Abstract
Orchids are one of the most species-rich families in the world, and many species are under threat in numerous countries. Biodiversity research focusing on the relationship between the richness of orchid species and ecological factors was performed across the Cerová vrchovina Mts (Western Carpathians) testing impact of 26 explanatory variables. We aimed to determine the main ecological predictors controlling species richness and to predict potential species richness patterns. Altogether, 19 orchid species were found in the studied area, with Cephalanthera damasonium and Epipactis microphylla being the most common. Four environmental predictors (minimal longitude, carbonate-containing sediments, maximal yearly solar irradiation, and agricultural land) had statistically significant effects on orchid richness following regression analysis. Predictive models for the nine most frequent species using MaxEnt software showed (i) that land cover and geological substrate had the highest contribution to the explained variance in the models and (ii) strong potential for occurrence of given orchids in several poorly mapped parts of the studied area.
1. Introduction
The family Orchidaceae, covering epiphytic and terrestrial species, is one of the most species-rich flowering plant families in the world. Terrestrial orchids occur across almost all continents, with Indochina, Southwest Australia, Europe, Northern Asia, and North America being the most important hot-spots of their diversity [1,2,3]. In Europe, their biodiversity center is in Southern Europe, mainly in the Mediterranean region [4] which served as plant refugial area during the last glacial periods [5,6] and many orchid species are categorized as endangered. Many species are listed in national plant red lists and protected by national legislations in numerous countries. Their high biodiversity and conservation values are closely coupled with unique life strategies and ecological conditions. The most commonly published threats for orchids are direct destruction of habitats or other negative human and global impacts such as climate change, pollution, tourism, and recreation activities [7].
Intensive orchid conservation research in taxonomy, phylogeny, evolution, and genetic diversity has been performed during last decades e.g., [8,9], including research on mycorrhizal symbionts e.g., [10,11] and propagation techniques [12], aimed at finding consensus in their conservation strategy [13]. One of the previously identified knowledge gaps is the ecology of threatened orchid species including their interactions with fungi, pollinators, habitats, and threats [13]. However, there are only few studies associated with these aims in the region of Central Europe [14,15,16].
The role of ecological factors in the distribution and abundance of terrestrial orchids is subject of intensive research activities on various geographical scales [17]. Historical factors and macroclimate are the main drivers of orchid diversity on broad scales, while regional and local ecological factors have strong effect on their richness and abundance in small scales [17,18]. Among them, geographical characteristics such as latitude, longitude, or altitude; climate represented by temperature, precipitation, humidity, and light; and geological substrates and soil properties play important roles e.g., [17,18,19,20,21]. It has been also found that the distribution, diversity, and abundance of orchids is tightly related to habitat types e.g., [17,22,23].
Existing European studies focusing on the relationship between diversity of terrestrial orchids and ecological factors covered mainly broad geographical ranges e.g., [18,21,24], while regional or local studies were less frequent e.g., [25,26]. Fine-scale studies have a potential to be important for better understanding of the association between species richness and environmental variables [13,24]. Their results can contribute to suitable local conservation and landscape management efforts.
Our case study was performed in a small area represented by the Protected Landscape Area Cerová vrchovina (PLA; Slovakia). Activities focusing on mapping of orchids in this area in the last years have resulted in considerable knowledge about the distribution of orchid species. We decided to use this new data matrix to study the relationship between orchid diversity and ecological variables with the aims to (i) show the distribution pattern and species richness of the species in this area and (ii) determine the main ecological predictors controlling species richness. In addition, there are still some insufficiently mapped parts of the studied area, where orchids can occur. Therefore, we would also like to predict potential species richness of the species in these areas using environmental variables [27].
2. Materials and Methods
2.1. Study Area
