The Effect of a Dam Construction on Subtidal Nematode Communities in the Ba Lai Estuary, Vietnam
Abstract
:1. Introduction
2. Materials and Methods
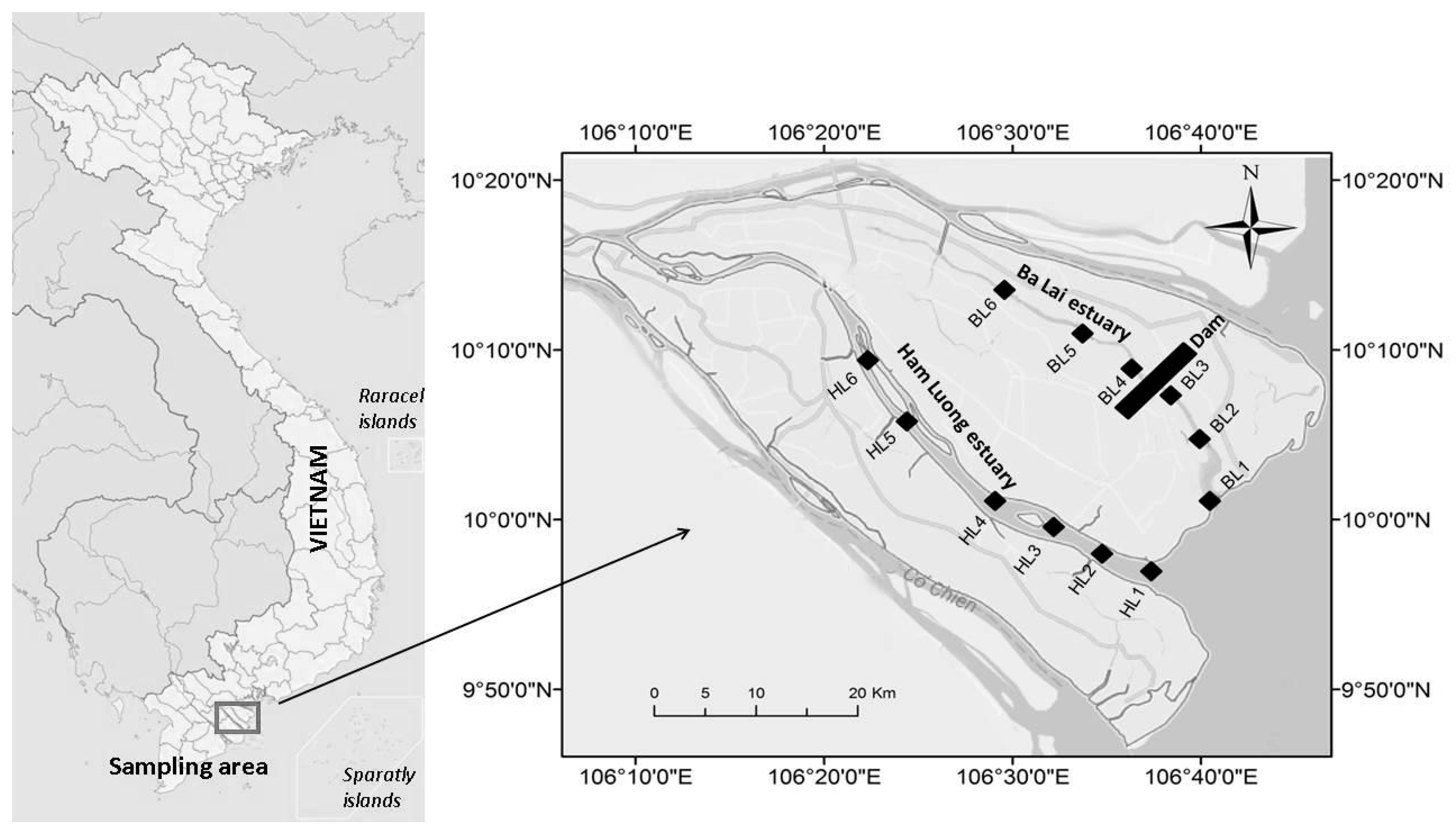
2.1. Study Area and Sampling Location
2.2. Sampling and Environmental Variable Analysis
2.3. Sampling and Analysis of Nematodes
2.4. Data Processing and Statistical Analysis
3. Results
3.1. Environmental Characteristics of Ba Lai and Ham Luong Estuaries
3.1.1. Water Environmental Characteristics
3.1.2. Sediment Environmental Characteristics
3.2. Nematode Assemblages in Ba Lai and Ham Luong Estuaries
3.2.1. Density of Nematode Communities in Ba Lai and Ham Luong Estuaries
3.2.2. Diversity of Nematode Communities in Ba Lai and Ham Luong Estuaries
3.2.3. Nematode Community Composition in Ba Lai and Ham Luong Estuaries
3.3. Correlation Between Nematode Communities and Environmental Variables
4. Discussion
4.1. Differences in Environmental Variables Related to Dam Effects
4.2. Differences in Nematode Communities Explained by Differences in Environmental Conditions related to Dam Effects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Dammed Estuary and Reference Estuary (68.29%) | |||||
|---|---|---|---|---|---|
| Parodontophora | 7.2 | Metalinhomoeus | 3.56 | Monhystrella | 2.13 |
| Theristus | 6.33 | Viscosia | 3.47 | Mononchulus | 2.12 |
| Terschellingia | 3.92 | Sphaerotheristus | 3.21 | Comesoma | 1.89 |
| Daptonema | 3.74 | Monhystera | 2.68 | Desmodora | 1.89 |
| Rhabdolaimus | 3.63 | Mesodorylaimus | 2.58 | Halalaimus | 1.86 |
| DD and DU (71.45%) | DD and RD (70.17%) | DD and RU (71.76%) | |||
| Theristus | 6.67 | Parodontophora | 6.07 | Parodontophora | 6.59 |
| Parodontophora | 5.39 | Pseudochromadora | 3.28 | Theristus | 5.86 |
| Rhabdolaimus | 3.48 | Metalinhomoeus | 3.15 | Daptonema | 3.86 |
| Metalinhomoeus | 3.25 | Viscosia | 3.06 | Pseudochromadora | 3.38 |
| Pseudochromadora | 3.18 | Theristus | 2.7 | Terschellingia | 3.34 |
| Terschellingia | 3.02 | Comesoma | 2.5 | Sphaerotheristus | 3.27 |
| Monhystera | 2.54 | Trissonchulus | 2.48 | Viscosia | 2.42 |
| Mesodorylaimus | 2.16 | Daptonema | 2.42 | Rhabdolaimus | 2.22 |
| Daptonema | 2.14 | Marylynnia | 2.15 | Linhystera | 2.11 |
| Halalaimus | 2.11 | Terschellingia | 2.03 | Halalaimus | 2.07 |
| Linhystera | 2.05 | Amphimonhystrella | 2 | Amphimonhystrella | 2 |
| Monhystrella | 2.02 | Halalaimus | 1.99 | Cobbia | 1.77 |
| Viscosia | 1.97 | Rhabdolaimus | 1.95 | Oncholaimus | 1.75 |
| Udonchus | 1.97 | Desmodora | 1.92 | Mesodorylaimus | 1.72 |
| Amphimonhystrella | 1.9 | Sabatieria | 1.88 | Sabatieria | 1.59 |
| Afrodorylaimus | 1.76 | Mesodorylaimus | 1.86 | Microlaimus | 1.52 |
| Sphaerotheristus | 1.69 | Linhystera | 1.81 | Oxystomina | 1.49 |
| Cobbia | 1.67 | Rhynchonema | 1.75 | Desmodora | 1.49 |
