Chamois (Rupicapra pyrenaica ornata). Boll. Zool. 1986, 53, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Nesti, I.; Posillico, M.; Lovari, S. Ranging behaviour and habitat selection of Alpine chamois. Ethol. Ecol. Evol. 2010, 22, 215–231. [Google Scholar] [CrossRef]
- Bocci, A.; Canavese, G.; Lovari, S. Even mortality patterns of the two sexes in a polygynous, near-monomorphic species: Is there a flaw? J. Zool. 2010, 280, 379–386. [Google Scholar] [CrossRef]
- Gonzalez, G.; Crampe, J.-P. Mortality patterns in a protected population of isards (Rupicapra pyrenaica). Can. J. Zool. 2001, 79, 2072–2079. [Google Scholar] [CrossRef]
- Jonas, T.; Geiger, F.; Jenny, H. Mortality pattern of the Alpine chamois: The influence of snow-meteorological factors. Ann. Glaciol. 2008, 49, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Rughetti, M.; Festa-Bianchet, M. Seasonal changes in sexual size dimorphism in northern chamois. J. Zool. 2011, 284, 257–264. [Google Scholar] [CrossRef]
- Willisch, C.S.; Bieri, K.; Struch, M.; Franceschina, R.; Schnidrig-Petrig, R.; Ingold, P. Climate effects on demographic parameters in an unhunted population of Alpine chamois (Rupicapra rupicapra). J. Mammal. 2013, 94, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Papaioannou, H.; Sgardelis, S.; Chondropoulos, B.; Vassilakis, D.; Kati, V.; Dimopoulos, P. Demographic characteristics, seasonal range and habitat topography of Balkan chamois population in its southernmost limit of its distribution (Giona mountain, Greece). J. Nat. Hist. 2015, 49, 327–345. [Google Scholar] [CrossRef]
- Boschi, C.; Nievergelt, B. The spatial patterns of Alpine chamois (Rupicapra rupicapra rupicapra) and their influence on population dynamics in the Swiss National Park. Mamm. Biol. 2003, 68, 16–30. [Google Scholar] [CrossRef]
- Corlatti, L.; Caroli, M.; Pietrocini, V.; Lovari, S. Rutting behaviour of territorial and nonterritorial male chamois: Is there a home advantage? Behav. Process. 2013, 92, 118–124. [Google Scholar] [CrossRef]
- Mustoni, A.; Pedrotti, L.; Zanon, E.; Tosi, G. Ungulati delle Alpi. Biologia-Riconoscimento—Gestione; Nitida Immagine Editrice: Cles, Italy, 2002; p. 539. [Google Scholar]
- Unterthiner, S.; Ferretti, F.; Rossi, L.; Lovari, S. Sexual and seasonal differences of space use in Alpine chamois. Ethol. Ecol. Evol. 2012, 24, 257–274. [Google Scholar] [CrossRef]
- Allaine, D.; Houssin, H.; Gaillard, J.M. Étude de la variabilité spatio-temporelle d’un indice de reproduction dans une population de chamois (Rupicapra rupicapra). Gibier Faune Sauvage 1990, 7, 85–94. [Google Scholar]
- Tosi, G.; Pedrotti, L.; Monaco, A.; Scherini, G. Progetto Camoscio Monte Baldo; Universita degli studi di Milano: Varese, Italy, 1996; p. 276. [Google Scholar]
- Schröder, W. Untersuchungen zur Ökologie des Gamswildes (Rupicapra rupicapra L.) in einem Vorkommen der Alpen—II. Teil. Zeitschrift Jagdwissenschaft 1971, 17, 197–235. [Google Scholar] [CrossRef]
- Schröder, W. Untersuchungen zur Ökologie des Gamswildes (Rupicapra rupicapra L.) in einem Vorkommen der Alpen. I, Teil. Zeitschrift Jagdwissenschaft 1971, 17, 113–168. [Google Scholar] [CrossRef]
- Corlatti, L.; Lebl, K.; Filli, F.; Ruf, T. Unbiased sex-specific survival in Alpine chamois. Mamm. Biol. 2012, 77, 135–139. [Google Scholar] [CrossRef]
- Hamr, J. Seasonal home range size and utilisation by female chamois (Rupicapra rupicapra L.) in Northern Tyrol. In The Biology and Management of Mountain Ungulates; Lovari, S., Ed.; Croom Helm: London, UK, 1985; Volume 12, pp. 106–116. [Google Scholar]
- Lovari, S.; Sacconi, F.; Trivellini, G. Do alternative strategies of space use occur in male Alpine chamois? Ethol. Ecol. Evol. 2006, 18, 221–231. [Google Scholar] [CrossRef]