The Cerová vrchovina Mts are situated in Slovakia (Central Europe) in the southern part of the Western Carpathians (Figure 1) between 48.12542°–48.29042° latitude and 19.78375°–20.20958° longitude in the altitudinal range of 173–722 m. The study area is located in a moderately warm to warm region [28] with average yearly temperature and yearly precipitation of 9.44 °C and 644 mm, respectively [29,30]. Geological bedrock is composed mainly of sandstones, siltstones, basalts, and their tuffs [19]. In 1989, a part of the area was declared a PLA to protect the landscape with specific geomorphology, geology, fauna, and flora [29]. This study covered the whole PLA with an area of 168.10 km2.
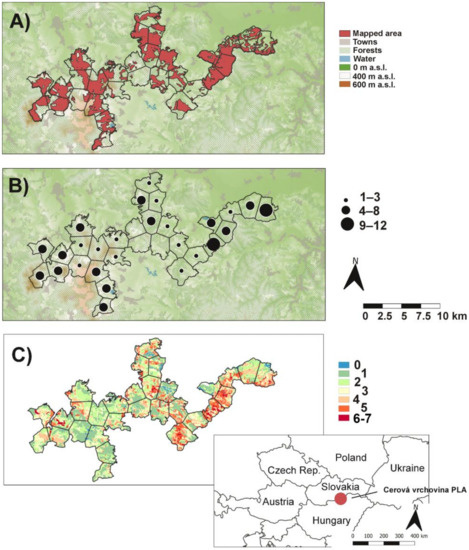
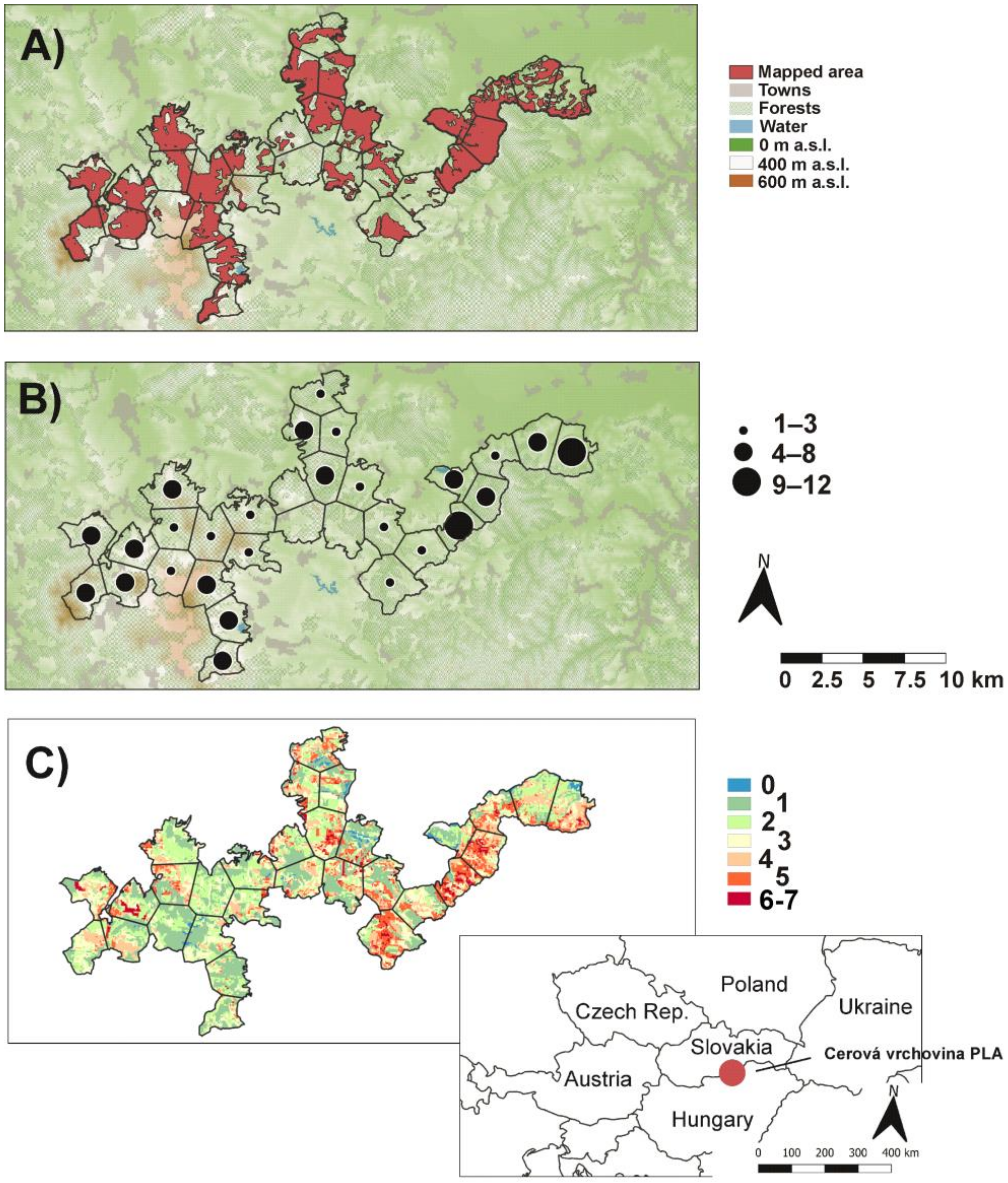
Figure 1.
Map of the Cerová vrchovina protected landscape area: (A) mapped area of orchid species, (B) orchid richness in polygons, and (C) number of orchids predicted by MaxEnt based on occurrence of 9 most frequent species (see Methods).
2.2. Data Sampling and Analyses
Intensive mapping of orchid species was carried out in the PLA under the program “Elaboration of pilot care programmes of the National park (further NP) Veľká Fatra, NP Muránska planina and PLA Cerová vrchovina in accordance with the reassessment of protected areas” in 2013–2015 and covered 46% of the studied area. Neottia nidus-avis was not mapped under this program due to its frequent occurrence in Slovakia [31]. Moreover, the species is not endangered and protected in Slovakia [32]. Records from this mapping (>80% from basic dataset) were completed by all available orchid findings reported by individual botanists from under-sampled (extensively mapped) areas from the years 2000–2018 (Figure 1A). Each record was characterized by geographical coordinates (latitude and longitude) in the WGS 84 system using field GPSs. Altogether, 912 records were included in the basic dataset. Only one record per species per 3ʺ (both latitude and longitude) was used prior to analyses. A geographical stratification thus reduced the basic dataset to 487 records.
The area of the PLA is highly irregular and splitting it into grids following the mapping method by Niklfeld [33] would be suboptimal, very different proportion of PLA area within grid cells: 0.16–98.49% and high proportion of grid cells with less than 30% of the PLA: 54%. Therefore, a one-hundred meter grid of points was created covering the area of the PLA; then these points were clustered into 30 groups by k-means clustering, and centroids of the clusters were obtained. The study area was then split by distance into 30 relatively homogeneous segments by centroid Voronoi tessellation (Figure 1).
Environmental variables derived from Geographic Information System (GIS) layers were provided by GeoModel Solar Company [30]. Initial variables (elevation: 3″ × 3″, yearly precipitation: 2′ × 2′, yearly solar irradiation: 15″ × 15″ and average yearly temperature: 30″ × ″) were accompanied by longitude, latitude, calculated temperature seasonality (as 100*standard deviation of monthly temperatures), average temperature of warmest and coldest months and their difference (similar to WorldClim bioclimatic variables 4 to 7), and xericity index following the formula proposed by Austin et al. [34] (cos (exposition − 202.5)*tan(slope)). They were rescaled to the same 3″ cell size, which was the highest resolution among the original GIS layers. Over the mapped parts of the 30 polygons we obtained median, minimal, and maximal values of these layers of environmental variables as well as their range (altogether 44 variables). We also produced three classes of land cover based on Corine Land Cover data (3″ × 3″), i.e., agricultural land (non-irrigated arable lands, vineyards, permanent crops, and complex cultivation patterns), pastures, forests [30], and four classes of geological substrate (3″ × 3″), i.e., Quaternary sediments, carbonate-containing siltstones and sandstones, other sediments, and volcanic rocks (http://apl.geology.sk/gm50js/). We also calculated the inverse Simpson index of these classes and the number of types of land cover and geological substrates (pre-clustering types) as measures of diversity (altogether 11 variables). Raster operations were done in QGIS 3.2.3 [35]. The initial set of variables contained 55 predictors.
We tested correlations among environmental variables (see Supplementary Material: Figure S1) to avoid multicollinearity using the “cor” function in R [36]. One variable from each pair of strongly correlated variables (Pearson correlation coefficient higher than 0.9) was removed. We retained the variable with higher pseudo-R2 for relationships with the number of species (see below). The set of variables was thus reduced to 26 predictors (Table 1).

Table 1.
Summary statistics of the polynomial regressions for variables which are arranged according to increasing p values. 1—geographical variables, 2—geological variables, 3—climatic variables, 4—landscape type characteristics. *—p < 0.05; ns—not significant.