| Mononchulus | 1.6 | Sphaerotheristus | 1.74 | Spilophorella | 1.44 |
| Cobbia | 1.72 | Achromadora | 1.44 | ||
| RD and RU (64.91%) | DU and RU (61.45%) | DU and RD (69.79%) | |||
| Parodontophora | 9.18 | Parodontophora | 9.05 | Theristus | 8.41 |
| Theristus | 8.3 | Theristus | 8.65 | Parodontophora | 7.34 |
| Daptonema | 5.77 | Terschellingia | 6.2 | Rhabdolaimus | 5.35 |
| Viscosia | 5.57 | Daptonema | 5.73 | Metalinhomoeus | 5.04 |
| Sphaerotheristus | 5.04 | Rhabdolaimus | 5.24 | Viscosia | 4.79 |
| Metalinhomoeus | 4.69 | Sphaerotheristus | 5.21 | Terschellingia | 4.4 |
| Comesoma | 4.34 | Metalinhomoeus | 5.08 | Comesoma | 3.67 |
| Terschellingia | 4.31 | Monhystera | 4.63 | Monhystera | 3.43 |
| Trissonchulus | 3.9 | Mesodorylaimus | 3.68 | Mesodorylaimus | 3.24 |
| Daptonema | 3.2 | ||||
| Trissonchulus | 3.1 | ||||
References
- MRCS. 2017 Lower Mekong Regional Water Quality Monitoring Report; Mekong River Commission: Vientiane, Lao PDR, 2019; p. 52. [Google Scholar]
- Le, A.T.; Le, V.; Du, L.; Tristan, S. Rapid integrated & ecosystem-based assessment of climate change vulnerability & adaptation for Ben Tre Province, Vietnam. J. Sci. Technol. 2014, 52, 287–293. [Google Scholar]
- VNCOLD Vietnam National Committee on Large Dam and Water Resources Development (VNCOLD). Available online: http://www.vncold.vn/en/web/default.aspx (accessed on 9 December 2018).
- Amanda, A.B.; Bristo, D. Impact of dams on global biodiversity: A scientometric analysis. Neotrop. Biol. Conserv. 2016, 11, 101–109. [Google Scholar]
- Diomande, D.; Kpai, N.N.; Kouadio, K.N.; Sébastino, K.; Costa, D.A.; Gourene, G. Spatial distribution and structure of benthic macroinvertebrates in an artificial reservoir: Taabo Lake (Côte d’ Ivoire). Int. J. Biol. Chem. Sci. 2013, 7, 1503–1514. [Google Scholar] [CrossRef] [Green Version]
- Oo, A.T.; Van Huylenbroecka, G.; Speelman, S. Differential impacts of an irrigation project: Case study of the Swar Dam Project in Yedashe, Bago region of Myanmar. J. Dev. Agric. Econ. 2017, 9, 178–189. [Google Scholar]
- Phan, P.D. Literature Review for the Development of Guideline for Prioritisation of Barriers to Fish Passage in Irrigation Schemes in Vietnam; Research Institute for Aquaculture No. 3: Nha Trang, Vietnam, 2015; p. 38. [Google Scholar]
- Wei, G.; Yang, Z.; Cui, B.; Li, B.; Chen, H.; Bai, J.; Dong, S. Impact of Dam Construction on Water Quality and Water Self-Purification Capacity of the Lancang River, China. Water Resour. Manag. 2009, 23, 1763–1780. [Google Scholar] [CrossRef]
- Wildi, W. Environmental hazards of dams and reservoirs. Terre Environ. 2010, 88, 187–197. [Google Scholar]
- Mattos, L.D.; Kruger, M.L.D.; Affonso, S.L.A.; Perbiche-Neves, G.; Junior, S.P. Small dams also change the benthic macroinvertebrates community in rocky rivers. Acta Limnol. Bras. 2017, 29. [Google Scholar] [CrossRef] [Green Version]