- Shank, C.C. Inter-and intra-sexual segregation of chamois (Rupicapra rupicapra) by altitude and habitat during summer. Zeitschrift Säugetierkunde 1985, 50, 117–125. [Google Scholar]
- Herrero, J.; Garin, I.; García-Serrano, A.; García-González, R. Habitat use in a Rupicapra pyrenaica pyrenaica forest population. Ecol. Manag. 1996, 88, 25–29. [Google Scholar] [CrossRef]
- Ciach, M.; Peksa, Ł. Human-induced environmental changes influence habitat use by an ungulate over the long term. Curr. Zool. 2018, 65, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Bačkor, P. Altitudinal distribution and morphological attributes of chamois (Rupicapra rupicapra tatrica) habitat in the Western Carpathians. Acta Zool. Lit. 2010, 20, 162–167. [Google Scholar] [CrossRef]
- Hamr, J. Disturbance behaviour of chamois in an Alpine tourist area of Austria. Mt. Res. Dev. 1988, 8, 65–73. [Google Scholar] [CrossRef]
- Selva, N.; Switalski, A.; Kreft, S.; Ibisch, P.L. Why keep areas road-free? The importance of roadless areas. In Handbook of Road Ecology; van Der Ree, R., Smith, D.J., Grilo, C., Eds.; John Wiley and Sons, Ltd.: Oxford, UK, 2015; pp. 10–26. [Google Scholar]
- Bleich, V.; Davis, J.; Marshal, J.; Torres, S.; Gonzales, B. Mining activity and habitat use by mountain sheep (Ovis canadensis). Eur. J. Wildl. Res. 2009, 55, 183–191. [Google Scholar] [CrossRef]
- Laurance, W.F.; Croes, B.M.; Tchignoumba, L.; Lahm, S.A.; Alonso, A.; Lee, M.E.; Campbell, P.; Ondzeano, C. Impacts of roads and hunting on central African rainforest mammals. Conserv. Biol. 2006, 20, 1251–1261. [Google Scholar] [CrossRef]
- Lian, X.; Li, X. Avoidance distances of four ungulates from roads in Kekexili and related protection suggestions. Chin. J. Ecol. 2012, 31, 81–86. [Google Scholar]
- Trepet, S.A.; Eskina, T.G. The influence of environmental factors on the dynamics of the size and spatial structure of the chamois (Rupicapra rupicapra caucasica) population on the Caucasian Reserve. Biol. Bull. 2013, 40, 698–707. [Google Scholar] [CrossRef]
- Putzu, N.; Bonetto, D.; Civallero, V.; Fenoglio, S.; Meneguz, P.G.; Preacco, N.; Tizzani, P. Temporal patterns of ungulate-vehicle collisions in a subalpine Italian region. Ital. J. Zool. 2014, 81, 463–470. [Google Scholar] [CrossRef]
- Gadd, M.E. Expected effects of a road across the serengeti. In Handbook of Road Ecology; van Der Ree, R., Smith, D.J., Grilo, C., Eds.; John Wiley and Sons, Ltd.: Oxford, UK, 2015; pp. 455–464. [Google Scholar]
- Slaght, J.C.; Milakovsky, B.; Maksimova, D.A.; Seryodkin, I.V.; Zaitsév, V.A.; Panichev, A.M.; Miquelle, D.G. Anthropogenic influences on the distribution of a Vulnerable coniferous forest specialist: Habitat selection by the Siberian musk deer Moschus moschiferus. Oryx 2019, 53, 174–180. [Google Scholar] [CrossRef]
- Papaioannou, H. Ungulates and their management in Greece. In European Ungulates and Their Management in the 21st Century; Apollonio, M., Andersen, R., Putman, R., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 540–562. [Google Scholar]
- Bounas, A.; Siarabi, S.; Toli, E.-A.; Sotiropoulos, K. DNA barcoding against poaching of Chamois (Rupicapra rupicapra), two confirmed cases from Greece. J. Wildl. Biodiv. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Iliopoulos, Y.; Youlatos, D.; Sgardelis, S. Wolf pack rendezvous site selection in Greece is mainly affected by anthropogenic landscape features. Eur. J. Wildl. Res. 2014, 60, 23–34. [Google Scholar] [CrossRef]
- Sokos, C.K.; Birtsas, P.K.; Connelly, J.W.; Papaspyropoulos, K.G. Hunting of migratory birds: Disturbance intolerant or harvest tolerant? Wildl. Biol. 2013, 19, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Analysis of legislation and practice of hunting in some EU countries. Available online: http://www.enpi-fleg.org (accessed on 11 March 2020).