The relationships between environmental variables and the number of orchid species in polygons were tested by a generalized linear model (GLM; quasi-Poisson family) in R [36]. The potential confounding effect of area mapped within polygons on species richness pattern was controlled in the regressions. A model where number of species depends only on the mapped area was used as a null model with calculating the pseudo-R2. A significance test of the quadratic term of the GLM was used to select whether quadratic regression (if p ≤ 0.05) or linear regression (if p > 0.05) is to be used. The final model for each variable is shown in Table 1. The Benjamini-Hochberg procedure was used to correct multiple testing. The goodness-of-fit of the models was expressed by McFadden’s pseudo-R2, where the null model was the number of species depending only on the area mapped.
We created models of the distribution of selected orchid species using MaxEnt [37] to model potential orchid richness in poorly mapped parts of PLA. The models were designed only for the nine the most frequent species with at least 12 records: Cephalanthera damasonium, C. longifolia, C. rubra, Epipactis microphylla, E. neglecta, E. pontica, E. purpurata, Orchis morio, and O. purpurea. For this purpose, we used the following environmental variables presented in Table 1: altitude, yearly solar irradiation, difference between the average temperature of the hottest and the coldest month, xericity, geological substrate, and land cover (the last two were represented as basic types, not grouped into classes). Areas of PLA mapped for orchid occurrence (Figure 1A) served as a bias mask for the model. The threshold of 10 percentile training presence was chosen to describe expected species presence. These expected presences for the 9 orchid species were summed up to get a map of predicted number of species for this group of species (Figure 1C).
Nomenclature of orchid species follows [38].
3. Results
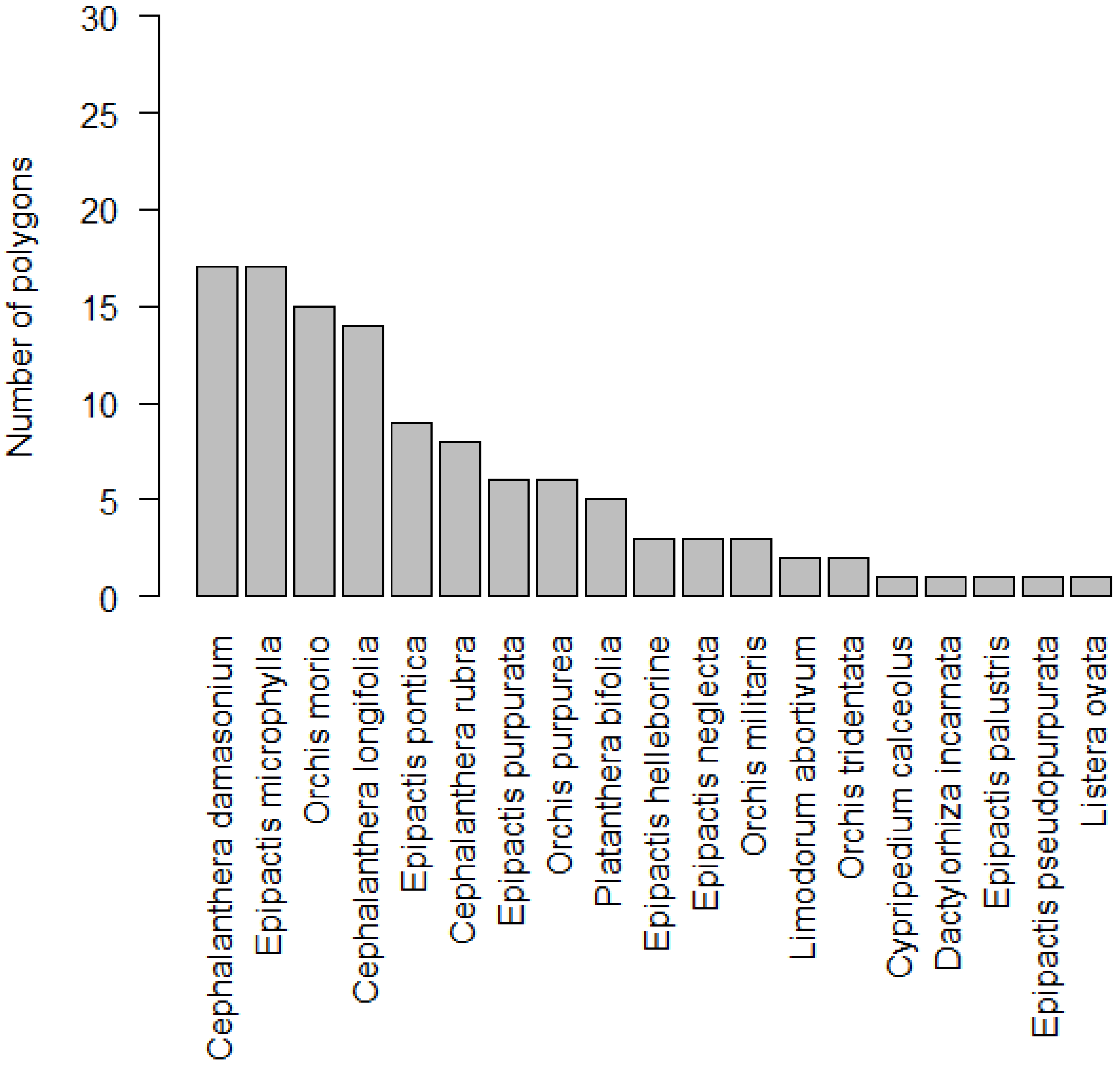
Altogether, 19 orchid species were found in the studied area (Appendix A). The most frequent species with occurrences in 17 polygons (~57%) were Cephalanthera damasonium and Epipactis microphylla, followed by Orchis morio (~50%) and C. longifolia (~47%), while 7 species, Limodorum abortivum, Orchis tridentata, Cypripedium calceolus, Dactylorhiza incarnata, Epipactis palustris, E. pseudopurpurata, and Listera ovata were found only in 1 or 2 polygons. The highest number of species was recorded within the Epipactis genus (7), followed by Orchis (4) and Cephalanthera (3) (Figure 2). Most polygons (~43%) contained 4–8 orchid species, but ~40% polygons contained 1 to 3 species. Only ~7% showed high species richness (9–12), and no orchid species was found in the rest of the polygons (10%; Figure 1B).