- Power, M.E.; Dietrich, W.E.; Finlay, J.C. Dams and Downstream Aquatic Biodiversity: Potential Food Web Consequences of Hydrologic and Geomorphic Change. Environ. Manag. Vol. 1996, 20, 887–895. [Google Scholar] [CrossRef]
- Lin, Q. Influence of Dams on River Ecosystem and Its Countermeasures. J. Water Resour. Prot. 2011, 3, 60–66. [Google Scholar] [CrossRef]
- Mueller, J.S.; Grabowski, T.B.; Brewer, S.K.; Worthington, T.A.; Mueller, J.S.; Hall, A. Effects of Temperature, Total Dissolved Solids, and Total Suspended Solids on Survival and Development Rate of Larval Arkansas River Shiner. J. Fish Wildl. Manag. 2017, 8, 79–88. [Google Scholar] [CrossRef]
- Quevedo, L.; Ibáñez, C.; Caiola, N.; Cid, N.; Hampel, H. Impact of a reservoir system on benthic macroinvertebrate and diatom communities of a large Mediterranean river (lower Ebro river, Catalonia, Spain). Limnetica 2018, 37, 209–228. [Google Scholar]
- Ngo, X.Q.; Ngo, T.T.; Nguyen, X.D.; Vanreusel, A. Initial study on impact of Ba Lai dam construction to humanity ecosystem in Binh Dai district, Ben tre province. In Proceedings of the Human Ecology and Sustainable Development from Theory to Practice, Ha Noi, Vietnam, 13 January 2017; pp. 254–274. [Google Scholar]
- Nguyen, S.T.; Nguyen, M.H. Study on deposition of Ba Lai estuary, Ben Tre province. J. Sci. Vietnam Natl. Univ. Nat. Sci. Technol. 2011, 1S, 211–217. [Google Scholar]
- Bongers, T. The Maturity Index: An Ecological Measure of Environmental Disturbance Based on Nematode Species Composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Vanreusel, A. Ecology of the free-living marine nematodes from the Voordelta (Southern Bight of the North Sea). I; Species composition and structure of the namatode communities. Cah. Biol. Mar. 1990, 31, 439–462. [Google Scholar]
- Moens, T.; Braeckman, U.; Derycke, S.; Fonseca, G.; Gallucci, F.; Gingold, R.; Guilini, K.; Ingels, J.; Leduc, D.; Vanaverbeke, J.; et al. Ecology of free-living marine nematodes. In Handbook of Zoology; De Gruyter: Berlin, Germany, 2013; pp. 109–152. ISBN 9783110274257. [Google Scholar]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera-an outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Yeates, G.W.; Ferris, H.; Moens, T.; Putten, W.H. Van Der The Role of Nematodes in Ecosystem. In Nematodes as Environmental Indicators; CAB International: Wallingford, UK, 2009; 341p, ISBN 9781845933852. [Google Scholar]
- Alves, A.S.; Adão, H.; Ferrero, T.J.; Marques, J.C.; Costa, M.J.; Patrício, J. Benthic meiofauna as indicator of ecological changes in estuarine ecosystems: The use of nematodes in ecological quality assessment. Ecol. Indic. 2013, 24, 462–475. [Google Scholar] [CrossRef]