- Switalski, T.A.; Nelson, C.R. Efficacy of road removal for restoring wildlife habitat: Black bear in the Northern Rocky Mountains, USA. Biol. Conserv. 2011, 144, 2666–2673. [Google Scholar] [CrossRef]
- EEA. The European environment—State and outlook 2020. In Knowledge for Transition to a Sustainable Europe; Publications Office of the European Union: Luxembourg; Brussels, Belgium, 2019; p. 496. [Google Scholar]


| Season | Area (ha) | Proportion (%) | Overlap (%) of Ranges (Core Areas) | |||||
|---|---|---|---|---|---|---|---|---|
| Range | Core | Range | Core | Winter | Spring | Summer | Autumn | |
| Winter | 3213 | 1323 | 49 | 47 | 100 | 59 (76) | 52 (31) | 49 (46) |
| Spring | 3414 | 1566 | 53 | 56 | 100 | 67 (37) | 59 (41) | |
| Summer | 4022 | 1385 | 62 | 50 | 100 | 81 (38) | ||
| Autumn | 3562 | 993 | 55 | 36 | 100 | |||
| Annual | 6491 | 2797 | 100 | 54 | ||||
| Demographic Parameters | 2002 | 2014 | 2017 | Mean (±StDev) |
|---|---|---|---|---|
| Population size (individuals) | 132 | 325 | 469 | - |
| Kids (%) | 23 | 29 | 26 | 26 (±3.00) |
| Yearlings (%) | 9 | 11 | 12 | 11 (±1.50) |
| Females (%) | 38 | 37 | 36 | 37 (±0.98) |
| Males (%) | 30 | 23 | 26 | 26 (±3.51) |
| Fecundity rate (kids/females) | 0.60 | 0.80 | 0.72 | 0.71 (±0.10) |
| Sex ratio (males/females) | 0.80 | 0.63 | 0.72 | 0.72 (±0.09) |
| Code | Environmental Variables | Occupied Grids | Available Grids | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2002 1 | 2014 | 2017 | ||||||||
| Winter | Spring | Summer | Autumn | Autumn | Autumn | |||||
| Topography | el | elevation (m) | A | 1384.98 | 1668.28 | 1910.00 | 1824.00 | 1977.47 | 1964.02 | 1449.70 |
| sl | slope (degrees) | A | 30.82 | 30.32 | 30.05 | 30.84 | 31.42 | 31.79 | 22.46 | |
| c | curvature (unitless) | A | 0.10 | 0.27 | 0.30 | 0.20 | 0.16 | 0.15 | 0.01 | |
| asp.N | North aspect | F | 17.24 | 26.61 | 19.11 | 21.85 | 12.50 | 18.68 | 9.75 | |
| asp.E | East aspect | F | 28.97 | 16.51 | 33.76 | 34.03 | 29.17 | 27.47 | 21.95 | |
| asp.S | South aspect | F | 4.83 | 11.01 | 9.55 | 4.20 | 9.72 | 9.72 | 30.95 | |
| asp.W | West aspect | F | 48.97 | 45.87 | 37.58 | 39.92 | 48.61 | 48.61 | 37.35 | |
| Habitat cover (%) | a | agriculture | A | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.74 |
| B | broad leaved forests | A | 5.70 | 7.28 | 3.75 | 2.99 | 1.39 | 2.66 | 14.41 | |
| g | grasslands | A | 24.01 | 25.62 | 33.75 | 39.02 | 53.77 | 41.65 | 46.06 | |
| p | pine forests | A | 62.20 | 50.23 | 30.68 | 29.95 | 6.92 | 9.21 | 15.10 | |
| r | rocks | A | 8.09 | 16.86 | 31.82 | 28.03 | 37.93 | 46.23 | 9.20 | |
| s | scrubs | A | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 | 14.49 | |
| Human disturbance | Ds | distance from closest settlements (m) | A | 5468.40 | 5413.86 | 5842.35 | 5650.77 | 4783.19 | 5209.57 | 3348.03 |
| Dr | distance from closest road (m) | A | 2472.70 | 2631.57 | 3105.44 | 3117.21 | 3144.69 | 2985.88 | 1482.34 | |
| hunt | area where hunting is allowed (binary) | F | 19.31 | 25.69 | 16.56 | 10.92 | 34.72 | 27.47 | 58.82 | |
| Type | Legal Rule | Area (km2) | Greek Land (%) | Chamois Distribution (%) | Data Source | Method |
|---|---|---|---|---|---|---|