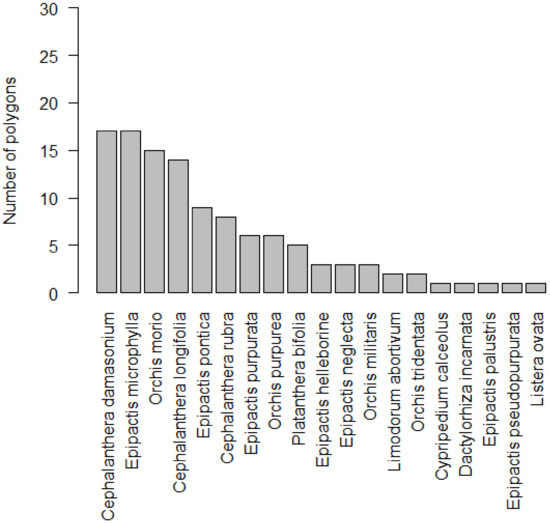
Figure 2.
Number of polygons where orchid species occurred in Protected Landscape Area Cerová vrchovina.
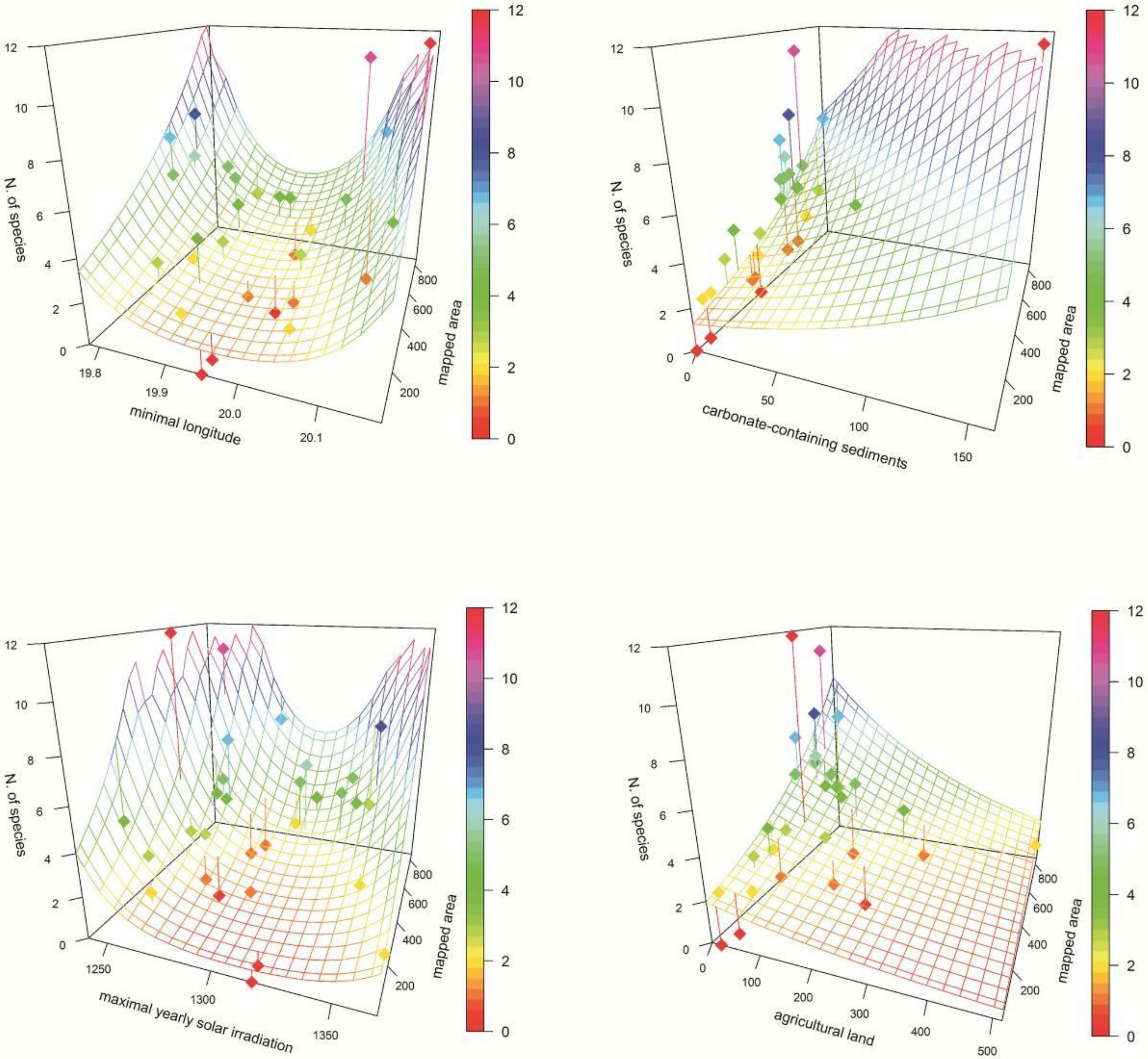
Results of the regressions suggested that only four environmental predictors (minimal longitude, carbonate-containing sediments, maximal yearly solar irradiation, and agricultural land) had statistically significant (p ˂ 0.05) effects on orchid richness. However, only the first three of them showed significance after correction for multiple testing (Table 1). Minimal longitude and maximal yearly solar irradiation showed a U-shaped relationship with the highest number of orchids in the margins of these gradients. Species richness decreased with the increasing cover of agricultural lands and increased on carbonate-containing sediments (only one, the most species-rich polygon, contained significant amounts of these sediments; Figure 3). In the initial dataset, however, minimal longitude showed a very strong correlation with other geographical and climatic variables. The other three variables showing significant relationship with orchid diversity showed no strong correlations with other environmental predictors.
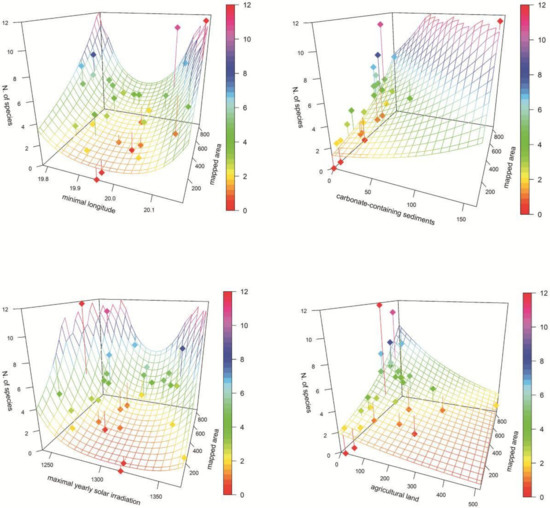
Figure 3.
Association of orchid richness and selected statistically significant (p > 0.05) ecological variables (see Table 1). Mapped area, geological and land cover areas are given in number of raster layer cells (3″). Dots—study polygons; surface—model; vertical lines—deviation of polygon number of species from the model; colour scale—number of orchid species.