- Ferrero, T.J.; Debenham, N.J.; Lambshead, P.J.D. The nematodes of the Thames estuary: Assemblage structure and biodiversity, with a test of Attrill’s linear model. Estuar. Coast. Shelf Sci. 2008, 79, 409–418. [Google Scholar] [CrossRef]
- Ngo, X.Q.; Nguyen, N.C.; Vanreusel, A. Nematode morphometry and biomass patterns in relation to community characteristics and environmental variables in the Mekong Delta, Vietnam. Raffles Bull. Zool. 2014, 62, 501–512. [Google Scholar]
- Ngo, X.Q.; Nguyen, N.C.; Smol, N.; Prozorova, L.; Vanreusel, A. Intertidal nematode communities in the Mekong estuaries of Vietnam and their potential for biomonitoring. Environ. Monit. Assess. 2016, 188, 91. [Google Scholar]
- Soetaert, K.; Vincx, M.; Wittoeck, J.; Tulkens, M. Meiobenthic distribution and nematode community structure in five European estuaries. Hydrobiologia 1995, 311, 185–206. [Google Scholar] [CrossRef]
- Smol, N.; Willems, K.A.; Govaere, J.C.R.; Sandee, A.J.J. Composition, distribution and biomass of meiobenthos in the Oosterschelde estuary (SW Netherlands). Hydrobiologia 1994, 282/283, 197–217. [Google Scholar] [CrossRef] [Green Version]
- Tita, G.; Vincxy, M.; Desrosiers, G. Size spectra, body width and morphotypes of intertidal nematode: An ecological interpretation. J. Mar. Biol. Assoc. UK 1999, 79, 1007–1015. [Google Scholar] [CrossRef]
- Tita, G.; Desrosiers, G.; Vincx, M.; Clément, M. Intertidal meiofauna of the St Lawrence estuary (Quebec, Canada): Diversity, biomass and feeding structure of nematode assemblages. J. Mar. Biol. Assoc. UK 2002, 82, 779–791. [Google Scholar] [CrossRef]
- Wojtasik, B. The influence of water power station in Niedzica on littoral zone meiobenthos of Czorsztyński and Sromowiecki dam reservoirs (Pieniny mountains, Poland). Teka Kom. Ochr. Kszt. Środ. Przyr.—OL PAN 2009, 6, 411–423. [Google Scholar]
- Georg, W.; Orendt, C.; Höss, S.; Großschartner, M.; Adamek, Z.; Jurajda, P.; Traunspurger, W.; de Deckere, E.; Liefferinge, C. van The macroinvertebrate and nematode community from soft sediments in impounded sections of the river Elbe near Pardubice, Czech Republic. Lauterbornia 2010, 69, 87–105. [Google Scholar]
- Nicholas, W.L.; Bird, A.F.; Beech, T.A.; Stewart, A.C. The nematode fauna of the Murray River estuary, South Australia; the effects of the barrages across its mouth. Hydrobiologia 1992, 234, 87–101. [Google Scholar] [CrossRef]
- Tiemann, J.S.; Gillette, D.P. Effects of Lowhead Dams on Riffle-Dwelling Fishes and Macroinvertebrates in a Midwestern River. Trans. Am. Fish. Soc. 2004, 133, 705–717. [Google Scholar] [CrossRef]
- Montagna, P.A.; Palmer, T.A.; Beseres Pollack, J. Hydrological Changes and Estuarine Dynamics; SpringerBriefs in Environmental Science; Springer: New York, NY, USA, 2013; Volume 8, ISBN 978-1-4614-5832-6. [Google Scholar]
- ISO-5667 ISO 5667-3:2018—Water Quality—Sampling—Part 3: Preservation and Handling of Water Samples. Available online: https://www.iso.org/standard/72370.html (accessed on 4 August 2019).