| Public safety ** | 250 m from cities and villages 150 m from houses 250 m from camping sites when operating Aarchaeological sites and monuments | 8482.67 | 6.425 | 0.030 | https://land.copernicus.eu/pan-european/corine-land-cover/clc2018?tab=download | Buffer (250 m) to the first CLC category of artificial surfaces3: 1.1.1, 1.1.2, 1.2.1., 1.2.3., 1.2.4., 1.4.1., 1.4.2. |
| 500 m from eastern borderline | 99.52 | 0.075 | 0.000 | https://geodata.gov.gr/dataset/aktogramme | Buffer to eastern borderline | |
| Subtotal 1: public safety | 8581.30 | 6.500 | 0.030 | Merging geospatial data | ||
| Wildlife conservation | Wildlife refuges | 10,630.50 | 8.052 | 29.490 | https://www.eea.europa.eu/data-and-maps/data/nationally-designated-areas-national-cdda-12/gis-data/cdda-shape-file | Area calculation |
| Core zones of the 10 national parks | 354.33 | 0.268 | 5.079 | Area calculation | ||
| Strict nature reserves | 118.14 | 0.089 | 1.057 | Area calculation | ||
| Nature reserve zones | 1960.90 | 1.485 | 9.285 | Area calculation | ||
| Subtotal 2: wildlife areas | 12,094.12 | 9.160 | 37.189 | Merging geospatial data | ||
| Game management | Game breeding station | 30.53 | 0.023 | 0.000 | https://www.eea.europa.eu/data-and-maps/data/nationally-designated-areas-national-cdda-12/gis-data/cdda-shape-file | Area calculation |
| Controlled hunting area | 1115.14 | 0.845 | 2.859 | Area calculation | ||
| Subtotal 3: game management areas | 1144.98 | 0.867 | 2.859 | Merging geospatial data | ||
| Private land | Private fenced land | n/a | n/a | n/a | Geospatial data not available | |
| Vineyards, cultivated land etc. in case that hunting harms crops. | n/a | n/a | n/a | Geospatial data not available | ||
| Permanent hunting ban areas | 21,469.89 | 16.210 | 40.062 | Merging geospatial data: subtotals 1–3 | ||
| Temporary hunting ban areas | 372.47 | 0.282 | n/a | Screening 37 Ministerial Decisions 2016-2019 | Sum of areas | |
| Total | 21,842.37 | 16.544 | 40.062 | Sum of permanent and temporary areas | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kati, V.; Kassara, C.; Vassilakis, D.; Papaioannou, H. Balkan Chamois (Rupicapra rupicapra balcanica) Avoids Roads, Settlements, and Hunting Grounds: An Ecological Overview from Timfi Mountain, Greece. Diversity 2020, 12, 124. https://doi.org/10.3390/d12040124
Kati V, Kassara C, Vassilakis D, Papaioannou H. Balkan Chamois (Rupicapra rupicapra balcanica) Avoids Roads, Settlements, and Hunting Grounds: An Ecological Overview from Timfi Mountain, Greece. Diversity. 2020; 12(4):124. https://doi.org/10.3390/d12040124
Chicago/Turabian StyleKati, Vassiliki, Christina Kassara, Dimitrios Vassilakis, and Haritakis Papaioannou. 2020. "Balkan Chamois (Rupicapra rupicapra balcanica) Avoids Roads, Settlements, and Hunting Grounds: An Ecological Overview from Timfi Mountain, Greece" Diversity 12, no. 4: 124. https://doi.org/10.3390/d12040124
APA StyleKati, V., Kassara, C., Vassilakis, D., & Papaioannou, H. (2020). Balkan Chamois (Rupicapra rupicapra balcanica) Avoids Roads, Settlements, and Hunting Grounds: An Ecological Overview from Timfi Mountain, Greece. Diversity, 12(4), 124. https://doi.org/10.3390/d12040124