The area under the curve (AUC) of predictive models for the nine most frequent orchid species ranged from 0.848 (for Orchis morio) to 0.951 (Epipactis neglecta) with an average of 0.896. Land cover and geological substrate showed highest contribution/importance in the models, while yearly solar irradiation showed lowest values (Table 2). Based on the nine models, species diversity showed strong potential for distribution of given orchids in several poorly mapped parts of the PLA, mainly in the central parts. The highest predicted number of species, seven, was only on 0.02% of the area of the PLA, with 6 species on 3.33% of the PLA, 5 species on 11.52%, 4 on 15.41%, 3 on 17.47%, 2 on 24.12%, and 1 on 26.6%, while the occurrence of none of the nine orchids was predicted on 1.54% of the PLA (Figure 1C).

Table 2.
MaxEnt model statistics and effects of variables. Threshold—10th percentile training presence; area—fractional predicted area (what % of PLA was predicted to be suitable for the species); dem—altitude; r13—yearly solar irradiation; bio7—difference between average temperature of hottest and coldest month; CLC—land cover; geo—geological substrate.
4. Discussion
4.1. Diversity of Orchids in a Central European Context
The study area is situated in Slovakia close to the border with Hungary in the southern part of the Western Carpathians and is strongly influenced by the warm climate of the Pannonian basin [29]. Both countries are characterized by a similar orchid species pool, i.e., 78 taxa identified in Slovakia and 70 in Hungary [31,39]. However, their distribution is influenced by different climatic, geographical, and geological characteristics specific for the two different biogeographical regions, the Carpathian region that prevails in Slovakia and the Pannonian region dominating in Hungary. Taxa with a preference for montane climate occur more frequently in Slovakia, while more thermophilous taxa are distributed mainly in Hungary [31,39]. Nineteen (twenty with Neottia nidus-avis) orchid taxa (~25% of all orchid taxa in Slovakia) typical for (sub)montane areas were found in our study. This number is relatively small but corresponds to an area with a limited altitudinal gradient and a low geological heterogeneity. The local orchid species pool is also driven by a strong decline of traditional agricultural management (regular mowing and grazing) during the last thirty years with gradual overgrowing of grasslands by trees and shrubs [29]. The most frequent recorded species in our study, Cephalanthera damasonium, Epipactis microphylla, Orchis morio, C. longifolia, E. pontica, and C. rubra are relatively commonly distributed across the whole of Slovakia [31,40]. While Orchis morio grows on grasslands, another five species prefer forest habitats [4,31,39]. Orchis morio is also one of the most frequent species in Hungary, but E. pontica is considered to be rare in Hungary. One of the two distribution centers of this species is situated in the Mátra, Medves, Karancs, and Bükk regions, adjacent to the studied PLA. The other four mentioned species are common in all (sub)montane regions of Hungary [39].
4.2. Effects of Ecological Factors on Orchids Diversity
Four environmental factors, namely minimal longitude, carbonate-containing sediments, maximal yearly solar irradiation, and agricultural land showed a statistically significant relationship with orchid richness in our study.
Longitude and its effect on species richness of orchids is only occasionally mentioned in large- and regional-scale studies. For example, total orchid richness in Greece showed an inversely unimodal trend along a longitudinal gradient [24]. Generally, geographical position expressed by altitude, latitude, and longitude is a proxy for local climatic conditions [17], which are the most substantive ecological variables influencing orchid richness. Effects of climatic factors, mainly air temperature and precipitation on orchid richness can be different, depending on altitude, latitude, phytogeography, orchid traits, or scale. For example, orchid species richness is lower in cold areas compared to warm climatic conditions in temperate regions [17]. At a regional scale, using orchid data from Greece, species richness showed different relations with climatic characteristics in the case of tuberous and intermediate orchids versus rhizomatous orchids [24]. Our results showed strong collinearity of minimal longitude and several variables, such as altitude, temperature, and precipitations, and this may be responsible for the relationship between longitude and orchid richness in our study.
Calcareous geological substrates were significant drivers of orchid species diversity in our case. This corroborated the results of previous studies that identified carbonates and their soils as to the most important substrates for the occurrence of terrestrial orchids [18,21,24]. The same can be stated about the Western Carpathians [31,41,42]. High orchid diversity was recorded in the eastern part of the study area where there is a high proportion of sandstones with an increased amount of calcium (see also [29]). Several species such as Orchis militaris, O. tridentata, or Cypripedium calceolus occurred only in this part of the PLA [43,44]; https://www.biomonitoring.sk. Indeed, these three species show a clear habitat affinity to substrates with a high calcium content and alkaline soil reaction elsewhere in Central Europe [31,39].
The light regime is also an important factor controlling patterns of orchid distribution and abundance [17], namely solar irradiation. It was shown as an important factor for the distribution of Platanthera bifolia in the Czech Republic, where this species occurred mainly in places with low solar radiation [26,45]. Our results showed a U-shaped response of orchid richness along the solar irradiation gradient, with the highest number on both margins of the gradient and decline towards mid-solar irradiation values. It means that high species richness occurs mainly in areas with high and low solar irradiation. This is in line with the fact that a substantial part of the detected orchids are either typical forest species (e.g., Epipactis species except E. palustris and Cephalanthera species) or grassland species (Orchis militaris, O. morio) [17,31,39,42].
Agricultural lands with intensive utilization, which in our study include non-irrigated arable lands, vineyards, permanent crops, and complex cultivation patterns, are unsuitable and/or suboptimal for orchid distribution [17]. This pattern is obvious in the studied area, where number of orchids decreased with increasing area of agricultural lands.
4.3. Prediction of Orchid Occurrence and Nature Conservation Consequences
Maxent is a useful statistical tool for predicting potential distribution of species [46], including orchids. Existing studies showed that several factors such as those associated with geography, landscape, and habitat characteristics are important for the prediction of orchid distributions [21,47,48]. Based on our results, the most important factors for the nine species were land cover and geological substrate, in accordance with other published data. Both these ecological variables are frequently considered crucial for orchid distribution, composition, and diversity [17].
Results of the predictions are helpful to determine potential localities of the most frequent orchid species in the study area, which all are listed as endangered in the red list of vascular plants of Slovakia [32]. These results will help to target floristic research in the future and subsequently lead to a better knowledge of the distribution, which can help with their conservation.