- ISO-5667 ISO 5667-12:2017—Water Quality—Sampling—Part 12: Guidance on Sampling of Bottom Sediments from Rivers, Lakes and Estuarine Areas. Available online: https://www.iso.org/standard/59903.html (accessed on 4 August 2019).
- Krumbein, W.C. Size Frequency Distributions of Sediments. SEPM J. Sediment. Res. 1934, 4, 65–77. [Google Scholar] [CrossRef]
- U.S. EPA Method 3050B: Acid Digestion of Sediments, Sludges, and Soils. J. Jpn. Soc. Bronchol. 1996, 12.
- Brown, K.A.; McGreer, E.R.; Taekema, B.; Cullen, J.T. Determination of Total Free Sulphides in Sediment Porewater and Artefacts Related to the Mobility of Mineral Sulphides. Aquat. Geochem. 2011, 17, 821–839. [Google Scholar] [CrossRef]
- Vincx, M. Meiofauna in marine and freshwater sediments. In Hall, G.S. Methods for the Examination of Organismal Diversity in Soils and Sediments; CAB International in association with United Nations Educational, Scientific, and Cultural Organization and the International Union of Biological Sciences: Wallingford, UK, 1996; pp. 187–195. ISBN 0851991491. [Google Scholar]
- De Grisse, A.T. Redescription ou modification de quelques techniques utilissée dans l’étude des nematodes phytoparasitaires. Meded. Rijksfac. der Landbouveten Gent 1969, 34, 351–369. [Google Scholar]
- Schmidt-Rhaesa, A. Gastrotricha, Cycloneuralia and Gnathifera. Volume 2, Nematoda; De Gruyter: Berlin, Germany, 2013; ISBN 9783110274257. [Google Scholar]
- Zulini, A. Identification Manual for Freshwater Nematode Genera. In Lecture Book for MSc; Nematology Ghent University: Ghent, Belgium, 2010; p. 112. [Google Scholar]
- Abebe, E.; Andrássy, I. The Handbook on Freshwater Nematodes: Ecology and Taxonomy; The CABI Publisher: Wallingford, UK, 2006. [Google Scholar]
- Warwick, R.M.; Platt, H.M.; Somerfield, P.J. Free-living Marine Nematodes Part III: Monhysterids; Kermack, D.M., Barnes, R.S.K., Eds.; The Linnean Society of London and the Estuarine & Coastal Sciences Association: London, UK, 1998; ISBN 1 85 153 260 9. [Google Scholar]
- Platt, H.M.; Warwick, R.M. Free-living Marine Nematodes. Part I: British Enoplids; Kermack, D.M., Barnes, R.S.K., Eds.; The Linnean Society of London and the Estuarine & Coastal Sciences Association: London, UK, 1983; ISBN 0 521 25422 1. [Google Scholar]
- Platt, H.M.; Warwick, R.M. Free-Living Marine Nematodes, Part II: British Chromadorids; Kermack, D.M., Barnes, R.S.K., Eds.; The Linnean Society & The Estuarine & Brackish-Water Sciences Association: London, UK, 1988; ISBN 90 04 085955. [Google Scholar]
- Nguyen, V.T. Fauna of Vietnam. Free-living Nematodes Orders Monhysterida, Araeolaimida, Chromadorida, Rhabditida, Enoplida, Mononchida and Dorylaimida; Science Technology: Ha Noi, Vietnam, 2007. [Google Scholar]