Our study showed that carbonate-containing sediments, even though they actually contain only low-to-average amounts of carbonates [49], are important for the preservation of orchid diversity. Grasslands, mainly dry, semi-dry, and wet meadows of Festuco-Brometea and Molinio-Arrhenatheretea classes, which broadly occur in the eastern part of PLA with a significant portion of this geological substrate [29], need traditional management practices (mowing and/or pasture) due to their high importance for orchid distribution. Recently, huge parts of these grasslands are gradually getting overgrown by trees and shrubs and this endangers the existence of typical grassland or wet-meadow orchids such as Orchis militaris, O. tridentata, or Dactylorhiza inacarnata, which are strictly limited to this area.
5. Conclusions
The results of our study showed that the main ecological variables affecting the diversity of orchids were carbonate-containing sediments that occurred only in the eastern part of the studied area. In addition, prediction of orchid richness revealed new areas where additional mapping of orchids is needed. Ensuring there is sufficient habitat availability seems to be effective for long-term persistence of this endangered plant group due to their complex biology. Our results further suggest that conservation actions should be addressed to species groups with similar ecological niches or habitat preference (e.g., forest- or tree-less habitats). It is important to note that some environmental predictors (for example light conditions and landscape structure) can be directly influenced by forest or landscape management activities.
Supplementary Materials
The following are available online at https://www.mdpi.com/1424-2818/12/4/154/s1.
Author Contributions
Conceptualization, R.H.; methodology, R.H. and M.H.; data preparing, M.H., V.R. and D.S.; data analysis, M.H; writing—original draft preparation, R.H., M.H., M.S. and D.G.; writing—contributed writing the manuscript, all authors; writing—review and editing, R.H. and M.H. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Our thanks belong to Martin Bažány, Mário Duchoň, Pavol Eliáš ml., Tomáš Figura, Katarína Gaálová, Rastislav Gális, Pavel Kliment, Rastislav Lasák, Branislav Lizoň, Martin Saksa and Ľudovít Vaško for providing data.
Conflicts of Interest
The authors declare no conflict of interest.
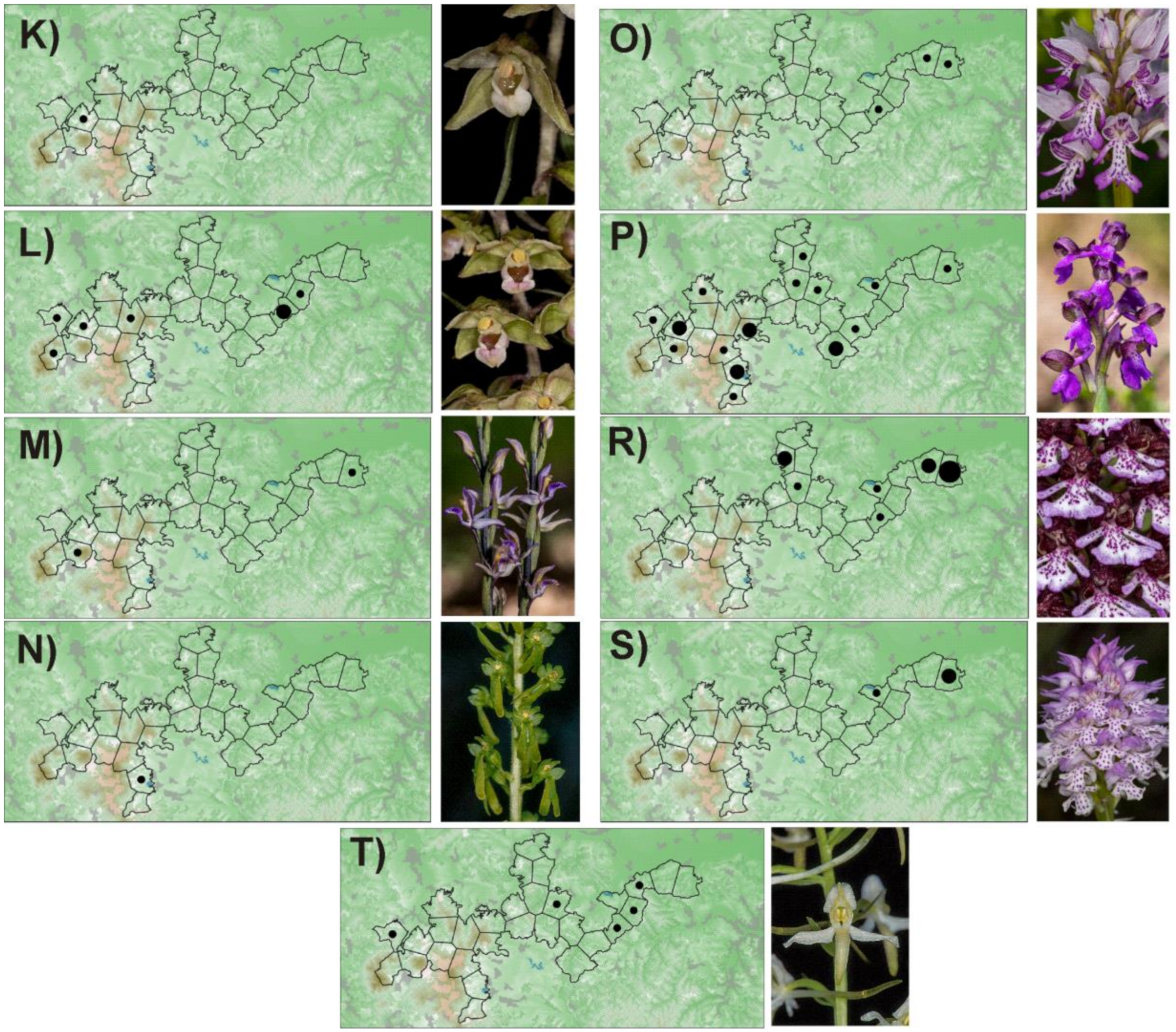
Appendix A
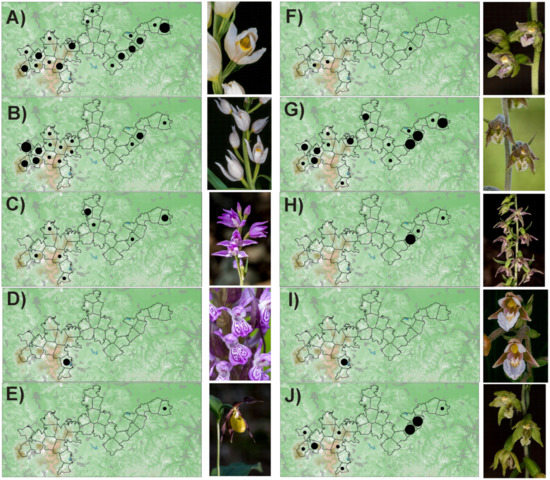
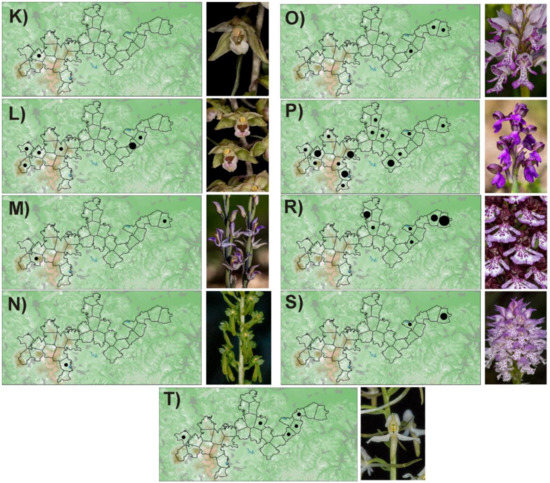
Figure A1.
Distribution of orchids in the PLA Cerová vrchovina: A—Cephalanthera damasonium, B—C. lonogifolia, C—C. rubra, D—Dactylorhiza incarnata, E—Cypripedium calceolus, F—Epipactis helleborine, G—E. microphylla, H—E. neglecta, I—E. palustris, J—E. pontica, K—E. pseudopurpurata, L—E. purpurata, M—Limodorum abortivum, N—Listera ovata, O—Orchis militaris, P—O. morio, R—O. purpurea, S—O. tridentata, T—Platanthera bifolia. Number of localities in polygon: ● 1–2, ● 3–10, ● more than 10.
Figure A1.
Distribution of orchids in the PLA Cerová vrchovina: A—Cephalanthera damasonium, B—C. lonogifolia, C—C. rubra, D—Dactylorhiza incarnata, E—Cypripedium calceolus, F—Epipactis helleborine, G—E. microphylla, H—E. neglecta, I—E. palustris, J—E. pontica, K—E. pseudopurpurata, L—E. purpurata, M—Limodorum abortivum, N—Listera ovata, O—Orchis militaris, P—O. morio, R—O. purpurea, S—O. tridentata, T—Platanthera bifolia. Number of localities in polygon: ● 1–2, ● 3–10, ● more than 10.

References
- Dressler, R.L. The Orchids: Natural History and Classification; Harvard University Press: Cambridge, MA, USA, 1981; pp. 1–332. [Google Scholar]
- Swarts, N.D.; Dixon, K.W. Terrestrial orchid conservation in the age of extinction. Ann. Bot. 2009, 104, 543–556. [Google Scholar] [CrossRef]
- Chase, M.; Christenhusz, M.; Mirenda, T. The Book of Orchids: A Life-Size Guide to Six Hundred Species from around the World; Ivy Press: London, UK, 2017; pp. 1–656. [Google Scholar]
- Delforge, P. Orchids of Europe, North Africa and the Middle East; A & C Black: London, UK, 2006; pp. 1–640. [Google Scholar]
- Comes, H.P.; Kadereit, J.W. The effect of Quaternary climatic changes on plant distribution and evolution. Trends Plant Sci. 1998, 3, 432–438. [Google Scholar] [CrossRef]
- Médail, F.; Diadema, K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009, 36, 1333–1345. [Google Scholar] [CrossRef]
- Wraith, J.; Pickering, C. Quantifying anthropogenic threats to orchids using the IUCN Red List. Ambio 2018, 47, 307–317. [Google Scholar] [CrossRef]
- Aceto, S.; Caputo, P.; Cozzolina, S.; Gaudio, L.; Moretti, A. Phylogeny and Evolution of Orchis and Allied Genera Based on ITS DNA Variation: Morphological Gaps and Molecular Continuity. Mol. Phylog. Evol. 1999, 13, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Devos, N.; Tyteca, D.; Raspé, O.; Wesselingh, R.A.; Jaquemart, A.-L. Patterns of chloroplast diversity among western European Dactylorhiza species (Orchidaceae). Plant Syst. Evol. 2003, 243, 85–97. [Google Scholar] [CrossRef]
- Bayman, P.; González, E.J.; Fumero, J.J.; Tremblay, R.L. Are fungi necessary? How fungicides affect growth and survival of the orchid Lepanthes rupestris in the field. J. Ecol. 2002, 90, 1002–1008. [Google Scholar] [CrossRef]
- Rasmussen, H.N. Recent developments in the study of orchid mycorrhiza. Plant Soil 2002, 244, 149–163. [Google Scholar] [CrossRef]
- Yam, T.W.; Arditti, J. History of orchid propagation: A mirror of the history of biotechnology. Plant Biotechnol. Rep. 2009, 3, 1. [Google Scholar] [CrossRef]
- Wraith, J.; Norman, P.; Pickering, K. Orchid conservation and research: An analysis of gaps and priorities for globally Red Listed species. Ambio 2020. [Google Scholar] [CrossRef]
- Sonkoly, J.; Vojtkó, A.E.; Tökölyi, J.; Török, P.; Sramkó, G.; Illyés, Z.; Molnár, V.A. Higher seed number compensates for lower fruit set in deceptive orchids. J. Ecol. 2016, 104, 343–351. [Google Scholar] [CrossRef]
- Fantinato, E.; Del Vecchio, S.; Baltieri, M.; Fabris, B.; Buffa, G. Are food-deceptive orchid species really functionally specialized for pollinators? Ecol. Res. 2017, 32, 951–959. [Google Scholar] [CrossRef]
- Štípková, Z.; Tsiftsis, S.; Kindlmann, P. Pollination mechanisms are driving orchid distribution in space. Sci. Rep. 2020, 10, 850. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S. The role of ecological factors in distribution and abundance of terrestrial orchids. In Orchids Phytochemistry, Biology and Horticulture; Reference Series in Phytochemistry; Mérillion, J.-M., Kodja, H., Eds.; Springer International Publishing: Basel, Switzerland, 2020; pp. 1–71. [Google Scholar] [CrossRef]
- Djordjević, V.; Tsiftsis, S. Patterns of orchid species richness and composition in relation to geological substrates. Wulfenia 2019, 26, 1–21. [Google Scholar]
- Dijk, E.; Willems, J.H.; van Andel, J. Nutrient responses as a key factor to the ecology of orchid species. Acta Bot. Neerl. 1997, 46, 339–363. [Google Scholar] [CrossRef]
- Janečková, P.; Wotavová, K.; Schödelbaureová, I.; Jersáková, J.; Kindlmann, P. Relative effects of management and environmental conditions on performance and survival of populations of a terrestrial orchids. Biol. Conserv. 2006, 129, 40–49. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Tsiripidis, I.; Karagiannakidou, V.; Alifragis, D. Niche analysis and conservation of the orchids of east Macedonia (NE Greece). Acta Oecol. 2018, 33, 27–35. [Google Scholar] [CrossRef]