- Bezerra, T.N.; Decraemer, W.; Eisendle-Flöckner, U.; Hodda, M.; Holovachov, O.; Leduc, D.; Miljutin, D.; Mokievsky, V.; Peña Santiago, R.; Sharma, J.; et al. Nemys: World Database of Nematodes. Available online: http://nemys.ugent.be/ (accessed on 4 August 2019).
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A language and Environment for Statistical Computing. Vienna, Austria. 2018. Available online: https//:www.R project/ (accessed on 1 July 2019).
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation. PRlMER-E Ltd, 2nd ed.; Plymouth. Marine Laboratory: Plymouth, UK, 2001. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ongley, E.D. Pollution by sediments. In Control of Water Pollution from Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1996; pp. 19–37. [Google Scholar]
- Burton, G.A. Sediment quality criteria in use around the world. Limnology 2002, 3, 65–75. [Google Scholar] [CrossRef]
- Phuong, P.K.; Tu, N.D.; Thanh, N.V. Heavy metals status in sediment at Can Gio mangrove, Ho Chi Minh city, Vietnam. J. Biol. 2011, 33, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.S.; Ngo, X.Q.; Vanreusel, A.; Smol, N. The nematode community distribution in two estuaries of the Mekong delta: Cung Hau and Ham Luong, South Vietnam. J. Biol. 2012, 34, 1–12. [Google Scholar]
- Ngo, X.Q.; Smol, N.; Vanreusel, A. The meiofauna distribution in correlation with environmental characteristics in 5 Mekong estuaries, Vietnam. Cah. Biol. Mar. 2013, 54, 71–83. [Google Scholar]
- Tran, T.T.; Nguyen, L.Q.L.; Nguyen, T.M.Y.; Vanreusel, A.; Ngo, X.Q. Free-living nematode communities in Ba Lai river, Ben Tre province. Vietnam J. Sci. Technol. 2018, 56, 224–235. [Google Scholar]
- Vanreusel, A.; De Groote, A.; Gollner, S.; Bright, M. Ecology and Biogeography of Free-Living Nematodes Associated with Chemosynthetic Environments in the Deep Sea: A Review. PLoS ONE 2010, 5, 16. [Google Scholar] [CrossRef]
- Fonseca, G.; Gallucci, F. The need of hypothesis-driven designs and conceptual models in impact assessment studies: An example from the free-living marine nematodes. Ecol. Indic. 2016, 71, 79–86. [Google Scholar] [CrossRef]
- Adão, H.; Alves, A.S.; Patrício, J.; Neto, J.M.; Costa, M.J.; Marques, J.C. Spatial distribution of subtidal Nematoda communities along the salinity gradient in southern European estuaries. Acta Oecologica 2009, 35, 287–300. [Google Scholar] [CrossRef]
- Heip, C.; Vincx, M.; Vranken, G. The Ecology of Marine Nematodes. Oceanogr. Mar. Biol. 1985, 23, 399–489. [Google Scholar]
- Schratzberger, M.; Warr, K.; Rogers, S.I. Functional diversity of nematode communities in the southwestern North Sea. Mar. Environ. Res. Elsevier 2007, 63, 368–389. [Google Scholar] [CrossRef] [Green Version]
- Attrill, M.J. A testable in estuaries linear model for diversity trends. J. Anim. Ecol. 2002, 71, 262–269. [Google Scholar] [CrossRef]