- Hrivnák, R.; Gömöry, D.; Cvachová, A. Inter-annual variability of the abundance and morphology of Dactylorhiza majalis (Orchidaceae-Orchideae) in two permanent plots of a mire in Slovakia. Phyton Horn 2006, 46, 27–44. [Google Scholar]
- Slaviero, A.; Del Vecchio, S.; Pierce, S.; Fantinato, E.; Buffa, G. Plant community attributes affect dry grassland orchid establishment. Plant Ecol. 2016, 217, 1533–1543. [Google Scholar] [CrossRef]
- Tsiftsis, S.; Štípková, Z.; Kindlmann, P. Role of way of life, latitude, elevation and climate on the richness and distribution of orchid species. Biodiv. Conserv. 2019, 28, 75–96. [Google Scholar] [CrossRef]
- Procházka, A.; Mikita, T.; Jelínek, P. The relationship between some forest stand properties and the occurrence of orchids in the central parts of the Moravian Karst Protected Landscape Area. Acta Univ. Silvic. Mendel. Brun. 2017, 65, 919–931. [Google Scholar] [CrossRef]
- Štípková, Z.; Ramportl, D.; Černocká, V.; Kindlmann, P. Factors associated with the distributions of orchids in the Jeseníky Mountains, Czech Republic. Eur. J. Environ. Sci. 2017, 7, 135–145. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Lapin, M.; Faško, P.; Melo, M.; Štastný, P.; Tomlain, J. Climatic districts. Map No. 27. In Landscape Atlas of the Slovak Republici, 1st ed.; Miklóš, L., Ed.; Ministry of Environment Slovak Republic: Bratislava, Slovakia, 2002; p. 95. [Google Scholar]
- Kiss, G. (Ed.) A Karancs-Medves és a Csered-hegység Tájvédelmi Körzet. Nógrád és Gömör határán; Bükki Nemzeti Park Igazgatóság: Eger, Hungary, 2007; pp. 1–382. [Google Scholar]
- GeoModel Solar. GIS Data and Maps for Europe, Turkey and North Africa. Technical Report No. 121-01/2014; Šúri, M., Ed.; GeoModel Solar: Bratislava, Slovakia, 2014; pp. 1–58. [Google Scholar]
- Vlčko, J.; Dítě, D.; Kolník, M. Vstavačovité Slovenska; ZO SZOPK Orchidea: Zvolen, Slovakia, 2003; pp. 1–120. [Google Scholar]
- Eliáš, P.; Dítě, D.; Kliment, J.; Hrivnák, R.; Feráková, V. Red list of ferns and flowering plants of Slovakia, 5th edition (October 2014). Biologia 2015, 70, 218–228. [Google Scholar] [CrossRef]
- Niklfeld, H. Bericht über die Kartierung der Flora Mitteleuropas. Taxon 1971, 20, 545–571. [Google Scholar] [CrossRef]
- Austin, M.P.; Cunningham, R.B.; Fleming, P.N. New approaches to direct gradient analysis using environmental scalars and statistical curve-fitting procedures. Vegetatio 1984, 55, 11–27. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System; Open Source Geospatial Foundation Project. 2020. Available online: qgis.osgeo.org (accessed on 6 February 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: www.R-project.org (accessed on 6 February 2020).
- Steven, J.P.; Dudík, M.; Schapire, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.1). 2020. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 6 February 2020).
- Marhold, K.; Hindák, F. (Eds.) Checklist of Non-Vascular and Vascular Plants of Slovakia; Veda: Bratislava, Slovakia, 1998; pp. 1–687. [Google Scholar]
- Molnár, V.A. Magyarország Orchideáinak Atlasza; Kossuth Kiadó: Budapest, Hungary, 2011; pp. 1–510. [Google Scholar]
- Hrivnák, R.; Hrivnák, M.; Slezák, M.; Vlčko, J.; Baltiarová, J.; Svitok, M. Distribution and eco-coenotic patterns of the forest orchid Epipactis pontica in Slovakia. Ann. For. Res. 2014, 57, 55–69. [Google Scholar] [CrossRef]
- Potůček, O. Kľúč na Určovanie Vstavačovitých Československa; Rosalia, Mimoriadne Vydanie: Nitra, Slovakia, 1990; pp. 1–154. [Google Scholar]
- Jatiová, M.; Šmiták, J. Rozšíření a Ochrana Orchidejí na Moravě a ve Slezku; ArcaJiMfa: Třebíč, Czech Republic, 1996; pp. 1–545. [Google Scholar]
- Hrivnák, R.; Palkovič, J. Orchis Tridentata Scop. v Cerovej vrchovine. Bull. Slov. Bot. Spoločn. 1996, 18, 106–107. [Google Scholar]
- Slezák, M.; Hrivnák, R.; Belanová, E.; Jarčuška, B. Komentovaný prehľad zaujímavých nálezov cievnatých rastlín z územia stredného Slovenska. Bull. Slov. Bot. Spoločn. 2010, 32, 59–71. [Google Scholar]
- Štípková, Z.; Kosánová, K.; Romportl, D.; Kindlmann, P. Determinants of Orchid Occurrence: A Czech Example, Selected Studies in Biodiversity; IntechOpen: London, UK, 2018. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Naczk, A.M.; Kolanowska, M. Glacial Refugia and Future Habitat Coverage of Selected Dactylorhiza Representatives (Orchidaceae). PLoS ONE 2015, 10, e0143478. [Google Scholar] [CrossRef] [PubMed]
- Martinis, A.; Chaideftou, E.; Minotou, C.; Poirazidis, K. Spatial analysis of orchids diversity unveils hot-spots: The case of Zante Island, Greece. J. Agricult. Inform. 2018, 9, 26–40. [Google Scholar] [CrossRef]
- Vass, D. (Ed.) Geológia Lučenskej kotliny a Cerovej vrchoviny; Štátny geologický ústav Dioníza Štúra: Bratislava, Slovakia, 2007; pp. 1–284. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).