- Vanaverbeke, J.; Merckx, B.; Degraer, S.; Vincx, M. Sediment-related distribution patterns of nematodes and macrofauna: Two sides of the benthic coin? Mar. Environ. Res. 2010, 71, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T. Seasonal and Spatial Patterns in Meiofauna Community Structure of the Can Gio Mangrove Forest (Vietnam) with a Focus on Nematoda and Their Role as Bioindicator; Ghent University: Ghent, Belgium, 2009; 242p. [Google Scholar]
- Rzeznik-Orignac, J.; Fichet, D.; Boucher, G. Spatio-temporal structure of the nematode assemblages of the Brouage mudflat (Marennes Oléron, France). Estuar. Coast. Shelf Sci. 2003, 58, 77–88. [Google Scholar] [CrossRef]
- Gyedu-Ababio, T.K.; Furstenberg, J.P.; Baird, D.; Vanreusel, A. Nematodes as indicators of pollution: A case study from the Swartkops River system, South Africa. Hydrobiologia 1999, 397, 155–169. [Google Scholar] [CrossRef]







| P Value | |||
|---|---|---|---|
| Variable | pe | ps | Post Hoc Test |
| Salinity | 0.028 | DD ≠ DU: 0.034, DU ≠ RD: 0.035 | |
| pH | 0.035 | DD ≠ RU: 0.034, RD ≠ RU: 0.048 | |
| DO | 0.037 | 0.026 | |
| TSS | 0.034 | ||
| TDS | 0.028 | DD ≠ DU: 0.034, DU ≠ RD: 0.035 | |
| Pb | 0.035 | ||
| Hg | 0.035 | 0.034 | DD ≠ DU: 0.049, DU ≠ RD: 0.044 |
| Variable | p Value | |||
|---|---|---|---|---|
| pe&s | pe | ps | Post hoc Test | |
| Density | 0.0466 | 0.028 | DD ≠ DU: 0.034, DU ≠ RD: 0.035 | |
| Genus richness | 0.0466 | 0.035 | DD ≠ DU: 0.0001, DD ≠ RD: 0.00026, DD ≠ RU: 0.000003 | |
| H’ | 0.0015 | 0.026 | DD ≠ DU: 0.014, DD ≠ RD: 0.0065, DD ≠ RU: 0.0025 | |
| Genus composition (square-root transformation) | 0.0003 | 0.0001 | DD ≠ DU: 0.0001, DD ≠ RD: 0.0002, DD ≠ RU: 0.0001, DU ≠ RD: 0.0003, DU ≠ RU: 0.0039, RD ≠ RU: 0.0001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, N.T.M.; Vanreusel, A.; Lins, L.; Thai, T.T.; Nara Bezerra, T.; Quang, N.X. The Effect of a Dam Construction on Subtidal Nematode Communities in the Ba Lai Estuary, Vietnam. Diversity 2020, 12, 137. https://doi.org/10.3390/d12040137
Yen NTM, Vanreusel A, Lins L, Thai TT, Nara Bezerra T, Quang NX. The Effect of a Dam Construction on Subtidal Nematode Communities in the Ba Lai Estuary, Vietnam. Diversity. 2020; 12(4):137. https://doi.org/10.3390/d12040137
Chicago/Turabian StyleYen, Nguyen Thi My, Ann Vanreusel, Lidia Lins, Tran Thanh Thai, Tania Nara Bezerra, and Ngo Xuan Quang. 2020. "The Effect of a Dam Construction on Subtidal Nematode Communities in the Ba Lai Estuary, Vietnam" Diversity 12, no. 4: 137. https://doi.org/10.3390/d12040137
APA StyleYen, N. T. M., Vanreusel, A., Lins, L., Thai, T. T., Nara Bezerra, T., & Quang, N. X. (2020). The Effect of a Dam Construction on Subtidal Nematode Communities in the Ba Lai Estuary, Vietnam. Diversity, 12(4), 137. https://doi.org/10.3390/d12040137






